Stratification of Oligometastatic Prostate Cancer Patients by Liquid Biopsy: Clinical Insights from a Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients Selection and Treatment
2.2. Biological Experimental Plan
2.2.1. cfDNA Extraction and Sequencing
2.2.2. Library Preparation and NGS Analysis
2.3. Statistical Analysis
Normality of the Distributions as Assessed Using the Kolmogorov–Smirnov Test
3. Results
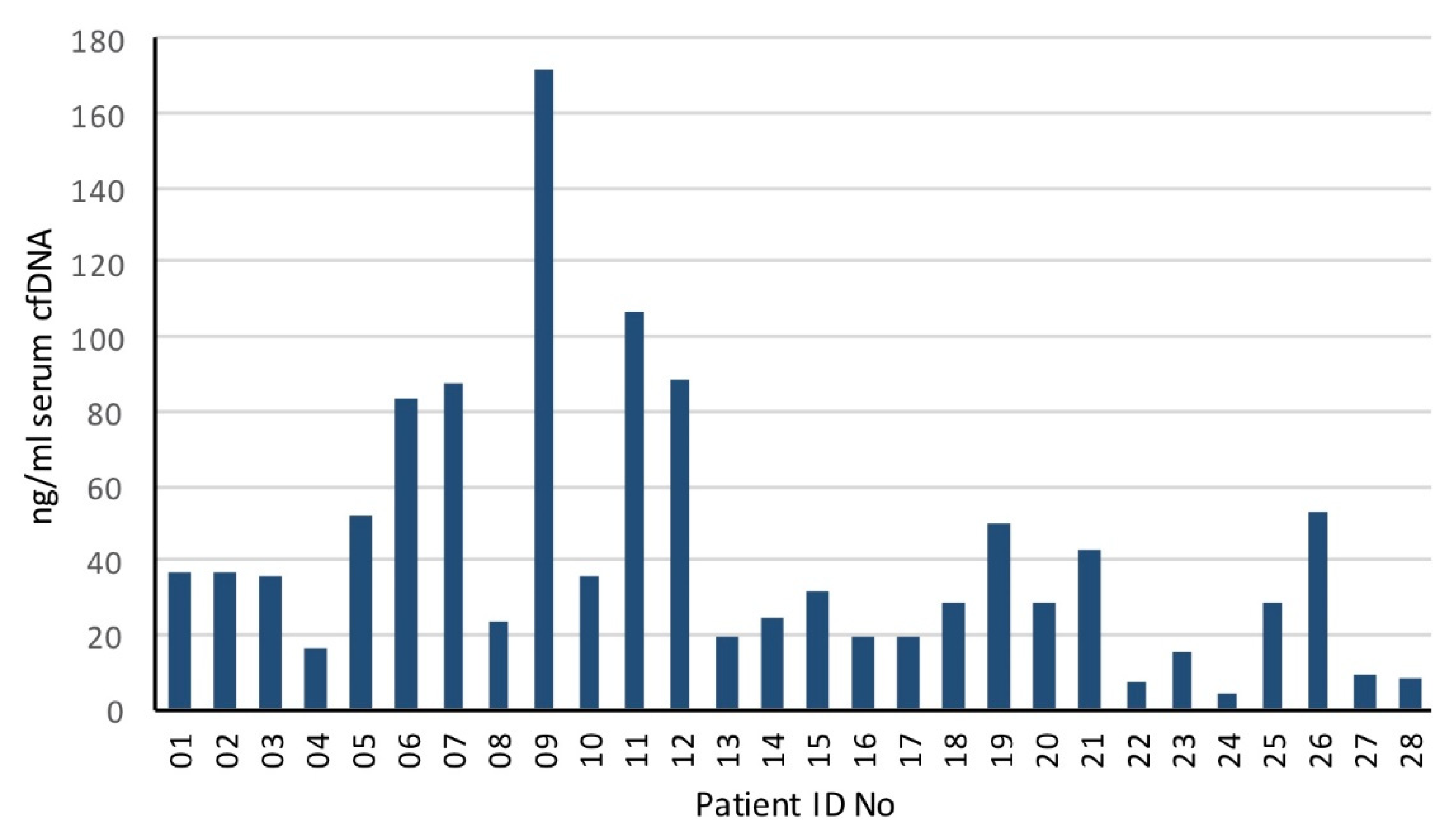
3.1. Population Clinical Characteristics and Outcomes
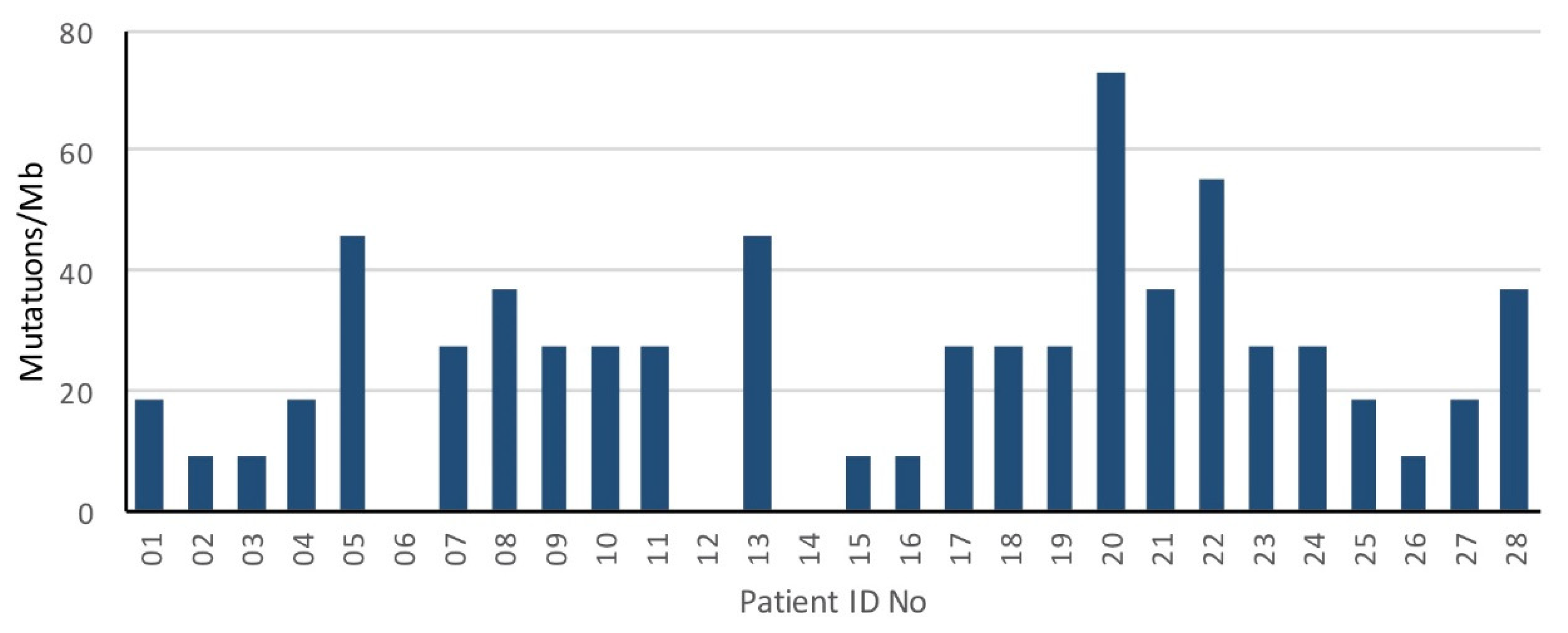
3.2. Genomic Landscape of Oligometastatic Prostate Cancer
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
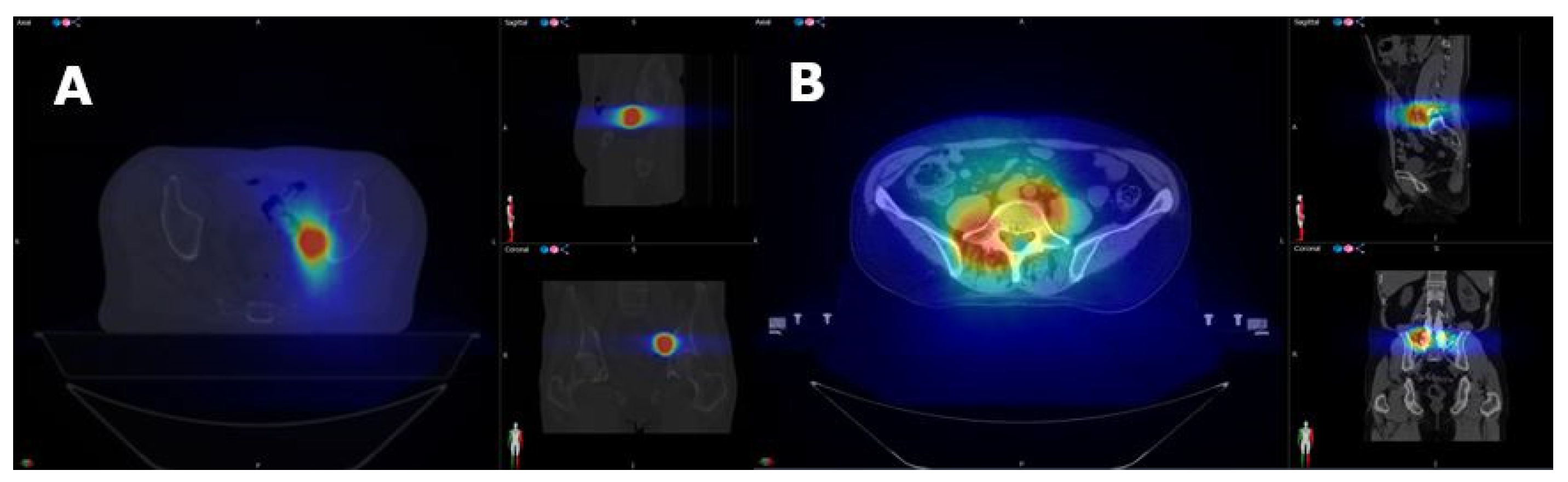
Appendix A.1. Radiotherapy Procedures
Appendix A.1.1. Target Volume Delineation
Appendix A.1.2. Dose Prescription
Appendix A.1.3. Radiotherapy Planning
| Abdominal targets | Value |
| Spine | Dmax < 18 Gy |
| Kidneys | V15Gy < 35% |
| Stomach | V36Gy < 3%, |
| Duodenum | V36Gy < 1% |
| Bowel bag | V36Gy < 3% |
| Liver | V15Gy < critical volume (700 cc) |
| Pelvic targets | |
| Bowel bag | D1cc < 21 Gy |
| Rectum | Dmax < 100% of prescription dose |
| Bladder/Urethra | Dmax < 120% of prescription dose |
| Bone targets | |
| Spine | Dmax < 21.9 Gy |
| D0.035cc < 18 Gy | |
| D1-2cc 12.3 Gy | |
| Spinal nerve roots | D3cc < 20.4 Gy |
| D0.035cc < 24 Gy | |
| Descending aorta | Dmax < 30 Gy |
| Ileum | Dmax < 25.2 Gy |
| Spine | D5cc < 17.7 Gy |
Appendix A.1.4. Follow Up
Appendix B
| Sample | Chromosome | Start | End | Reference Nucleotide | Altered Nucleotide | Gene | Effect | Evolution Disease |
|---|---|---|---|---|---|---|---|---|
| 1 | chr11 | 108272729 | 108272729 | C | G | ATM | missense | Polymetastatic disease |
| chrX | 67545317 | 67545319 | GCA | - | AR | nonframeshift deletion | ||
| 2 | chr2 | 47512394 | 47512394 | G | A | MSH2 | missense | Oligometastatic disease |
| 3 | chr11 | 108249096 | 108249096 | T | C | ATM | missense | Polymetastatic disease |
| 4 | chr11 | 108310287 | 108310287 | A | G | ATM | missense | Oligometastatic disease |
| chr14 | 37594904 | 37594904 | C | G | FOXA1 | missense | ||
| 5 | chr5 | 151663550 | 151663550 | C | T | SPARC | missense | Oligometastatic disease |
| chr11 | 108249096 | 108249096 | T | C | ATM | missense | ||
| chr12 | 11891545 | 11891545 | C | T | ETV6 | UTR3 | ||
| chrX | 67546515 | 67546529 | GGCGGCGGCGGCGGC | - | AR | nonframeshift deletion | ||
| chrX | 67546518 | 67546529 | GGCGGCGGCGGC | - | AR | nonframeshift deletion | ||
| 6 | - | - | - | - | - | - | - | Oligometastatic disease |
| 7 | chr2 | 208243577 | 208243577 | A | G | IDH1 | missense | Oligometastatic disease |
| chr17 | 43074471 | 43074471 | G | T | BRCA1 | missense | ||
| chrX | 67545316 | 67545316 | - | GCA | AR | nonframeshift insertion | ||
| 8 | chr9 | 72952984 | 72952984 | C | T | ALDH1A1 | missense | Oligometastatic disease |
| chr13 | 32329468 | 32329469 | TG | - | BRCA2 | frameshift deletion | ||
| chr13 | 32340099 | 32340099 | C | T | BRCA2 | missense | ||
| chrX | 67545317 | 67545322 | GCAGCA | - | AR | nonframeshift deletion | ||
| 9 | chr11 | 108244873 | 108244873 | C | T | ATM | stopgain | Oligometastatic disease |
| chr12 | 11890993 | 11890993 | C | G | ETV6 | missense | ||
| chr13 | 32332592 | 32332592 | A | C | BRCA2 | missense | ||
| 10 | chr14 | 37591441 | 37591441 | G | A | FOXA1 | missense | Polymetastatic disease |
| chr17 | 35106468 | 35106468 | G | A | RAD51D | missense | ||
| chrX | 67545317 | 67545322 | GCAGCA | - | AR | nonframeshift deletion | ||
| 11 | chr13 | 32332592 | 32332592 | A | C | BRCA2 | missense | Polymetastatic disease |
| chr17 | 43092412 | 43092412 | G | A | BRCA1 | missense | ||
| chr19 | 50413766 | 50413766 | G | A | POLD1 | missense | ||
| 12 | - | - | - | - | - | - | Oligometastatic disease | |
| 13 | chr7 | 81745064 | 81745064 | T | G | HGF | missense | Polymetastatic disease |
| chr11 | 108272849 | 108272849 | A | G | ATM | missense | ||
| chr11 | 108304735 | 108304735 | G | A | ATM | missense | ||
