Combined Treatment of Nitrated [6,6,6]Tricycles Derivative (SK2)/Ultraviolet C Highly Inhibits Proliferation in Oral Cancer Cells In Vitro
Abstract
1. Introduction
2. Materials and Methods
2.1. SK2 Synthesis, Reagents, and UVC Irradiation
2.2. Cell Culture and Cell Viability
2.3. Reactive Oxygen Species (ROS), Mitochondrial Superoxide (MitoSOX), and Mitochondrial Membrane Potential (MMP)
2.4. Cell Cycle
2.5. Apoptosis
2.6. DNA Damages
2.7. Statistics
3. Results
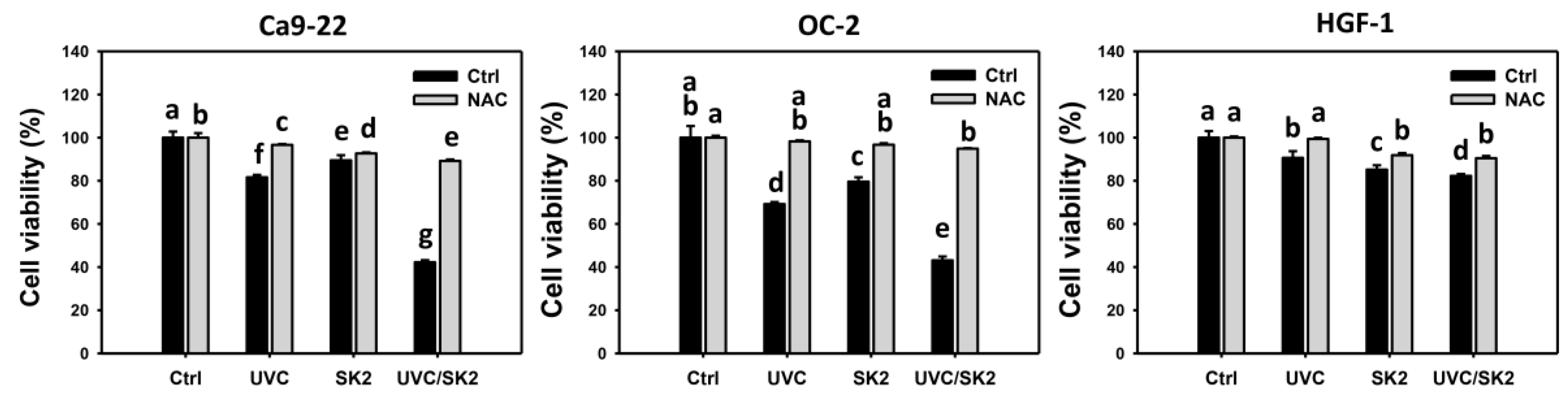
3.1. UVC/SK2 Combination Treatment Versus Single Treatment on Anti-Proliferation
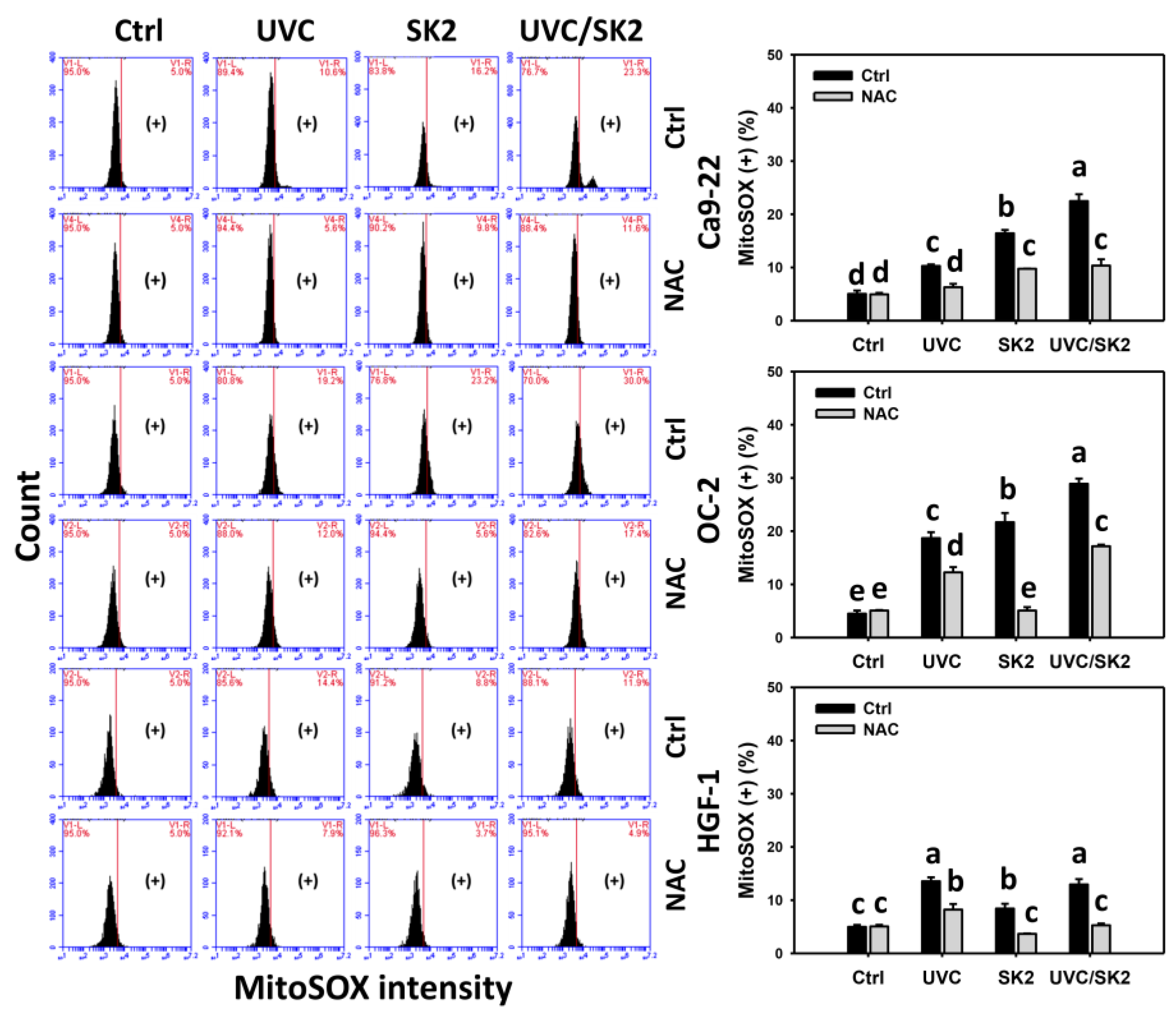
3.2. UVC/SK2 Combination Treatment Versus Single Treatment on ROS/MitoSOX
3.3. UVC/SK2 Combination Treatment versus Single Treatment on MMP Destruction
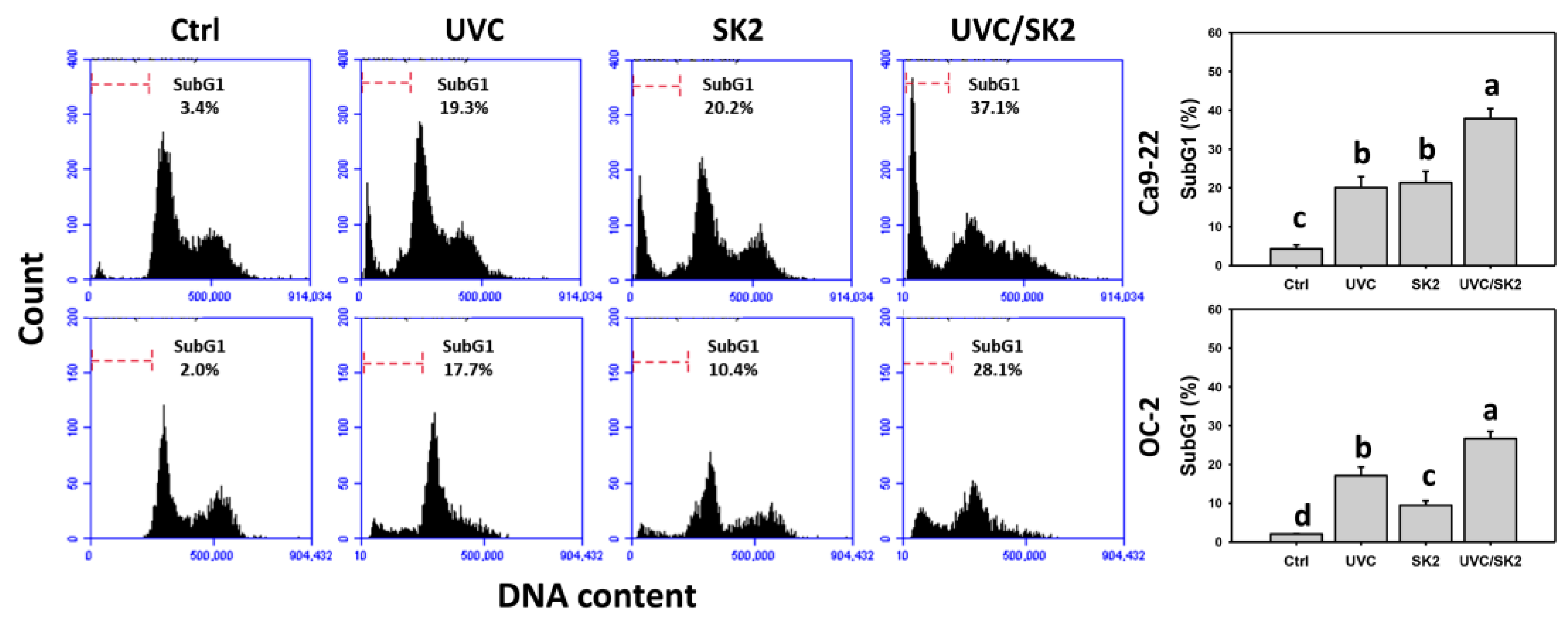
3.4. UVC/SK2 Combination Treatment versus Single Treatment on subG1 Increment
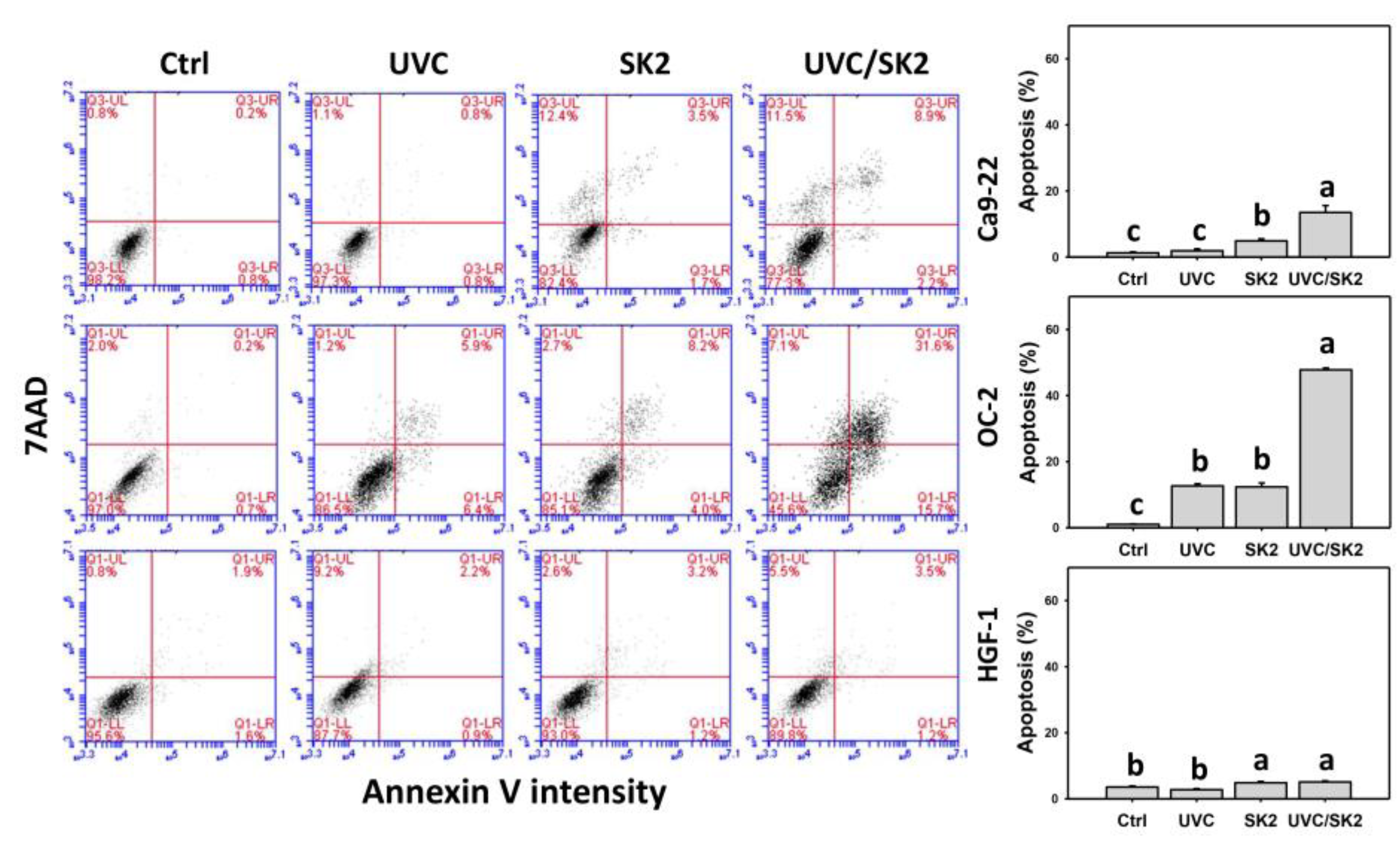
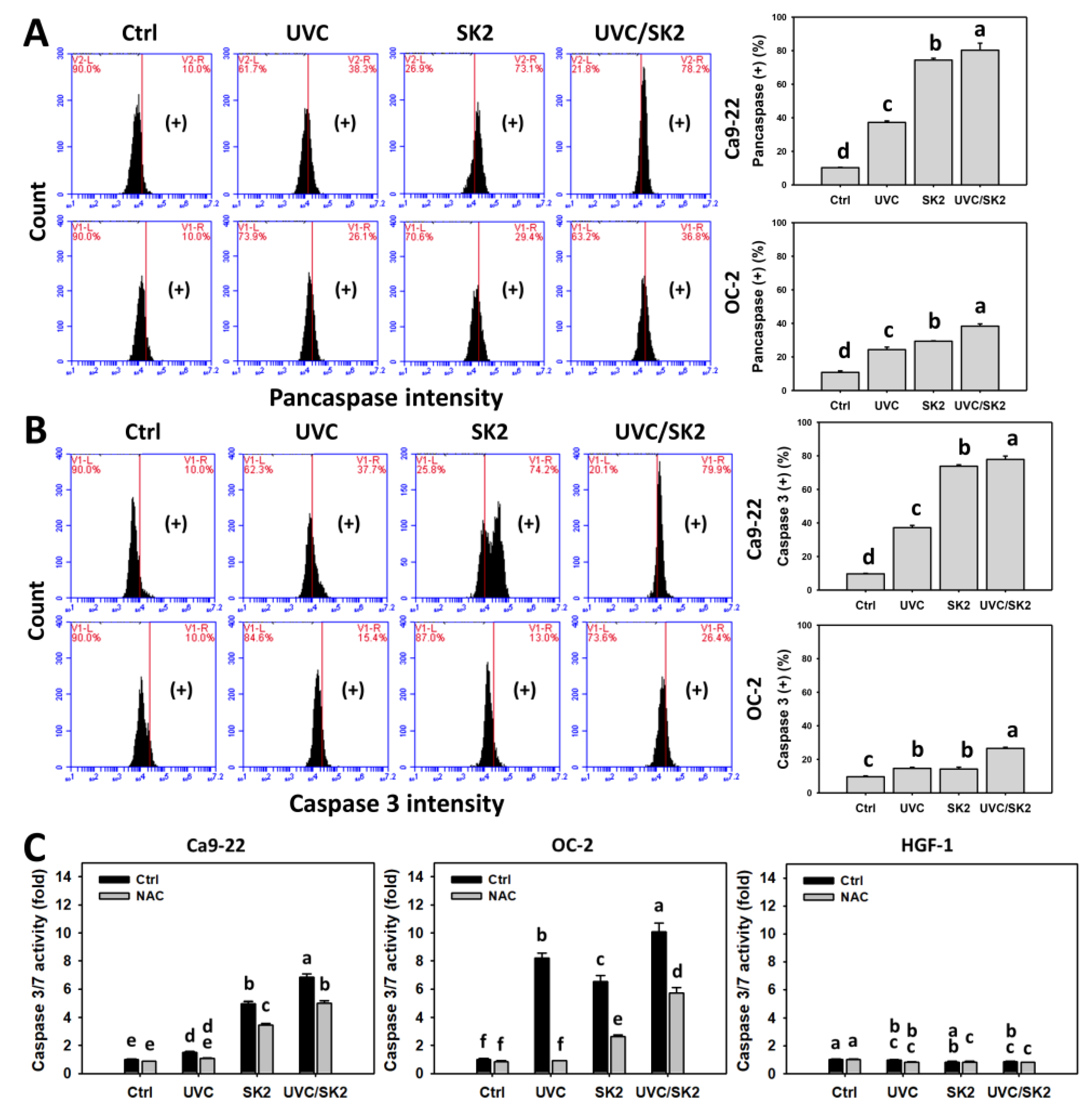
3.5. UVC/SK2 Combination Treatment versus Single Treatment on Apoptosis (Annexin V)
3.6. UVC/SK2 Combination Treatment versus Single Treatment on Caspase Activation
