Neurofibromatosis: New Clinical Challenges in the Era of COVID-19
Abstract
1. Introduction
2. Search Strategy
2.1. Methods
- Neurofibromatosis: Rare Disease, Rare Diseases, Neurofibromatosis, Neurofibromatoses, NF, NFs, NF1, NF2, Schwannomatosis.
- COVID-19: COVID-19, COVID19, COVID-19 Virus, COVID-19 Viruses, COVID-2019, SARS-CoV-2, SARS-CoV-2 Infection, Coronavirus, Coronaviruses.
- Telemedicine: Telemedicine, Telehealth, Telecare, Teleconsultation, e-health, Telerehabilitation, Video conference, remote support, phone calls.
2.2. Findings
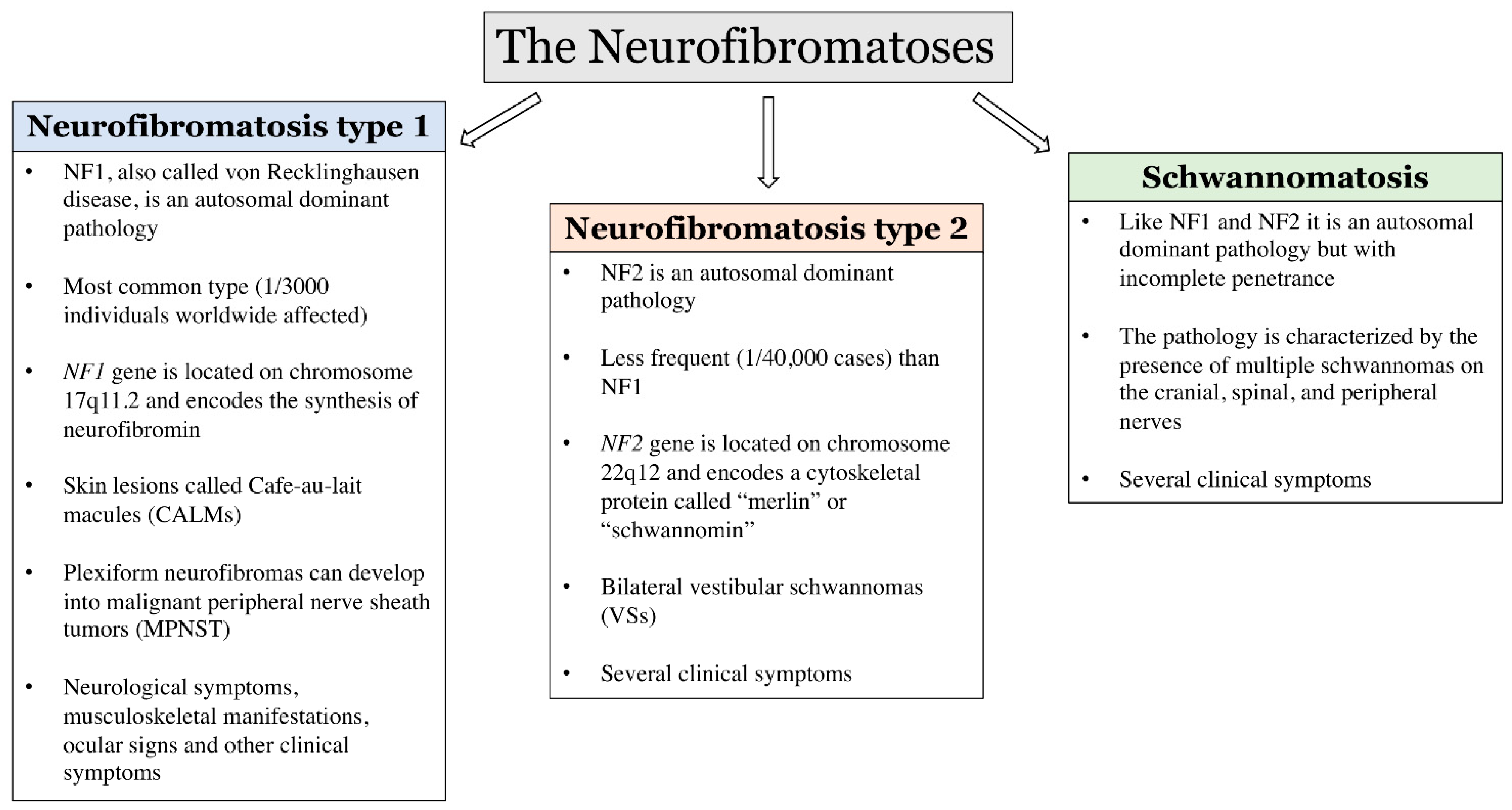
3. Neurofibromatosis
4. COVID-19
5. Influence of COVID-19 Pandemic Emergency on Neurofibromatosis Clinical Features
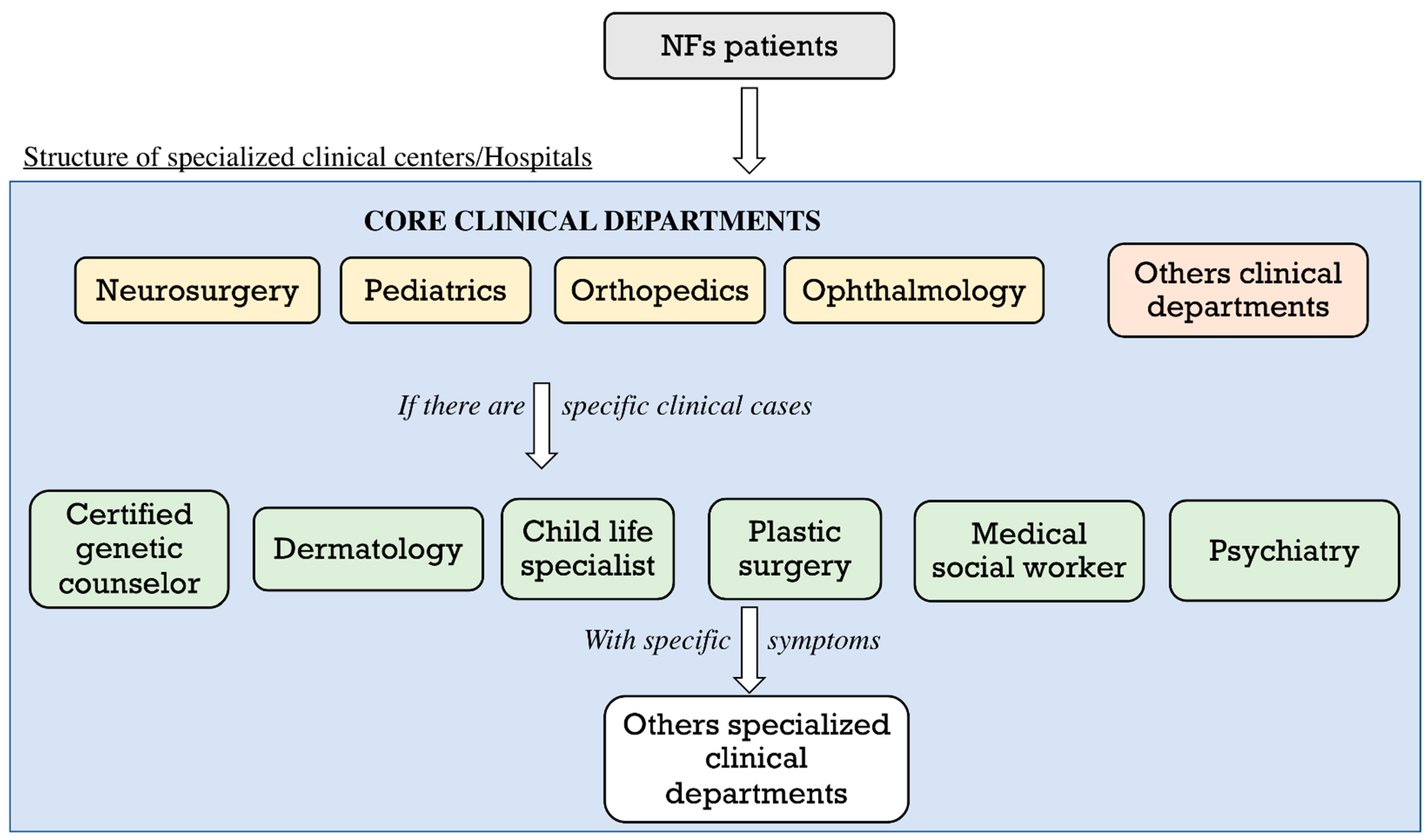
5.1. Impact of the COVID-19 Pandemic on the Clinical Care of Neurofibromatosis Patients
5.2. New Strategies to Fight the Impact of the COVID-19 Pandemic
6. Limitations
7. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Richter, T.; Nestler-Parr, S.; Babela, R.; Khan, Z.M.; Tesoro, T.; Molsen, E.; Hughes, D.A. Rare Disease Terminology and Definitions-A Systematic Global Review: Report of the ISPOR Rare Disease Special Interest Group. Value Health 2015, 18, 906–914. [Google Scholar] [CrossRef]
- Svenstrup, D.; Jorgensen, H.L.; Winther, O. Rare disease diagnosis: A review of web search, social media and large-scale data-mining approaches. Rare Dis. 2015, 3, e1083145. [Google Scholar] [CrossRef] [PubMed]
- Pogue, R.E.; Cavalcanti, D.P.; Shanker, S.; Andrade, R.V.; Aguiar, L.R.; de Carvalho, J.L.; Costa, F.F. Rare genetic diseases: Update on diagnosis, treatment and online resources. Drug Discov. Today 2018, 23, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Eurordis, A.K.; Faurisson, F. The Voice of 12,000 Patients. Experiences and Expectations of Rare Disease Patients on Diagnosis and Care in Europe; European Organisation for Rare Diseases: Paris, France, 2009. [Google Scholar]
- Ferreira, C.R. The burden of rare diseases. Am. J. Med. Genet. Part A 2019, 179, 885–892. [Google Scholar] [CrossRef] [PubMed]
- Nguengang Wakap, S.; Lambert, D.M.; Olry, A.; Rodwell, C.; Gueydan, C.; Lanneau, V.; Murphy, D.; Le Cam, Y.; Rath, A. Estimating cumulative point prevalence of rare diseases: Analysis of the Orphanet database. Eur. J. Hum. Genet. 2020, 28, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Kyle, J.E.; Stratton, K.G.; Zink, E.M.; Kim, Y.M.; Bloodsworth, K.J.; Monroe, M.E.; Undiagnosed Diseases Network; Waters, K.M.; Webb-Robertson, B.M.; Koeller, D.M.; et al. A resource of lipidomics and metabolomics data from individuals with undiagnosed diseases. Sci. Data 2021, 8, 114. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.E.; Singleton, K.S.; Wallin, M.; Faundez, V. Rare Genetic Diseases: Nature’s Experiments on Human Development. IScience 2020, 23, 101123. [Google Scholar] [CrossRef]
- Beaulieu, C.L.; Majewski, J.; Schwartzentruber, J.; Samuels, M.E.; Fernandez, B.A.; Bernier, F.P.; Brudno, M.; Knoppers, B.; Marcadier, J.; Dyment, D. FORGE Canada Consortium: Outcomes of a 2-year national rare-disease gene-discovery project. Am. J. Hum. Genet. 2014, 94, 809–817. [Google Scholar] [CrossRef]
- Brittain, H.K.; Scott, R.; Thomas, E. The rise of the genome and personalised medicine. Clin. Med. 2017, 17, 545–551. [Google Scholar] [CrossRef]