| chr13 | 32338416 | 32338416 | C | T | BRCA2 | missense | ||
| chrX | 67545317 | 67545328 | GCAGCAGCAGCA | - | AR | nonframeshift deletion | ||
| 14 | - | - | - | - | - | - | Polymetastatic disease | |
| 15 | chr11 | 108304735 | 108304735 | G | A | ATM | missense | Polymetastatic disease |
| 16 | chr14 | 37592342 | 37592342 | C | G | FOXA1 | missense | Oligometastatic disease |
| 17 | chr11 | 108289623 | 108289623 | C | T | ATM | missense | Oligometastatic disease |
| chr11 | 108279497 | 108279497 | C | - | ATM | frameshift deletion | ||
| chr13 | 32332592 | 32332592 | A | C | BRCA2 | missense | ||
| 18 | chr12 | 132677409 | 132677409 | C | T | POLE | missense | Polymetastatic disease |
| chr7 | 81729735 | 81729735 | G | A | HGF | missense | ||
| chr17 | 43093454 | 43093454 | G | A | BRCA1 | missense | ||
| 19 | chr13 | 32332592 | 32332592 | A | C | BRCA2 | missense | Oligometastatic disease |
| chr17 | 35106468 | 35106468 | G | A | RAD51D | missense | ||
| chrX | 67545317 | 67545322 | GCAGCA | - | AR | nonframeshift deletion | ||
| 20 | chr11 | 108272729 | 108272729 | C | G | ATM | missense | Oligometastatic disease |
| chr11 | 108267276 | 108267276 | T | C | ATM | missense | ||
| chr12 | 132677409 | 132677409 | C | T | POLE | missense | ||
| chr13 | 32332592 | 32332592 | A | C | BRCA2 | missense | ||
| chr17 | 43093454 | 43093454 | G | A | BRCA1 | missense | ||
| chr17 | 35106468 | 35106468 | G | A | RAD51D | missense | ||
| chrX | 67546514 | 67546514 | - | GGC | AR | nonframeshift insertion | ||
| chrX | 67545319 | 67545319 | A | T | AR | missense | ||
| 21 | chr7 | 81729735 | 81729735 | G | A | HGF | missense | Oligometastatic disease |
| chr12 | 132676174 | 132676174 | T | G | POLE | missense | ||
| chr13 | 32332592 | 32332592 | A | C | BRCA2 | missense | ||
| chrX | 67545317 | 67545325 | GCAGCAGCA | - | AR | nonframeshift deletion | ||
| 22 | chr11 | 108304735 | 108304735 | G | A | ATM | missense | Oligometastatic disease |
| chr12 | 132677409 | 132677409 | C | T | POLE | missense | ||
| chr17 | 43070958 | 43070958 | G | A | BRCA1 | missense | ||
| chr17 | 35106468 | 35106468 | G | A | RAD51D | missense | ||
| chrX | 67546515 | 67546520 | GGCGGC | - | AR | nonframeshift deletion | ||
| chrX | 67545317 | 67545334 | GCAGCAGCAGCAGCAGCA | - | AR | nonframeshift deletion | ||
| 23 | chr12 | 132677409 | 132677409 | C | T | POLE | missense | Oligometastatic disease |
| chr13 | 32336400 | 32336401 | TC | - | BRCA2 | frameshift deletion | ||
| chr13 | 32338613 | 32338613 | G | T | BRCA2 | missense | ||
| 24 | chr11 | 108304735 | 108304735 | G | A | ATM | missense | Oligometastatic disease |
| chr13 | 32332592 | 32332592 | A | C | BRCA2 | missense | ||
| chr17 | 43528665 | 43528665 | C | T | ETV4 | missense | ||
| 25 | chr11 | 108304735 | 108304735 | G | A | ATM | missense | Oligometastatic disease |
| chr17 | 43093454 | 43093454 | G | A | BRCA1 | missense | ||
| 26 | chrX | 67545317 | 67545346 | GCAGCAGCAGCAGCAGCAGCAGCAGCAGCA | - | AR | nonframeshift deletion | Oligometastatic disease |
| 27 | chr11 | 108254034 | 108254034 | T | C | ATM | missense | Oligometastatic disease |
| chr17 | 43533863 | 43533863 | T | C | ETV4 | missense | ||
| chrX | 67545317 | 67545322 | GCAGCA | - | AR | nonframeshift deletion | ||
| 28 | chr11 | 108304735 | 108304735 | G | A | ATM | missense | Oligometastatic disease |
| chr13 | 32332456 | 32332456 | C | A | BRCA2 | missense | ||
| chr13 | 32332592 | 32332592 | A | C | BRCA2 | missense | ||
| chrX | 67545317 | 67545328 | GCAGCAGCAGCA | - | AR | nonframeshift deletion |
References
- Foster, C.C.; Weichselbaum, R.R.; Pitroda, S.P. Oligometastatic Prostate Cancer: Reality or Figment of Imagination? Cancer 2019, 125, 340–352. [Google Scholar] [CrossRef] [PubMed]
- Hellman, S.; Weichselbaum, R.R. Oligometastases. J. Clin. Oncol. 1995, 13, 8–10. [Google Scholar] [CrossRef] [PubMed]
- Hellman, S.; Weichselbaum, R.R. Oligometastases Revised. Nat. Rev. Clin. Oncol. 2011, 8, 378–382. [Google Scholar] [CrossRef]