3.7. UVC/SK2 Combination Treatment versus Single Treatment on Extrinsic and Intrinsic Signaling Activation
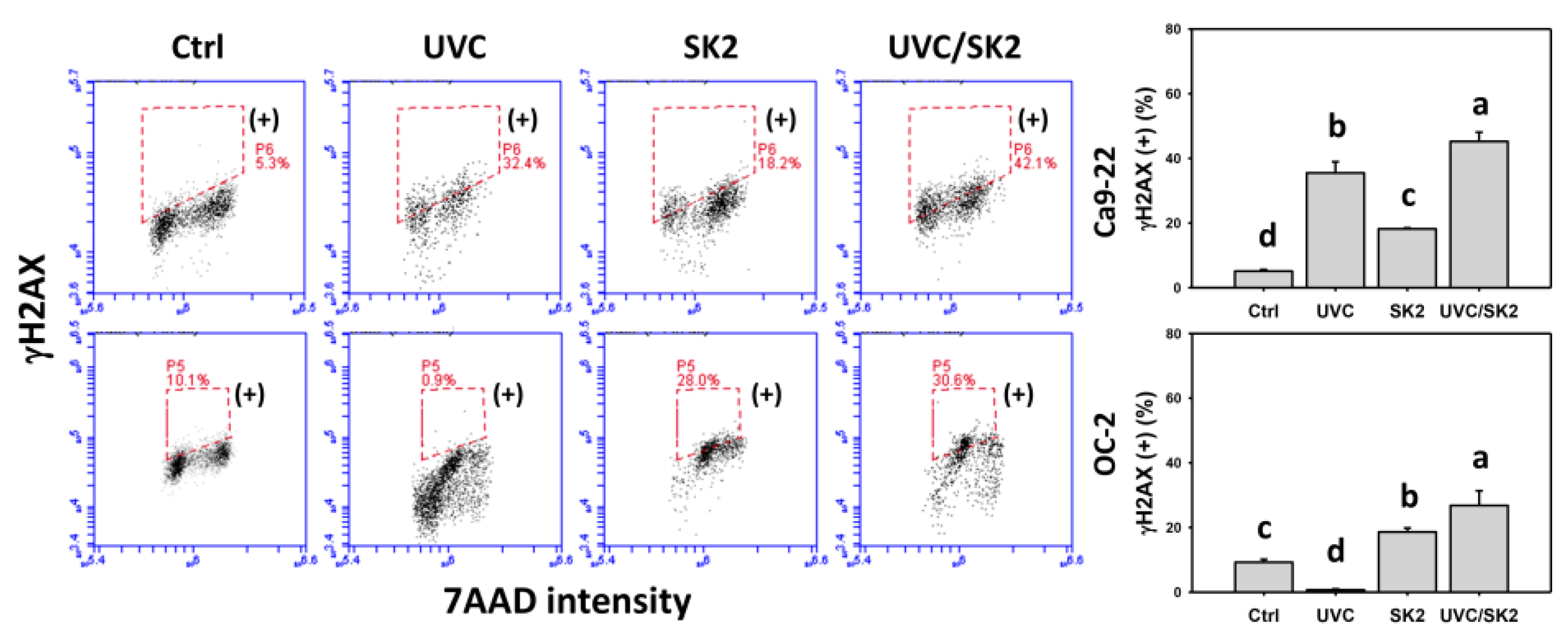
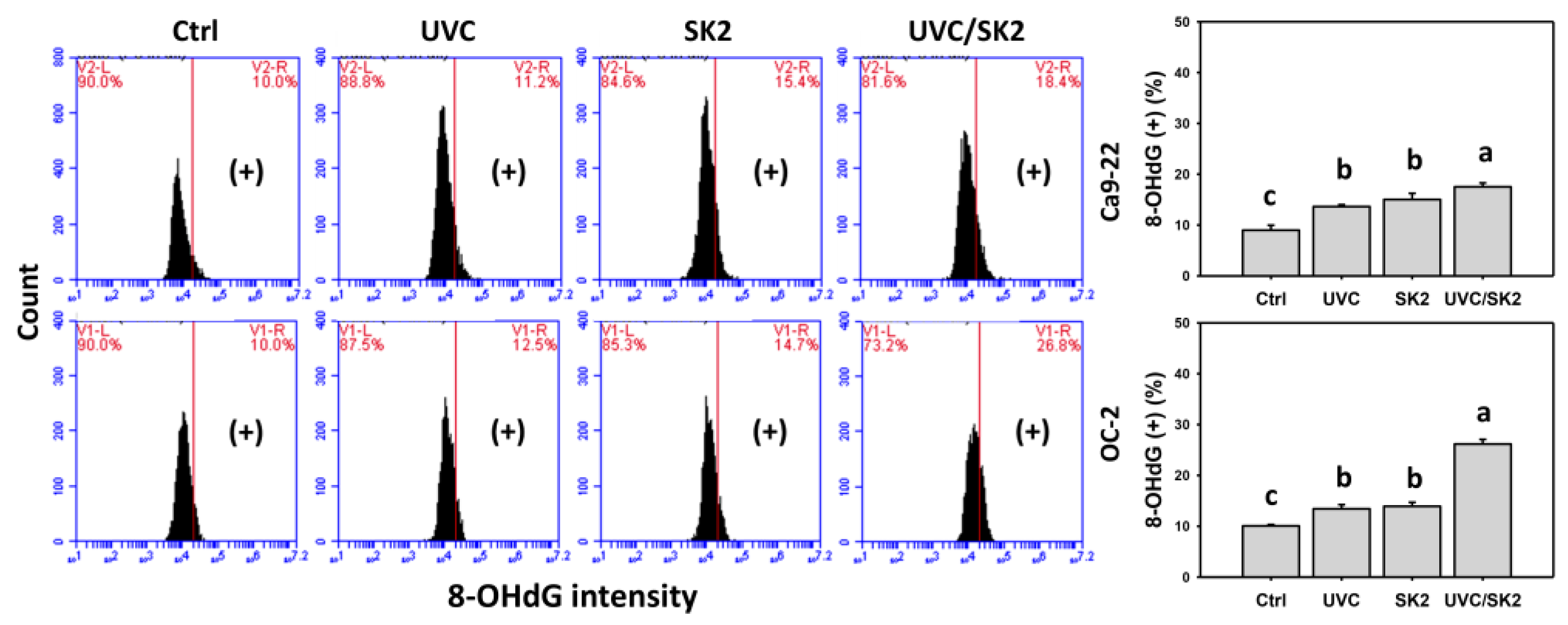
3.8. UVC/SK2 Combination Treatment versus Single Treatment on DNA Damage
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SK2 | IUPAC name 6-n-butoxy-10-nitro-12,13-dioxa-11-azatricyclo[7.3.1.02,7]trideca-2,4,6,10-tetrae |
| UVC | Ultraviolet C |
| ROS | Reactive oxygen species |
| MitoSOX | Mitochondrial superoxide |
| MMP | Mitochondrial membrane potential |
| NAC | N-Acetylcysteine |
| 8-OHdG | 8-Hydroxy-2′-deoxyguanosine |
| DMSO | Dimethyl sulfoxide |
| H2DCF-DA | 2′,7′-Dichlorodihydrofluorescein diacetate |
| 7AAD | 7-Aminoactinomycin D |
| NRF2 | Nuclear erythroid-like factor 2 |
References
- Warnakulasuriya, S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. 2009, 45, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Petersen, P.E. Oral cancer prevention and control—The approach of the World Health Organization. Oral Oncol. 2009, 45, 454–460. [Google Scholar] [CrossRef]