- Raynal, C.; Corvol, H. Variant classifications, databases and genotype-phenotype correlations. Arch. Pediatr. 2020, 27 (Suppl. S1), eS13–eS18. [Google Scholar] [CrossRef]
- Scott, S.A. Personalizing medicine with clinical pharmacogenetics. Genet. Med. 2011, 13, 987–995. [Google Scholar] [CrossRef] [PubMed]
- Davies, K. The era of genomic medicine. Clin. Med. 2013, 13, 594–601. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Gonzalez, X.; Cabaleiro, T.; Herrero, M.J.; McLeod, H.; Lopez-Fernandez, L.A. Clinical implementation of pharmacogenetics. Drug Metab. Pers. Ther. 2016, 31, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Anderson, H.D.; Crooks, K.R.; Kao, D.P.; Aquilante, C.L. The landscape of pharmacogenetic testing in a US managed care population. Genet. Med. 2020, 22, 1247–1253. [Google Scholar] [CrossRef]
- Oates, J.T.; Lopez, D. Pharmacogenetics: An Important Part of Drug Development with A Focus on Its Application. Int. J. Biomed. Investig. 2018, 1, 111. [Google Scholar] [CrossRef] [PubMed]
- Ventola, C.L. Role of pharmacogenomic biomarkers in predicting and improving drug response: Part 1: The clinical significance of pharmacogenetic variants. Pharm. Ther. 2013, 38, 545–560. [Google Scholar]
- Birkmeyer, J.D.; Barnato, A.; Birkmeyer, N.; Bessler, R.; Skinner, J. The Impact Of The COVID-19 Pandemic On Hospital Admissions In The United States. Health Aff. 2020, 39, 2010–2017. [Google Scholar] [CrossRef]
- Hartnett, K.P.; Kite-Powell, A.; DeVies, J.; Coletta, M.A.; Boehmer, T.K.; Adjemian, J.; Gundlapalli, A.V.; National Syndromic Surveillance Program Community of Practice. Impact of the COVID-19 Pandemic on Emergency Department Visits—United States, January 1, 2019–May 30, 2020. Morb. Mortal. Wkly. Rep. 2020, 69, 699–704. [Google Scholar] [CrossRef]
- Rosenbaum, L. The untold toll—the pandemic’s effects on patients without COVID-19. N. Engl. J. Med. 2020, 382, 2368–2371. [Google Scholar] [CrossRef]
- Chowdhury, S.F.; Sium, S.M.A.; Anwar, S. Research and Management of Rare Diseases in the COVID-19 Pandemic Era: Challenges and Countermeasures. Front. Public Health 2021, 9, 640282. [Google Scholar] [CrossRef]
- Armocida, B.; Formenti, B.; Ussai, S.; Palestra, F.; Missoni, E. The Italian health system and the COVID-19 challenge. Lancet Public Health 2020, 5, e253. [Google Scholar] [CrossRef]
- Blumenthal, D.; Fowler, E.J.; Abrams, M.; Collins, S.R. COVID-19—implications for the health care system. N. Engl. J. Med. 2020, 383, 1483–1488. [Google Scholar] [CrossRef] [PubMed]
- Talarico, R.; Marinello, D.; Cannizzo, S.; Gaglioti, A.; Ticciati, S.; Carta, C.; Kodra, Y.; Azadegan, M.; Taruscio, D.; Mosca, M.; et al. Shaping the Future of Rare Diseases after a Global Health Emergency: Organisational Points to Consider. Int. J. Environ. Res. Public Health 2020, 17, 8694. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.C.; Wong, W.H.; Fung, J.L.; Hong Kong, R.D.; Chung, B.H. Impact of COVID-19 pandemic on patients with rare disease in Hong Kong. Eur. J. Med. Genet. 2020, 63, 104062. [Google Scholar] [CrossRef]
- Radtke, H.B.; Klein-Tasman, B.P.; Merker, V.L.; Knight, P.; Ullrich, N.J.; Jordan, J.T.; Korf, B.; Plotkin, S.R. The impact of the COVID-19 pandemic on neurofibromatosis clinical care and research. Orphanet J. Rare Dis. 2021, 16, 61. [Google Scholar] [CrossRef]