- Reyes, D.K.; Pienta, K.J. The biology and treatment of oligometastatic cancer. Oncotarget 2015, 6, 8491–8524. [Google Scholar] [CrossRef] [PubMed]
- Soloway, M.S.; Hardeman, S.W.; Hickey, D.; Todd, B.; Soloway, S.; Raymond, J.; Moinuddin, M. Stratification of patients with metastatic prostate cancer based on extent of disease on initial bone scan. Cancer 1988, 61, 195–202. [Google Scholar] [CrossRef]
- Singh, D.; Yi, W.S.; A Brasacchio, R.; Muhs, A.G.; Smudzin, T.; Williams, J.P.; Messing, E.; Okunieff, P. Is there a favorable subset of patients with prostate cancer who develop oligometastases? Int. J. Radiat. Oncol. Biol. Phys. 2004, 58, 3–10. [Google Scholar] [CrossRef]
- Tosoian, J.J.; Gorin, M.A.; Ross, A.E.; Pienta, K.J.; Tran, P.T.; Schaeffer, E.M. Oligometastatic prostate cancer: Definitions, clinical outcomes, and treatment considerations. Nat. Rev. Urol. 2017, 14, 15–25. [Google Scholar] [CrossRef]
- Gillessen, S.; Omlin, A.; Attard, G.; de Bono, J.S.; Efstathiou, E.; Fizazi, K.; Halabi, S.; Nelson, P.S.; Sartor, O.; Smith, M.R.; et al. Management of patients with advanced prostate cancer: Recommendations of the St Gallen Advanced Prostate Cancer Consensus Conference (APCCC). Ann. Oncol. 2015, 26, 1589–1604. [Google Scholar] [CrossRef]
- Casamassima, F.; Masi, L.; Menichelli, C.; Bonucci, I.; Casamassima, E.; Lazzeri, M.; Gulisano, M.; Aterini, S. Efficacy of eradicative radiotherapy for limited nodal metastases detected with choline PET scan in prostate cancer patients. Tumori 2011, 97, 49–55. [Google Scholar] [CrossRef]
- Jereczek-Fossa, B.A.; Beltramo, G.; Fariselli, L.; Fodor, C.; Santoro, L.; Vavassori, A.; Zerini, D.; Gherardi, F.; Ascione, C.; Bossi-Zanetti, I.; et al. Robotic image-guided stereotactic radiotherapy, for isolated recurrent primary, lymph node or metastatic prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, 889–897. [Google Scholar] [CrossRef]
- Muacevic, A.; Kufeld, M.; Rist, C.; Wowra, B.; Stief, C.; Staehler, M. Safety and feasibility of image-guided robotic radiosurgery for patients with limited bone metastases of prostate cancer. Urol. Oncol. 2013, 31, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, K.A.; Barney, B.M.; Davis, B.J.; Park, S.S.; Kwon, E.D.; Olivier, K.R. Stereotactic body radiation therapy in the treatment of oligometastatic prostate cancer. Front. Oncol. 2013, 2, 215. [Google Scholar] [CrossRef] [PubMed]
- Berkovic, P.; De Meerleer, G.; Delrue, L.; Lambert, B.; Fonteyne, V.; Lumen, N.; Decaestecker, K.; Villeirs, G.; Vuye, P.; Ost, P. Salvage stereotactic body radiotherapy for patients with limited prostate cancer metastases: Deferring androgen deprivation therapy. Clin. Genitourin. Cancer 2013, 11, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Schick, U.; Jorcano, S.; Nouet, P.; Rouzaud, M.; Vees, H.; Zilli, T.; Ratib, O.; Weber, D.C.; Miralbell, R. Androgen deprivation and high-dose radiotherapy for oligometastatic prostate cancer patients with less than five regional and/or distant metastases. Acta Oncol. 2013, 52, 1622–1628. [Google Scholar] [CrossRef] [PubMed]
- Decaestecker, K.; De Meerleer, G.; Lambert, B.; Delrue, L.; Fonteyne, V.; Claeys, T.; De Vos, F.; Huysse, W.; Hautekiet, A.; Maes, G.; et al. Repeated stereotactic body radiotherapy for oligometastatic prostate cancer recurrence. Radiat. Oncol. 2014, 9, 135. [Google Scholar] [CrossRef]
- Detti, B.; Bonomo, P.; Masi, L.; Doro, R.; Cipressi, S.; Iermano, C.; Bonucci, I.; Franceschini, D.; Di Brina, L.; Bakhi, M.; et al. Stereotactic radiotherapy for isolated nodal recurrence of prostate cancer. World J. Urol. 2015, 33, 1197–1203. [Google Scholar] [CrossRef]
- Ost, P.; Jereczek-Fossa, B.; Van As, N.; Zilli, T.; Muacevic, A.; Brown, L.; Casamassima, F.; Orecchia, R.; Henderson, D.; Olivier, K.; et al. A multi-institutional analysis of stereotactic body radiotherapy for oligometastatic prostate cancer recurrence. Eur. Urol. Suppl. 2015, 14, e447. [Google Scholar] [CrossRef]