- Myoung, H.; Hong, S.-P.; Yun, P.-Y.; Lee, J.-H.; Kim, M.-J. Anti-cancer effect of genistein in oral squamous cell carcinoma with respect to angiogenesis and in vitro invasion. Cancer Sci. 2003, 94, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Yang, E.S.; Choi, M.J.; Kim, J.H.; Choi, K.S.; Kwon, T.K. Combination of withaferin A and X-ray irradiation enhances apoptosis in U937 cells. Toxicol. Vitr. 2011, 25, 1803–1810. [Google Scholar] [CrossRef] [PubMed]
- Bigdeli, B.; Goliaei, B.; Masoudi-Khoram, N.; Jooyan, N.; Nikoofar, A.; Rouhani, M.; Haghparast, A.; Mamashli, F. Enterolactone: A novel radiosensitizer for human breast cancer cell lines through impaired DNA repair and increased apoptosis. Toxicol. Appl. Pharmacol. 2016, 313, 180–194. [Google Scholar] [CrossRef]
- Chang, H.-W.; Tang, J.-Y.; Yen, C.-Y.; Chang, H.-S.; Huang, H.-W.; Chung, Y.-A.; Chen, I.-S.; Huang, M.-Y. Synergistic anti-oral cancer effects of UVC and methanolic extracts of Cryptocarya concinna roots via apoptosis, oxidative stress and DNA damage. Int. J. Radiat. Biol. 2016, 92, 263–272. [Google Scholar] [CrossRef]
- Kawaguchi, J.; Adachi, S.; Yasuda, I.; Yamauchi, T.; Nakashima, M.; Ohno, T.; Shimizu, M.; Yoshioka, T.; Itani, M.; Kozawa, O.; et al. Cisplatin and ultra-violet-C synergistically down-regulate receptor tyrosine kinases in human colorectal cancer cells. Mol. Cancer 2012, 11, 45. [Google Scholar] [CrossRef]