- Wolters, P.L.; Reda, S.; Martin, S.; Al Ghriwati, N.; Baker, M.; Berg, D.; Erickson, G.; Franklin, B.; Merker, V.L.; Oberlander, B.; et al. Impact of the coronavirus pandemic on mental health and health care in adults with neurofibromatosis: Patient perspectives from an online survey. Am. J. Med. Genet. Part A 2022, 188, 71–82. [Google Scholar] [CrossRef]
- Shimoyama, K.; Azuma, K.; Oda, J. A patient with COVID-19 and bleeding complications due to neurofibromatosis type 1 during VV-ECMO: A case report. Medicine 2021, 100, e28094. [Google Scholar] [CrossRef]
- Wakamatsu, I.; Yatomi, M.; Uno, S.; Oishi, Y.; Ikeuchi, H.; Hanazato, C.; Sawada, Y.; Saito, H.; Yamaguchi, K.; Kasahara, N.; et al. A case of a patient with neurofibromatosis type I who developed pneumothorax and eosinophilic pleural effusion after suffering from COVID-19 pneumonia. Radiol. Case Rep. 2021, 16, 3504–3508. [Google Scholar] [CrossRef]
- Tatemoto, T.; Mukaino, M.; Kumazawa, N.; Tanabe, S.; Mizutani, K.; Katoh, M.; Saitoh, E.; Otaka, Y. Overcoming language barriers to provide telerehabilitation for COVID-19 patients: A two-case report. Disabil. Rehabil. Assist. Technol. 2021, 1–8. [Google Scholar] [CrossRef]
- Van Koningsbruggen-Rietschel, S.; Dunlevy, F.; Bulteel, V.; Downey, D.G.; Dupont, L. SARS-CoV-2 disrupts clinical research: The role of a rare disease-specific trial network. Eur. Respir. J. 2020, 56, 2002114. [Google Scholar] [CrossRef]
- Nishida, Y.; Ikuta, K.; Natsume, A.; Ishihara, N.; Morikawa, M.; Kidokoro, H.; Muramatsu, Y.; Nonobe, N.; Ishizuka, K.; Takeichi, T.; et al. Establishment of in-hospital clinical network for patients with neurofibromatosis type 1 in Nagoya University Hospital. Sci. Rep. 2021, 11, 11933. [Google Scholar] [CrossRef] [PubMed]
- Le, K.; Nguyen, M. The psychological consequences of COVID-19 lockdowns. Int. Rev. Appl. Econ. 2021, 35, 147–163. [Google Scholar] [CrossRef]
- Orgilés, M.; Morales, A.; Delvecchio, E.; Mazzeschi, C.; Espada, J.P. Immediate psychological effects of the COVID-19 quarantine in youth from Italy and Spain. Front. Psychol. 2020, 11, 2986. [Google Scholar] [CrossRef]
- Sanchez-Garcia, J.C.; Cortes-Martin, J.; Rodriguez-Blanque, R.; Marin-Jimenez, A.E.; Montiel-Troya, M.; Diaz-Rodriguez, L. Depression and Anxiety in Patients with Rare Diseases during the COVID-19 Pandemic. Int. J. Environ. Res. Public Health 2021, 18, 3234. [Google Scholar] [CrossRef] [PubMed]
- Portnoy, J.; Waller, M.; Elliott, T. Telemedicine in the era of COVID-19. J. Allergy Clin. Immunol. Pract. 2020, 8, 1489–1491. [Google Scholar] [CrossRef]
- Bashshur, R.; Doarn, C.R.; Frenk, J.M.; Kvedar, J.C.; Woolliscroft, J.O. Telemedicine and the COVID-19 Pandemic, Lessons for the Future; Mary Ann Liebert, Inc.: Larchmont, NY, USA, 2020. [Google Scholar]
- Bokolo, A.J. Exploring the adoption of telemedicine and virtual software for care of outpatients during and after COVID-19 pandemic. Ir. J. Med. Sci. 2021, 190, 1–10. [Google Scholar] [CrossRef]
- Korf, B.R. Neurofibromatosis. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2013; Volume 111, pp. 333–340. [Google Scholar] [CrossRef]
- Karaconji, T.; Whist, E.; Jamieson, R.V.; Flaherty, M.P.; Grigg, J.R.B. Neurofibromatosis Type 1: Review and Update on Emerging Therapies. Asia-Pac. J. Ophthalmol. 2019, 8, 62–72. [Google Scholar] [CrossRef]