- Henderson, D.R.; Tree, A.; Taylor, H.; Khoo, V.; Van As, N.J. Oligometastatic prostate cancer: An evaluation of stereotactic body radiotherapy (SBRT) as an alternative to palliative androgen deprivation therapy. J. Clin. Oncol. 2015, 33 (Suppl. 1), 696–702. [Google Scholar] [CrossRef]
- Muldermans, J.L.; Romak, L.B.; Kwon, E.D.; Park, S.S.; Olivier, K.R. Stereotactic Body Radiation Therapy for Oligometastatic Prostate Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2016, 95, 696–702. [Google Scholar] [CrossRef]
- Ost, P.; Jereczek-Fossa, B.A.; Van As, N.; Zilli, T.; Muacevic, A.; Olivier, K.; Henderson, D.; Casamassima, F.; Orecchia, R.; Surgo, A.; et al. Progression-free Survival Following Stereotactic Body Radiotherapy for Oligometastatic Prostate Cancer Treatment-naive Recurrence: A Multi-institutional Analysis. Eur. Urol. 2016, 69, 9–12. [Google Scholar] [CrossRef]
- Ost, P.; Jereczek-Fossa, B.A.; Van As, N.; Zilli, T.; Tree, A.; Henderson, D.; Orecchia, R.; Casamassima, F.; Surgo, A.; Miralbell, R.; et al. Pattern of Progression after Stereotactic Body Radiotherapy for Oligometastatic Prostate Cancer Nodal Recurrences. Clin. Oncol. 2016, 28, e115–e120. [Google Scholar] [CrossRef] [PubMed]
- Tran, P.; Radwan, N.; Ross, A.; Reyes, D.; Wright, J.; Song, D.; Deville, C.; Deweese, T.; Carducci, M.; Schaeffer, E.; et al. Stereotactic ablative radiation therapy for the treatment of oligometastatic prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 2016, 96 (Suppl. 1), E248. [Google Scholar] [CrossRef][Green Version]
- Oehler, C.; Zimmermann, M.; Curschmann, J.; Sumila, M.; Strebel, R.; Li, Q.; Schneider, U.; Zwahlen, D. Factors predicting the benefit of stereotactic body radiation therapy for oligometastatic lymph node recurrence in prostate cancer: A single institution experience. Int. J. Radiat. Oncol. Biol. Phys. 2017, 99 (Suppl. 1), E282. [Google Scholar] [CrossRef]
- Jereczek-Fossa, B.A.; Fanetti, G.; Fodor, C.I.; Ciardo, D.; Santoro, L.; Francia, C.M.; Muto, M.; Surgo, A.; Zerini, D.; Marvaso, G.; et al. Salvage Stereotactic Body Radiotherapy for Isolated Lymph Node Recurrent Prostate Cancer: Single Institution Series of 94 Consecutive Patients and 124 Lymph Nodes. Clin. Genitourin. Cancer 2017, 15, e623–e632. [Google Scholar] [CrossRef]
- Ingrosso, G.; Trippa, F.; Maranzano, E.; Maranzano, E.; Carosi, A.; Ponti, E.; Arcidiacono, F.; Draghini, L.; Di Murro, L.; Lancia, A.; et al. Stereotactic body radiotherapy in oligometastatic prostate cancer patients with isolated lymph nodes involvement: A two-institution experience. World J. Urol. 2017, 35, 45–49. [Google Scholar] [CrossRef]
- Triggiani, L.; Alongi, F.; Buglione, M.; Detti, B.; Santoni, R.; Bruni, A.; Maranzano, E.; Lohr, F.; D’Angelillo, R.; Magli, A.; et al. Efficacy of stereotactic body radiotherapy in oligorecurrent and in oligoprogressive prostate cancer: New evidence from a multicentric study. Br. J. Cancer 2017, 116, 1520–1525. [Google Scholar] [CrossRef]
- Ponti, E.; Lancia, A.; Ost, P.; Trippa, F.; Triggiani, L.; Detti, B.; Ingrosso, G. Exploring All Avenues for Radiotherapy in Oligorecurrent Prostate Cancer Disease Limited to Lymph Nodes: A Systemic Review of the Role of Stereotactic Body Radiotherapy. Eur. Urol. Focus 2017, 3, 538–544. [Google Scholar] [CrossRef]
- Triggiani, L.; Mazzola, R.; Tomasini, D.; Bruni, A.; Alicino, G.; Matrone, F.; Bortolus, R.; Francolini, G.; Detti, B.; Magli, A.; et al. Upfront metastasis-directed therapy in oligorecurrent prostate cancer does not decrease the time from initiation of androgen deprivation therapy to castration resistance. Med. Oncol. 2021, 38, 72. [Google Scholar] [CrossRef]
- Mazzola, R.; Francolini, G.; Triggiani, L.; Napoli, G.; Cuccia, F.; Nicosia, L.; Livi, L.; Magrini, S.M.; Salgarello, M.; Alongi, F. Metastasis-directed Therapy (SBRT) Guided by PET-CT 18F-CHOLINE Versus PET-CT 68Ga-PSMA in Castration-sensitive Oligorecurrent Prostate Cancer: A Comparative Analysis of Effectiveness. Clin. Genitourin. Cancer 2021, 19, 230–236. [Google Scholar] [CrossRef]