- Murray, D.; Mirzayans, R. Cellular responses to platinum-based anticancer drugs and UVC: Role of p53 and implications for cancer therapy. Int. J. Mol. Sci. 2020, 21, 5766. [Google Scholar] [CrossRef]
- Wang, S.-C.; Wang, Y.-Y.; Lin, L.-C.; Chang, M.-Y.; Yuan, S.-S.F.; Tang, J.-Y.; Chang, H.-W. Combined treatment of sulfonyl chromen-4-ones (CHW09) and ultraviolet-C (UVC) enhances proliferation inhibition, apoptosis, oxidative stress, and DNA damage against oral cancer cells. Int. J. Mol. Sci. 2020, 21, 6443. [Google Scholar] [CrossRef]
- Imani, R.; Veranic, P.; Iglic, A.; Kreft, M.E.; Pazoki, M.; Hudoklin, S. Combined cytotoxic effect of UV-irradiation and TiO2 microbeads in normal urothelial cells, low-grade and high-grade urothelial cancer cells. Photochem. Photobiol. Sci. 2015, 14, 583–590. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Aiguade, J.; Hao, J.; Forsyth, C.J. Synthesis of a 2,9-dioxabicyclo[3.3.1]nonane via double intramolecular Hetero-Michael addition: Entry to the F−G ring system of the azaspiracids. Org. Lett. 2001, 3, 979–982. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, N.C.; Mondal, P.; Roy, S. A mild efficient iodine-catalyzed synthesis of novel anticoagulants with 2,8-dioxabicyclo[3.3.1]nonane core. Tetrahedron Lett. 2013, 54, 2386–2390. [Google Scholar] [CrossRef]
- Talontsi, F.M.; Dittrich, B.; Schüffler, A.; Sun, H.; Laatsch, H. Epicoccolides: Antimicrobial and antifungal polyketides from an endophytic fungus Epicoccum sp. associated with Theobroma cacao. Eur. J. Org. Chem. 2013, 2013, 3174–3180. [Google Scholar] [CrossRef]
- El Amrani, M.; Lai, D.; Debbab, A.; Aly, A.H.; Siems, K.; Seidel, C.; Schnekenburger, M.; Gaigneaux, A.; Diederich, M.; Feger, D.; et al. Protein kinase and HDAC inhibitors from the endophytic fungus Epicoccum nigrum. J. Nat. Prod. 2014, 77, 49–56. [Google Scholar] [CrossRef]
- Deshmukh, S.K.; Gupta, M.K.; Prakash, V.; Saxena, S. Endophytic fungi: A source of potential antifungal compounds. J. Fungi 2018, 4, 77. [Google Scholar] [CrossRef]
- Wang, S.-C.; Chang, M.-Y.; Shiau, J.-P.; Farooqi, A.A.; Huang, Y.-H.; Tang, J.-Y.; Chang, H.-W. Antiproliferation- and apoptosis-inducible effects of a novel nitrated [6,6,6]tricycle derivative (SK2) on oral cancer cells. Molecules 2022, 27, 1576. [Google Scholar] [CrossRef]