- Evans, D.G.R.; Salvador, H.; Chang, V.Y.; Erez, A.; Voss, S.D.; Druker, H.; Scott, H.S.; Tabori, U. Cancer and Central Nervous System Tumor Surveillance in Pediatric Neurofibromatosis 2 and Related Disorders. Clin. Cancer Res. 2017, 23, e54–e61. [Google Scholar] [CrossRef]
- Ghalayani, P.; Saberi, Z.; Sardari, F. Neurofibromatosis type I (von Recklinghausen’s disease): A family case report and literature review. Dent. Res. J. 2012, 9, 483–488. [Google Scholar]
- Bergqvist, C.; Servy, A.; Valeyrie-Allanore, L.; Ferkal, S.; Combemale, P.; Wolkenstein, P.; Network, N.F.F. Neurofibromatosis 1 French national guidelines based on an extensive literature review since 1966. Orphanet J. Rare Dis. 2020, 15, 37. [Google Scholar] [CrossRef]
- Kiuru, M.; Busam, K.J. The NF1 gene in tumor syndromes and melanoma. Lab. Investig. 2017, 97, 146–157. [Google Scholar] [CrossRef]
- Serra, G.; Antona, V.; Corsello, G.; Zara, F.; Piro, E.; Falsaperla, R. NF1 microdeletion syndrome: Case report of two new patients. Ital. J. Pediatr. 2019, 45, 138. [Google Scholar] [CrossRef] [PubMed]
- Cassiman, C.; Legius, E.; Spileers, W.; Casteels, I. Ophthalmological assessment of children with neurofibromatosis type 1. Eur. J. Pediatr. 2013, 172, 1327–1333. [Google Scholar] [CrossRef] [PubMed]
- Mahalingam, M. NF1 and Neurofibromin: Emerging Players in the Genetic Landscape of Desmoplastic Melanoma. Adv. Anat. Pathol. 2017, 24, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Rabab’h, O.; Gharaibeh, A.; Al-Ramadan, A.; Ismail, M.; Shah, J. Pharmacological Approaches in Neurofibromatosis Type 1-Associated Nervous System Tumors. Cancers 2021, 13, 3880. [Google Scholar] [CrossRef]
- Ly, K.I.; Blakeley, J.O. The Diagnosis and Management of Neurofibromatosis Type 1. Med. Clin. N. Am. 2019, 103, 1035–1054. [Google Scholar] [CrossRef] [PubMed]
- Bergoug, M.; Doudeau, M.; Godin, F.; Mosrin, C.; Vallee, B.; Benedetti, H. Neurofibromin Structure, Functions and Regulation. Cells 2020, 9, 2365. [Google Scholar] [CrossRef]
- Bayat, M.; Bayat, A. Neurological manifestations of neurofibromatosis: A review. Neurol. Sci. 2020, 41, 2685–2690. [Google Scholar] [CrossRef]
- Filopanti, M.; Verga, U.; Ulivieri, F.M.; Giavoli, C.; Rodari, G.; Arosio, M.; Natacci, F.; Spada, A. Trabecular Bone Score (TBS) and Bone Metabolism in Patients Affected with Type 1 Neurofibromatosis (NF1). Calcif. Tissue Int. 2019, 104, 207–213. [Google Scholar] [CrossRef]
- Ferner, R.E.; Gutmann, D.H. Neurofibromatosis type 1 (NF1): Diagnosis and management. Handb. Clin. Neurol. 2013, 115, 939–955. [Google Scholar] [CrossRef]
- Ozarslan, B.; Russo, T.; Argenziano, G.; Santoro, C.; Piccolo, V. Cutaneous Findings in Neurofibromatosis Type 1. Cancers 2021, 13, 463. [Google Scholar] [CrossRef] [PubMed]
- Isaacs, H., Jr. Perinatal neurofibromatosis: Two case reports and review of the literature. Am. J. Perinatol. 2010, 27, 285–292. [Google Scholar] [CrossRef]
- Jha, S.K.; Mendez, M.D. Cafe Au Lait Macules. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Cannon, A.; Chen, M.J.; Li, P.; Boyd, K.P.; Theos, A.; Redden, D.T.; Korf, B. Cutaneous neurofibromas in Neurofibromatosis type I: A quantitative natural history study. Orphanet J. Rare Dis. 2018, 13, 31. [Google Scholar] [CrossRef]