- Mazzola, R.; Cuccia, F.; Pastorello, E.; Salgarello, M.; Francolini, G.; Livi, L.; Triggiani, L.; Magrini, S.M.; Ingrosso, G.; Aristei, C.; et al. PSMA-guided metastases directed therapy for bone castration sensitive oligometastatic prostate cancer: A multi-institutional study. Clin. Exp. Metastasis 2022, 39, 443–448. [Google Scholar] [CrossRef]
- Decaestecker, K.; Meerleer, G.D.; Ameye, F.; Fonteyne, V.; Lambert, B.; Joniau, S.; Delrue, L.; Billiet, I.; Duthoy, W.; Junius, S.; et al. Surveillance or Metastasis-Directed Therapy for Oligometastatic Prostate Cancer Recurrence (STOMP): Study protocol for a randomized Phase II Trial. BMC Cancer 2014, 14, 671. [Google Scholar] [CrossRef] [PubMed]
- Ost, P.; Reynders, D.; Decaestecker, K.; Fonteyne, V.; Lumen, N.; De Bruycker, A.; Lambert, B.; Delrue, L.; Bultijnck, R.; Claeys, T.; et al. Surveillance or Metastasis-Directed Therapy for Oligometastatic Prostate Cancer Recurrence: A Prospective, Randomized, Multicenter Phase II Trial. J. Clin. Oncol. 2018, 36, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Phillips, R.; Shi, W.Y.; Deek, M.; Radwan, N.; Lim, S.J.; Antonarakis, E.S.; Rowe, S.P.; Ros, A.E.; Gorin, M.A.; Deville, C.; et al. Outcomes of Observation vs. Stereotactic Ablative Radiation for Oligometastatic Prostate Cancer: The ORIOLE Phase 2 Randomized Clinical Trial. JAMA Oncol. 2020, 6, 650–659. [Google Scholar] [CrossRef] [PubMed]
- Cornford, P.; van den Bergh, R.C.N.; Briers, E.; Van den Broeck, T.; Cumberbatch, M.G.; De Santis, M.; Fanti, S.; Fossati, N.; Gandaglia, G.; Gillessen, S.; et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer. Part II-2020 Update: Treatment of Relapsing and Metastatic Prostate Cancer. Eur. Urol. 2021, 79, 263–282. [Google Scholar] [CrossRef] [PubMed]
- The Cancer Genome Atlas Research Network. The Molecular Taxonomy of Primary Prostate Cancer. Cell 2015, 163, 1011–1025. [Google Scholar] [CrossRef] [PubMed]
- Foroni, C.; Zarovni, N.; Bianciardi, L.; Bernardi, S.; Triggiani, L.; Zocco, D.; Venturella, M.; Chiesi, A.; Valcamonico, F.; Berruti, A. When Less Is More: Specific Capture and Analysis of Tumor Exosomes in Plasma Increases the Sensitivity of Liquid Biopsy for Comprehensive Detection of Multiple Androgen Receptor Phenotypes in Advanced Prostate Cancer Patients. Biomedicine 2020, 8, 131. [Google Scholar] [CrossRef] [PubMed]
- Bardelli, A.; Pantel, K. Liquid Biopsies, What We Do Not Know (Yet). Cancer Cell 2017, 31, 172–179. [Google Scholar] [CrossRef]
- Shyr, D.; Liu, Q. Next generation sequencing in cancer research and clinical application. Biol. Proced. Online 2013, 15, 4. [Google Scholar] [CrossRef]
- Beltran, H.; Yelensky, R.; Frampton, G.M.; Park, K.; Downing, S.R.; MacDonald, T.Y.; Jarosz, M.; Lipson, D.; Tagawa, S.T.; Nanus, D.M.; et al. Targeted Next-generation Sequencing of Advanced Prostate Cancer Identifies Potential Therapeutic Targets and Disease Heterogeneity. Eur. Urol. 2013, 63, 920–926. [Google Scholar] [CrossRef]
- Triggiani, L.; Bardoscia, L.; Colosini, A.; Bernardi, S.; Bresciani, R.; Foroni, C.; Pasinetti, N.; Borghetti, P.; Caraffini, B.; Orizio, F.; et al. Oligometastatic prostate cancer patients stratification: A molecular signature identified by liquid biopsy. J. Clin. Oncol. 2018, 36 (Suppl. 6), TPS400. [Google Scholar] [CrossRef]
- World Medical Association. Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef] [PubMed]
- Cornford, P.; Bellmunt, J.; Bolla, M.; Briers, E.; De Santis, M.; Gross, T.; Henry, A.M.; Joniau, S.; Lam, T.B.; Mason, M.D.; et al. EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part II: Treatment of Relapsing, Metastatic, and Castration-Resistant Prostate Cancer. Eur. Urol. 2017, 71, 630–642. [Google Scholar] [CrossRef] [PubMed]
- Van Etten, J.L.; Dehm, S.M. Clonal origin and spread of metastatic prostate cancer. Endocr. Relat. Cancer 2016, 23, R207–R217. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, S.; Farina, M.; Zanaglio, C.; Cattina, F.; Polverelli, N.; Schieppati, F.; Re, F.; Foroni, C.; Malagola, M.; Dunbar, A.J.; et al. ETV6: A Candidate Gene for Predisposition to “Blend Pedigrees”? A Case Report from the NEXT-Famly Clinical Trial. Case Rep Hematol. 2020, 2020, 2795656. [Google Scholar] [CrossRef] [PubMed]