- Chan, C.K.; Tsai, Y.L.; Chang, M.Y. Construction of nitrated benzo[3.3.1]bicyclic acetal/ketal core via nitration of o-carbonyl allylbenzenes. Org. Lett. 2017, 19, 1358–1361. [Google Scholar] [CrossRef]
- Hung, J.-H.; Chen, C.-Y.; Omar, H.A.; Huang, K.-Y.; Tsao, C.-C.; Chiu, C.-C.; Chen, Y.-L.; Chen, P.-H.; Teng, Y.-N. Reactive oxygen species mediate Terbufos-induced apoptosis in mouse testicular cell lines via the modulation of cell cycle and pro-apoptotic proteins. Environ. Toxicol. 2016, 31, 1888–1898. [Google Scholar] [CrossRef]
- Huang, C.-H.; Yeh, J.-M.; Chan, W.-H. Hazardous impacts of silver nanoparticles on mouse oocyte maturation and fertilization and fetal development through induction of apoptotic processes. Environ. Toxicol. 2018, 33, 1039–1049. [Google Scholar] [CrossRef]
- Wang, T.-S.; Lin, C.-P.; Chen, Y.-P.; Chao, M.-R.; Li, C.-C.; Liu, K.-L. CYP450-mediated mitochondrial ROS production involved in arecoline N-oxide-induced oxidative damage in liver cell lines. Environ. Toxicol. 2018, 33, 1029–1038. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.Y.-K.; Chang, K.-W.; Chen, C.-F.; Chang, R.C.-S. Characterization of two new cell lines derived from oral cavity human squamous cell carcinomas—OC1 and OC2. J. Oral Maxillofac. Surg. 1990, 48, 385–390. [Google Scholar] [CrossRef]