- Woertler, K. Tumors and tumor-like lesions of peripheral nerves. Semin. Musculoskelet. Radiol. 2010, 14, 547–558. [Google Scholar] [CrossRef]
- Ortonne, N.; Wolkenstein, P.; Blakeley, J.O.; Korf, B.; Plotkin, S.R.; Riccardi, V.M.; Miller, D.C.; Huson, S.; Peltonen, J.; Rosenberg, A.; et al. Cutaneous neurofibromas: Current clinical and pathologic issues. Neurology 2018, 91, S5–S13. [Google Scholar] [CrossRef] [PubMed]
- Fasih, S.; Suppiyah, S.; Barron, J.; Barnett-Tapia, C.; Avery, R.; Dickson, B.; Ferguson, P.; Swallow, C.; Zadeh, G.; Gupta, A.A. Malignant transformation of plexiform neurofibroma to MPNST while on MEK inhibitor. Neuro-Oncol. Adv. 2021, 3, vdab033. [Google Scholar] [CrossRef] [PubMed]
- Darrigo, L.G., Jr.; Geller, M.; Bonalumi Filho, A.; Azulay, D.R. Prevalence of plexiform neurofibroma in children and adolescents with type I neurofibromatosis. J. Pediatr. 2007, 83, 571–573. [Google Scholar] [CrossRef][Green Version]
- Mautner, V.F.; Friedrich, R.E.; von Deimling, A.; Hagel, C.; Korf, B.; Knofel, M.T.; Wenzel, R.; Funsterer, C. Malignant peripheral nerve sheath tumours in neurofibromatosis type 1: MRI supports the diagnosis of malignant plexiform neurofibroma. Neuroradiology 2003, 45, 618–625. [Google Scholar] [CrossRef]
- Gupta, G.; Maniker, A. Malignant peripheral nerve sheath tumors. Neurosurg. Focus 2007, 22, E12. [Google Scholar] [CrossRef]
- Friedrich, R.E.; Hartmann, M.; Mautner, V.F. Malignant peripheral nerve sheath tumors (MPNST) in NF1-affected children. Anticancer Res. 2007, 27, 1957–1960. [Google Scholar]
- Patel, N.B.; Stacy, G.S. Musculoskeletal manifestations of neurofibromatosis type 1. Am. J. Roentgenol. 2012, 199, W99–W106. [Google Scholar] [CrossRef]
- Souza, M.L.R.; Jansen, A.K.; Rodrigues, L.O.C.; Vilela, D.L.S.; Kakehasi, A.M.; Martins, A.S.; Souza, J.F.; Rezende, N.A. Reduced bone mineral content and density in neurofibromatosis type 1 and its association with nutrient intake. Rev. Assoc. Med. Bras. 2020, 66, 666–672. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Du, K.; Sun, F.; Xu, H.; Li, J. Case analysis of short stature complicated with neurofibromatosis type 1. J. Neurosurg. Sci. 2018, 62, 779–781. [Google Scholar] [CrossRef] [PubMed]
- Schindera, C.; Wingeier, K.; Goeggel Simonetti, B.; Diepold, M.; Nauer, C.B.; Fleischhauer, J.; Steinlin, M. Macrocephaly in neurofibromatosis type 1: A sign post for optic pathway gliomas? Child′s Nerv. Syst. 2011, 27, 2107–2111. [Google Scholar] [CrossRef][Green Version]
- Campen, C.J.; Gutmann, D.H. Optic Pathway Gliomas in Neurofibromatosis Type 1. J. Child Neurol. 2018, 33, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Senthilkumar, V.A.; Tripathy, K. Lisch Nodules. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Jutant, E.M.; Girerd, B.; Jais, X.; Savale, L.; O’Connell, C.; Perros, F.; Sitbon, O.; Humbert, M.; Montani, D. Pulmonary hypertension associated with neurofibromatosis type 1. Eur. Respir. Rev. 2018, 27, 180053. [Google Scholar] [CrossRef]
- Raborn, J.; McCafferty, B.J.; Gunn, A.J.; Moawad, S.; Mahmoud, K.; Aal, A.K.A.; Saddekni, S. Endovascular Management of Neurofibromatosis Type I-Associated Vasculopathy: A Case Series and Brief Review of the Literature. Vasc. Endovasc. Surg. 2020, 54, 182–190. [Google Scholar] [CrossRef]