- Arora, K.; Barbieri, C.E. Molecular Subtypes of Prostate Cancer. Curr. Oncol. Rep. 2018, 20, 58. [Google Scholar] [CrossRef]
- Robinson, D.; Van Allen, E.M.; Wu, Y.M.; Schultz, N.; Lonigro, R.J.; Mosquera, J.M.; Montgomery, B.; Taplin, M.E.; Pritchard, C.C.; Attard, G.; et al. Integrative Clinical Genomics of Advanced Prostate Cancer. Cell 2015, 161, 1215–1228. [Google Scholar] [CrossRef]
- Sumanasuriya, S.; De Bono, J. Treatment of Advanced Prostate Cancer—A Review of Current Therapies and Future Promise. Cold Spring Harb. Perspect. Med. 2018, 8, a030635. [Google Scholar] [CrossRef]
- Alix-Panabières, C.; Pantel, K. Liquid Biopsy: From Discovery to Clinical Application. Cancer Discov. 2021, 11, 858–873. [Google Scholar] [CrossRef]
- Clarke, N.; Wienchno, P.; Alekseev, B.; Sala, N.; Jones, R.; Kocak, I.; Chiuri, V.E.; Jassem, J.; Flechon, A.; Redfern, C.; et al. Olaparib combined with abiraterone in patients with metastatic castration-resistant prostate cancer: A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2018, 19, 975–986. [Google Scholar] [CrossRef]
- Carr, T.H.; Adelman, C.; Barnicle, A.; Kozarewa, I.; Luke, S.; Lai, Z.; Hollis, S.; Dougherty, B.; Harrington, E.A.; Kang, J.; et al. Homologous Recombination Repair Gene Mutation Characterization by Liquid Biopsy: A Phase II Trial of Olaparib and Abiraterone in Metastatic Castrate-Resistant Prostate Cancer. Cancers 2021, 13, 5830. [Google Scholar] [CrossRef]
- Zocco, D.; Bernardi, S.; Novelli, M.; Astrua, C.; Fava, P.; Zarovni, N.; Carpi, F.M.; Bianciardi, L.; Malavenda, O.; Quaglino, P.; et al. Isolation of extracellular vesicles improves the detection of mutant DNA from plasma of metastatic melanoma patients. Sci. Rep. 2020, 10, 15745. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, L.; Chapman, E.; Khoo, V.; Suh, Y.E.; Tunariu, N.; Wang, Y.; van As, N. Metastasis-directed Therapy in Prostate Cancer: Prognostic Significance of the ESTRO/EORTC Classification in Oligometastatic Bone Disease. Clin. Oncol. 2022, 34, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Stelcer, E.; Konkol, M.; Głeboka, A.; Suchorska, W.M. Liquid Biopsy in Oligometastatic Prostate Cancer—A Biologist’s Point of View. Front. Oncol. 2019, 9, 775. [Google Scholar] [CrossRef] [PubMed]
- Gorges, T.M.; Kuske, A.; Rock, K.; Mauermann, O.; Muller, V.; Peine, S.; Verpoort, K.; Novosadova, V.; Kubista, M.; Riethdorf, S.; et al. Accession of tumor heterogeneity by multiplex transcriptome profiling of single circulating tumor cells. Clin. Chem 2016, 62, 1504–1515. [Google Scholar] [CrossRef]
- Scher, H.I.; Graf, R.P.; Schreiber, N.A.; McLaughlin, B.; Jendrisak, A.; Wang, Y.; Lee, J.; Greene, S.; Krupa, R.; Lu, D.; et al. Phenotypic heterogeneity of circulating tumor cells informs clinical decisions between AR signaling inhibitors and taxanes in metastatic prostate cancer. Cancer Res. 2017, 77, 5687–5698. [Google Scholar] [CrossRef]
- Pereira, B.; Chen, C.T.; Goyal, L.; Walmsley, C.; Pinto, C.J.; Baiev, I.; Allen, R.; Henderson, L.; Saha, S.; Reyes, S.; et al. Cell-free DNA captures tumor heterogeneity and driver alterations in rapid autopsies with pre-treated metastatic cancer. Nat. Commun. 2021, 12, 3199. [Google Scholar] [CrossRef]
- Tsui, D.W.Y.; Cheng, M.L.; Shady, M.; Yang, J.L.; Stephens, D.; Won, H.; Srinivasan, P.; Huberman, K.; Meng, F.; Jing, X.; et al. Tumor fraction-guided cell-free DNA profiling in metastatic solid tumor patients. Genome Med. 2021, 13, 96. [Google Scholar] [CrossRef]
- Perkins, G.; Yap, T.A.; Pope, L.; Cassidy, A.M.; Dukes, J.P.; Riisnaes, R.; Massard, C.; Cassier, P.A.; Miranda, S.; Clark, J.; et al. Multi-Purpose Utility of Circulating Plasma DNA Testing in Patients with Advanced Cancers. PLoS ONE 2012, 7, e47020. [Google Scholar] [CrossRef]
- Tan, M.H.E.; Jun, L.I.; Xu, H.E.; Melcher, K.; Yong, E.L. Androgen receptor: Structure, role in prostate cancer and drug discovery. Acta Pharmacol. Sin. 2015, 36, 3–23. [Google Scholar] [CrossRef]