- Su, C.; Haskins, A.H.; Omata, C.; Aizawa, Y.; Kato, T.A. PARP inhibition by flavonoids induced selective cell killing to BRCA2-deficient cells. Pharmaceuticals 2017, 10, 80. [Google Scholar] [CrossRef] [PubMed]
- Zou, P.; Xia, Y.; Chen, T.; Zhang, J.; Wang, Z.; Chen, W.; Chen, M.; Kanchana, K.; Yang, S.; Liang, G. Selective killing of gastric cancer cells by a small molecule targeting ROS-mediated ER stress activation. Mol. Carcinog. 2016, 55, 1073–1086. [Google Scholar] [CrossRef]
- Tang, J.-Y.; Ou-Yang, F.; Hou, M.-F.; Huang, H.-W.; Wang, H.-R.; Li, K.-T.; Fayyaz, S.; Shu, C.-W.; Chang, H.-W. Oxidative stress-modulating drugs have preferential anticancer effects-involving the regulation of apoptosis, DNA damage, endoplasmic reticulum stress, autophagy, metabolism, and migration. Semin. Cancer Biol. 2019, 58, 109–117. [Google Scholar] [CrossRef]
- Galati, S.; Boni, C.; Gerra, M.C.; Lazzaretti, M.; Buschini, A. Autophagy: A player in response to oxidative stress and DNA damage. Oxidative Med. Cell. Longev. 2019, 2019, 5692958. [Google Scholar] [CrossRef]