- Roth, J.; Ber, R.; Constantini, S. Neurofibromatosis Type 1-Related Hydrocephalus: Treatment Options and Considerations. World Neurosurg. 2019, 128, e664–e668. [Google Scholar] [CrossRef]
- Ardern-Holmes, S.; Fisher, G.; North, K. Neurofibromatosis Type 2. J. Child Neurol. 2017, 32, 9–22. [Google Scholar] [CrossRef]
- Sato, T.; Sekido, Y. NF2/Merlin Inactivation and Potential Therapeutic Targets in Mesothelioma. Int. J. Mol. Sci. 2018, 19, 988. [Google Scholar] [CrossRef]
- Petrilli, A.M.; Fernandez-Valle, C. Role of Merlin/NF2 inactivation in tumor biology. Oncogene 2016, 35, 537–548. [Google Scholar] [CrossRef] [PubMed]
- Gronholm, M.; Teesalu, T.; Tyynela, J.; Piltti, K.; Bohling, T.; Wartiovaara, K.; Vaheri, A.; Carpen, O. Characterization of the NF2 protein merlin and the ERM protein ezrin in human, rat, and mouse central nervous system. Mol. Cell. Neurosci. 2005, 28, 683–693. [Google Scholar] [CrossRef]
- Mota, M.; Shevde, L.A. Merlin regulates signaling events at the nexus of development and cancer. Cell Commun. Signal. 2020, 18, 63. [Google Scholar] [CrossRef]
- Radek, M.; Tomasik, B.; Wojdyn, M.; Snopkowska-Wiaderna, D.; Blaszczyk, M.; Radek, A. Neurofibromatosis type 2 (NF 2) or schwannomatosis?--Case report study and diagnostic criteria. Neurol. Neurochir. Pol. 2016, 50, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Mahboubi, H.; Slattery, W.H., 3rd; Mehta, G.U.; Lekovic, G.P. Options and strategies for hearing restoration in pediatric neurofibromatosis type 2. Child′s Nerv. Syst. 2020, 36, 2481–2487. [Google Scholar] [CrossRef] [PubMed]
- Bachir, S.; Shah, S.; Shapiro, S.; Koehler, A.; Mahammedi, A.; Samy, R.N.; Zuccarello, M.; Schorry, E.; Sengupta, S. Neurofibromatosis Type 2 (NF2) and the Implications for Vestibular Schwannoma and Meningioma Pathogenesis. Int. J. Mol. Sci. 2021, 22, 690. [Google Scholar] [CrossRef]
- Chen, J.; Landegger, L.D.; Sun, Y.; Ren, J.; Maimon, N.; Wu, L.; Ng, M.R.; Chen, J.W.; Zhang, N.; Zhao, Y.; et al. A cerebellopontine angle mouse model for the investigation of tumor biology, hearing, and neurological function in NF2-related vestibular schwannoma. Nat. Protoc. 2019, 14, 541–555. [Google Scholar] [CrossRef] [PubMed]
- Wilding, A.; Ingham, S.L.; Lalloo, F.; Clancy, T.; Huson, S.M.; Moran, A.; Evans, D.G. Life expectancy in hereditary cancer predisposing diseases: An observational study. J. Med. Genet. 2012, 49, 264–269. [Google Scholar] [CrossRef]
- Farschtschi, S.; Mautner, V.F.; McLean, A.C.L.; Schulz, A.; Friedrich, R.E.; Rosahl, S.K. The Neurofibromatoses. Dtsch. Arztebl. Int. 2020, 117, 354–360. [Google Scholar] [CrossRef]
- Tamura, R. Current Understanding of Neurofibromatosis Type 1, 2, and Schwannomatosis. Int. J. Mol. Sci. 2021, 22, 5850. [Google Scholar] [CrossRef]
- Evans, D.G.; Bowers, N.L.; Tobi, S.; Hartley, C.; Wallace, A.J.; King, A.T.; Lloyd, S.K.W.; Rutherford, S.A.; Hammerbeck-Ward, C.; Pathmanaban, O.N.; et al. Schwannomatosis: A genetic and epidemiological study. J. Neurol. Neurosurg. Psychiatry 2018, 89, 1215–1219. [Google Scholar] [CrossRef] [PubMed]
- Scarpa, N.; Sghedoni, D.; Rosa, M.; per la Salute, B. COVID-19, la malattia da nuovo coronavirus (SARS-CoV-2). Quesiti Clin. 2020, 11, 1–50. [Google Scholar]
- World Health Organization. Managing Epidemics: Key Facts about Major Deadly Diseases; World Health Organization: Geneva, Switzerland, 2018.