- Oh, M.; Alkhushaym, N.; Fallatah, S.; Althagafi, A.; Aljadeed, R.; Alsowaida, Y.; Jeter, J.; Martin, J.R.; Babiker, H.M.; McBride, A.; et al. The association of BRCA1 and BRCA2 mutations with prostate cancer risk, frequency, and mortality: A meta-analysis. Prostate 2019, 79, 880–895. [Google Scholar] [CrossRef]
- Annala, M.; Vandekerkhove, G.; Khalaf, D.; Taavitsainen, S.; Beja, K.; Warner, E.W.; Sunderland, K.; Kollmannsberger, C.; Eigl, B.J.; Finch, D.; et al. Criculating Tumor DNA Genomics Correlate with Resistance to Abiraterone and Enzalutamide in Prostate Cancer. Cancer Discov. 2018, 8, 444–457. [Google Scholar] [CrossRef] [PubMed]
- Carter, H.B.; Helfand, B.; Mamawala, M.; Wu, Y.; Landis, P.; HYu, H.; Wiley, K.; Na, R.; Shi, Z.; Petkewicz, J.; et al. Germline Mutations in ATM and BRCA1/2 Are Associated with Grade Reclassification in Men on Active Surveillance for Prosate Cancer. Eur. Urol. 2019, 75, 743–749. [Google Scholar] [CrossRef] [PubMed]
- Meyer, A.; Wilhelm, B.; Dork, T.; Bremer, M.; Baumann, R.; Karstens, H.J.; Machtens, S. ATM missense variant P1054R predispose to prostate cancer. Radiother. Oncol. 2007, 83, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Pitroda, S.P.; Weichselbaum, R.R. Integrated molecular and clinical staging defines the spectrum of metastatic cancer. Nat Rev Clin. Oncol. 2019, 16, 581–588. [Google Scholar] [CrossRef]
- Bignardi, M.; Cozzi, L.; Fogliata, A.; Lattuada, P.; Mancosu, P.; Navarria, P.; Urso, G.; Vigorito, S.; Scorsetti, M. Critical appraisal of volumetric modulated arc therapy in stereotactic body radiation therapy for metastases to abdominal lymph nodes. Int. J. Radiat. Oncol. Biol. Phys. 2009, 75, 1570–1577. [Google Scholar] [CrossRef]
- Jereczek-Fossa, B.A.; Fariselli, L.; Beltramo, G.; Catalano, G.; Serafini, F.; Garibaldi, C.; Cambria, R.; Brait, L.; Possanzini, M.; Bianchi, L.C.; et al. Linac-based or robotic image-guided stereotactic radiotherapy for isolated lymph node recurrent prostate cancer. Radiother. Oncol. 2009, 93, 14–17. [Google Scholar] [CrossRef]
- Benedict, S.H.; Yenice, K.M.; Followill, D.; Galvin, J.M.; Hinson, W.; Kavanagh, B.; Keall, P.; Lovelock, M.; Meeks, S.; Papiez, L.; et al. Stereotactic body radiation therapy: The report of AAPM Task Group 101. Med. Phys. 2010, 37, 4078–4101. [Google Scholar] [CrossRef]
- Bignardi, M.; Navarria, P.; Mancosu, P.; Cozzi, L.; Fogliata, A.; Tozzi, A.; Castiglioni, S.; Carnaghi, C.; Tronconi, M.C.; Santoro, A.; et al. Clinical outcome of hypofractionated stereotactic radiotherapy for abdominal lymph node metastases. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, 831–838. [Google Scholar] [CrossRef]
- Alongi, F.; Fogliata, A.; Clerici, E.; Navarria, P.; Tozzi, A.; Comito, T.; Ascolese, A.M.; Clivio, A.; Lobefalo, F.; Reggiori, G.; et al. Volumetric modulated arc therapy with flattening filter free beams for isolated abdominal/pelvic lymph nodes: Report of dosimetric and early clinical results in oligometastatic patients. Radiat. Oncol. 2012, 7, 204. [Google Scholar] [CrossRef][Green Version]
- Jereczek-Fossa, B.A.; Piperno, G.; Ronchi, S.; Catalano, G.; Fodor, C.; Cambria, R.; Ing, P.F.; Gherardi, F.; Alterio, D.; Zerini, D.; et al. Linac-based stereotactic body radiotherapy for oligometastatic patients with single abdominal lymph node recurrent cancer. Am. J. Clin. Oncol. 2014, 37, 227–233. [Google Scholar] [CrossRef]
- Hanna, G.; Murray, L.; Patel, R.; Jain, S.; Aitken, K.; Franks, K.; van As, N.; Tree, A.; Hatfield, P.; Harrow, S.; et al. UK Consensus on Normal Tissue Dose Constraints for Stereotactic Radiotherapy. Clin. Oncol. 2018, 30, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Common Terminology Criteria for Adverse Events (CTCAE)—Version 4.0 Published: 28 May 2009 (v4.02: 15 September 2009)—U.S. Department of Health and Human Services, National Institutes of Health, National Cancer Institute. Available online: https://www.eortc.be/services/doc/ctc/ctcae_4.03_2010-06-14_quickreference_5x7.pdf (accessed on 28 May 2009).