- Rathore, V.; Galhotra, A.; Pal, R.; Sahu, K.K. COVID-19 Pandemic and Children: A Review. J. Pediatr. Pharmacol. Ther. 2020, 25, 574–585. [Google Scholar] [CrossRef]
- Shah, S.M.; Alsaab, H.O.; Rawas-Qalaji, M.M.; Uddin, M.N. A Review on Current COVID-19 Vaccines and Evaluation of Particulate Vaccine Delivery Systems. Vaccines 2021, 9, 1086. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Malviya, R.; Sharma, P.K. Corona virus: A review of COVID-19. Eurasian J. Med. Oncol. 2020, 4, 8–25. [Google Scholar]
- Kipshidze, N.; Dangas, G.; White, C.J.; Kipshidze, N.; Siddiqui, F.; Lattimer, C.R.; Carter, C.A.; Fareed, J. Viral Coagulopathy in Patients With COVID-19: Treatment and Care. Clin. Appl. Thromb. Hemost. 2020, 26, 118–144. [Google Scholar] [CrossRef]
- Jha, S.; Soni, A.; Siddiqui, S.; Batra, N.; Goel, N.; Dey, S.; Budhiraja, S.; Naithani, R. Prevalence of flu-like symptoms and COVID-19 in healthcare workers from India. J. Assoc. Physicians India 2020, 68, 27–29. [Google Scholar]
- Wan, S.; Xiang, Y.; Fang, W.; Zheng, Y.; Li, B.; Hu, Y.; Lang, C.; Huang, D.; Sun, Q.; Xiong, Y.; et al. Clinical features and treatment of COVID-19 patients in northeast Chongqing. J. Med. Virol. 2020, 92, 797–806. [Google Scholar] [CrossRef]
- Bolay, H.; Gul, A.; Baykan, B. COVID-19 is a Real Headache! Headache 2020, 60, 1415–1421. [Google Scholar] [CrossRef] [PubMed]
- Kingstone, T.; Taylor, A.K.; O’Donnell, C.A.; Atherton, H.; Blane, D.N.; Chew-Graham, C.A. Finding the ‘right’ GP: A qualitative study of the experiences of people with long-COVID. BJGP Open 2020, 4, bjgpopen20X101143. [Google Scholar] [CrossRef]
- Chakraborty, C.; Sharma, A.R.; Sharma, G.; Bhattacharya, M.; Lee, S.S. SARS-CoV-2 causing pneumonia-associated respiratory disorder (COVID-19): Diagnostic and proposed therapeutic options. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 4016–4026. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Deng, H.; Huang, L.; Xia, L.; Zhou, X. Analysis of Characteristics in Death Patients with COVID-19 Pneumonia without Underlying Diseases. Acad. Radiol. 2020, 27, 752. [Google Scholar] [CrossRef] [PubMed]
- Ella, K.M.; Mohan, V.K. Coronavirus vaccine: Light at the end of the tunnel. Indian Pediatr. 2020, 57, 407–410. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Marc, G.P.; Moreira, E.D.; Zerbini, C. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 2020, 384, 403–416. [Google Scholar] [CrossRef]
- Sadoff, J.; Gray, G.; Vandebosch, A.; Cárdenas, V.; Shukarev, G.; Grinsztejn, B.; Goepfert, P.A.; Truyers, C.; Fennema, H.; Spiessens, B. Safety and efficacy of single-dose Ad26. COV2. S vaccine against COVID-19. N. Engl. J. Med. 2021, 384, 2187–2201. [Google Scholar] [CrossRef]
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111. [Google Scholar] [CrossRef]
- Zieneldien, T.; Kim, J.; Cao, J.; Cao, C. COVID-19 Vaccines: Current Conditions and Future Prospects. Biology 2021, 10, 960. [Google Scholar] [CrossRef]
- Ali, I.; Alharbi, O.M.L. COVID-19: Disease, management, treatment, and social impact. Sci. Total Environ. 2020, 728, 138861. [Google Scholar] [CrossRef]
- Nicola, M.; Alsafi, Z.; Sohrabi, C.; Kerwan, A.; Al-Jabir, A.; Iosifidis, C.; Agha, M.; Agha, R. The socio-economic implications of the coronavirus pandemic (COVID-19): A review. Int. J. Surg. 2020, 78, 185–193. [Google Scholar] [CrossRef]
- Fagoni, N.; Perone, G.; Villa, G.F.; Celi, S.; Bera, P.; Sechi, G.M.; Mare, C.; Zoli, A.; Botteri, M. The Lombardy Emergency Medical System Faced with COVID-19: The Impact of Out-of-Hospital Outbreak. Prehosp. Emerg. Care 2021, 25, 1–7. [Google Scholar] [CrossRef]
- Chudasama, Y.V.; Gillies, C.L.; Zaccardi, F.; Coles, B.; Davies, M.J.; Seidu, S.; Khunti, K. Impact of COVID-19 on routine care for chronic diseases: A global survey of views from healthcare professionals. Diabetes Metab. Syndr. 2020, 14, 965–967. [Google Scholar] [CrossRef]
- Raymond, E.; Thieblemont, C.; Alran, S.; Faivre, S. Impact of the COVID-19 Outbreak on the Management of Patients with Cancer. Target. Oncol. 2020, 15, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Tobor-Swietek, E.; Sykut-Cegielska, J.; Bik-Multanowski, M.; Walczak, M.; Rokicki, D.; Kaluzny, L.; Wierzba, J.; Pac, M.; Jahnz-Rozyk, K.; Wiesik-Szewczyk, E.; et al. COVID-19 Pandemic and Patients with Rare Inherited Metabolic Disorders and Rare Autoinflammatory Diseases-Organizational Challenges from the Point of View of Healthcare Providers. J. Clin. Med. 2021, 10, 4862. [Google Scholar] [CrossRef] [PubMed]
- Whitworth, J. COVID-19: A fast evolving pandemic. Trans. R. Soc. Trop. Med. Hyg. 2020, 114, 241–248. [Google Scholar] [CrossRef]
- Spitzer, E.; Ren, B.; Brugts, J.J.; Daemen, J.; McFadden, E.; Tijssen, J.G.; Van Mieghem, N.M. Cardiovascular Clinical Trials in a Pandemic: Immediate Implications of Coronavirus Disease 2019. Card. Fail. Rev. 2020, 6, e09. [Google Scholar] [CrossRef] [PubMed]
- Rubio-San-Simon, A.; Verdu-Amoros, J.; Hladun, R.; Juan-Ribelles, A.; Molero, M.; Guerra-Garcia, P.; Perez-Martinez, A.; Castaneda, A.; Canete, A.; de Rojas, T.; et al. Challenges in early phase clinical trials for childhood cancer during the COVID-19 pandemic: A report from the new agents group of the Spanish Society of Paediatric Haematology and Oncology (SEHOP). Clin. Transl. Oncol. 2021, 23, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.W.; Ren, J.Y.; Gu, Y.H.; Li, Q.F.; Wang, Z.C. NF1, Neurofibromin and Gene Therapy: Prospects of Next-Generation Therapy. Curr. Gene Ther. 2020, 20, 100–108. [Google Scholar] [CrossRef]
- Wootton, R. Telemedicine. BMJ 2001, 323, 557–560. [Google Scholar] [CrossRef]
- Hjelm, N. Benefits and drawbacks of telemedicine. J. Telemed. Telecare 2005, 11, 60–70. [Google Scholar] [CrossRef]
| First Author | Year | Type of Study | Analysis/Outcomes | References |
|---|---|---|---|---|
| Chowdhury | 2021 | Literature Review | The article analyzed the impact of COVID-19 Pandemic on rare diseases patients | [21] |
| Armocida | 2020 | Editorial | Report of Italian health system and the COVID-19 challenge | [22] |
| Blumenthal | 2020 | Literature Review | Analysis of COVID-19 and implications for the Health Care System | [23] |
| Talarico | 2020 | Literature Review | Analysis of Rare Disease and Global Health Emergency | [24] |
| Chung | 2020 | Clinical research | Impact of COVID-19 pandemic on patients with rare disease in Hong Kong | [25] |
| Radtke | 2021 | Clinical survey | Sixty-three United States NF clinics online survey | [26] |
| Wolters | 2022 | Clinical survey | Anonymous online survey distributed to adults with NF. 613 adults (18–81 years; Mean = 45.7) with NF1 (77.8%), NF2 (14.2%), and schwannomatosis (7.8%). The analysis assess the impact of the pandemic on mental health and NF health care | [27] |
| Shimoyama | 2021 | Case report | A patient with COVID-19 and bleeding complications due to neurofibromatosis type 1 during VV-ECMO. | [28] |
| Wakamatsu | 2021 | Case report | A case of a patient with neurofibromatosis type I who developed pneumothorax and eosinophilic pleural effusion after suffering from COVID-19 pneumonia | [29] |
| Tatemoto | 2021 | Case report | Successful telerehabilitation delivery for patient (49-year-old man with NF) quarantined due to COVID-19 | [30] |
| van Koningsbruggen-Rietschel | 2020 | Editorial | The article analyzed how SARS-CoV-2 disrupts clinical research in the rare disease-specific trial network | [31] |
| Nishida | 2021 | Clinical article | The study analyzed the establishment of an in-hospital clinical network for patients with neurofibromatosis type 1 in Nagoya University Hospital | [32] |
| Le | 2021 | Clinical article | The study analyzed the psychological consequences of COVID-19 lockdowns | [33] |
| Orgilès | 2020 | Clinical survey | The study analyzed the psychological consequences of COVID-19 quarantines in young people | [34] |
| Sanchez-Garcia | 2021 | Online study | The study analyzed the depression and anxiety in patients with Rare Diseases during the COVID-19 Pandemic | [35] |
| Portnoy | 2020 | Editorial | The study analyzed the use of Telemedicine during the COVID-19 pandemic | [36] |
| Bashshur | 2020 | Editorial | The study analyzed the use of Telemedicine during the COVID-19 pandemic with future perspectives | [37] |
| Bokolo | 2021 | Literature Review | The study analyzed the adoption of Telemedicine and virtual software for care of outpatients during and after COVID-19 pandemic | [38] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ardizzone, A.; Capra, A.P.; Campolo, M.; Filippone, A.; Esposito, E.; Briuglia, S. Neurofibromatosis: New Clinical Challenges in the Era of COVID-19. Biomedicines 2022, 10, 940. https://doi.org/10.3390/biomedicines10050940
Ardizzone A, Capra AP, Campolo M, Filippone A, Esposito E, Briuglia S. Neurofibromatosis: New Clinical Challenges in the Era of COVID-19. Biomedicines. 2022; 10(5):940. https://doi.org/10.3390/biomedicines10050940
Chicago/Turabian StyleArdizzone, Alessio, Anna Paola Capra, Michela Campolo, Alessia Filippone, Emanuela Esposito, and Silvana Briuglia. 2022. "Neurofibromatosis: New Clinical Challenges in the Era of COVID-19" Biomedicines 10, no. 5: 940. https://doi.org/10.3390/biomedicines10050940
APA StyleArdizzone, A., Capra, A. P., Campolo, M., Filippone, A., Esposito, E., & Briuglia, S. (2022). Neurofibromatosis: New Clinical Challenges in the Era of COVID-19. Biomedicines, 10(5), 940. https://doi.org/10.3390/biomedicines10050940