| Inclusion Criteria |
|
| Exclusion criteria |
|
| TP53 | PIK3CA | mTOR | FOXA1 | FOXO1 | BRCA1 | BRCA2 |
| PTEN | CREB | AR | ETV1 | ETV3 | ETV4 | ETV6 |
| ALDH1A1 | ALDH3A1 | SPOP | FLI1 | IDH1 | ERG | SOX2 |
| cMET (HGFR) | HGF | SPARC | CAV1 | BMI1 | PARP1 | RB1 |
| ATM | CHEK2 | EGFR | POLE | POLD1 | MSH2 | MLH1 |
| RAD51D | SYK |
| Age | N | % | Residual Disease after Surgery | N | % | |
| <65 years | 13 | 46.4 | R0 (no) | 8 | 28.6 | |
| ≥65 years | 15 | 53.6 | R1 (microscopic) | 15 | 53.6 | |
| R2 (macroscopic) | 0 | 0.0 | ||||
| T stage | N | % | ||||
| T1c | 3 | 10.7 | EBRT 1 (total dose) | N | % | |
| T2c | 6 | 21.4 | 66 Gy | 6 | 21.4 | |
| T3a | 11 | 39.4 | 70 Gy | 18 | 64.2 | |
| T3b | 6 | 21.4 | 74 Gy | 1 | 3.6 | |
| T4 | 2 | 7.1 | ||||
| Elective node irradiation | 2 | 7.1 | ||||
| N stage | N | % | ||||
| N0 | 24 | 85.7 | Adjuvant ADT 1 | N | % | |
| N1 | 4 | 14.3 | No | 21 | 75.0 | |
| LHRH-analogue | 2 | 7.1 | ||||
| Gleason Grade Group | N | % | Antiandrogen | 4 | 14.3 | |
| 1 | 6 | 21.4 | Total Androgen Blockade | 1 | 3.6 | |
| 2 | 11 | 39.4 | ||||
| 3 | 3 | 10.7 | Biochemical relapse after primary treatment | N | % | |
| 4 | 2 | 7.1 | Yes | 27 | 96.4 | |
| 5 | 6 | 21.4 | No | 1 | 3.6 | |
| D’Amico Risk Class | N | % | Biochemical control duration | N | % | |
| Very low | 0 | 0,0 | <1 year | 5 | 17.9 | |
| Low | 2 | 7.1 | 1–5 years | 15 | 53.6 | |
| Favorable intermediate | 4 | 14.3 | >5 years | 8 | 28.5 | |
| Unfavorable intermediate | 2 | 7.1 | Median bRFS1 42.4mo (range 1.9–133.1) | |||
| High | 12 | 42.9 | ||||
| Very high | 8 | 28.6 | ADT 1 for biochemical relapse | N | % | |
| No relapse | 1 | 3.6 | ||||
| Primary treatment | N | % | No | 17 | 60.7 | |
| Surgery | 1 | 3.6 | LHRH-analogue | 6 | 21.4 | |
| EBRT 1 | 3 | 10.7 | Antiandrogen | 3 | 10.7 | |
| Brachytherapy (LDR 145 Gy) | 2 | 7.1 | Total Androgen Blockade | 1 | 3.6 | |
| Surgery+Adjuvant RT 1 | 9 | 32.2 | ||||
| Surgery+Salvage RT 1 | 13 | 46.4 | Number of treated lymph nodes | N | % | |
| 1 lymph node | 14 | 50.0 | ||||
| Oligorecurrence site | N | % | 2 lymph nodes | 5 | 17.8 | |
| Nodal | 20 | 71.5 | 3 lymph nodes | 2 | 7.1 | |
| Bone | 6 | 21.4 | 4 lymph nodes | 1 | 3.6 | |
| Both | 2 | 7.1 | ||||
| Bone SBRT 1 target | N | % | ||||
| Nodal SBRT 1 target | N | % | Axial | 4 | 14.3 | |
| Pelvic lymph nodes | 19 | 67.8 | Extra-axial | 4 | 14.3 | |
| Abdominal lymph nodes | 2 | 7.1 | Hip | 3 | 10.7 | |
| Both | 1 | 3.6 | Sternum/Ribs | 2 | 7.1 | |
| One nodal region | 18 | 64.2 | One site | 6 | 21.4 | |
| More than one nodal region | 4 | 14.3 | Two sites | 2 | 7.1 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colosini, A.; Bernardi, S.; Foroni, C.; Pasinetti, N.; Guerini, A.E.; Russo, D.; Bresciani, R.; Tomasi, C.; Magrini, S.M.; Bardoscia, L.; et al. Stratification of Oligometastatic Prostate Cancer Patients by Liquid Biopsy: Clinical Insights from a Pilot Study. Biomedicines 2022, 10, 1321. https://doi.org/10.3390/biomedicines10061321
Colosini A, Bernardi S, Foroni C, Pasinetti N, Guerini AE, Russo D, Bresciani R, Tomasi C, Magrini SM, Bardoscia L, et al. Stratification of Oligometastatic Prostate Cancer Patients by Liquid Biopsy: Clinical Insights from a Pilot Study. Biomedicines. 2022; 10(6):1321. https://doi.org/10.3390/biomedicines10061321
Chicago/Turabian StyleColosini, Antonella, Simona Bernardi, Chiara Foroni, Nadia Pasinetti, Andrea Emanuele Guerini, Domenico Russo, Roberto Bresciani, Cesare Tomasi, Stefano Maria Magrini, Lilia Bardoscia, and et al. 2022. "Stratification of Oligometastatic Prostate Cancer Patients by Liquid Biopsy: Clinical Insights from a Pilot Study" Biomedicines 10, no. 6: 1321. https://doi.org/10.3390/biomedicines10061321
APA StyleColosini, A., Bernardi, S., Foroni, C., Pasinetti, N., Guerini, A. E., Russo, D., Bresciani, R., Tomasi, C., Magrini, S. M., Bardoscia, L., & Triggiani, L. (2022). Stratification of Oligometastatic Prostate Cancer Patients by Liquid Biopsy: Clinical Insights from a Pilot Study. Biomedicines, 10(6), 1321. https://doi.org/10.3390/biomedicines10061321