- Takac, P.; Kello, M.; Vilkova, M.; Vaskova, J.; Michalkova, R.; Mojzisova, G.; Mojzis, J. Antiproliferative effect of acridine chalcone is mediated by induction of oxidative stress. Biomolecules 2020, 10, 345. [Google Scholar] [CrossRef]
- Kim, U.; Shin, C.; Kim, C.-Y.; Ryu, B.; Kim, J.; Bang, J.; Park, J.-H. Albendazole exerts antiproliferative effects on prostate cancer cells by inducing reactive oxygen species generation. Oncol. Lett. 2021, 21, 395. [Google Scholar] [CrossRef]
- Nasihun, T.; Widayati, E. Administration of purwoceng (Pimpinella alpina Molk) improves oxidative stress biomarker following UVC irradiation in spargue-dawley male rats. J. Nat. Remedies 2016, 16, 115–124. [Google Scholar] [CrossRef]
- Dunkern, T.R.; Fritz, G.; Kaina, B. Ultraviolet light-induced DNA damage triggers apoptosis in nucleotide excision repair-deficient cells via Bcl-2 decline and caspase-3/-8 activation. Oncogene 2001, 20, 6026–6038. [Google Scholar] [CrossRef]
- Uehara, F.; Miwa, S.; Tome, Y.; Hiroshima, Y.; Yano, S.; Yamamoto, M.; Efimova, E.; Matsumoto, Y.; Maehara, H.; Bouvet, M.; et al. Comparison of UVB and UVC effects on the DNA damage-response protein 53BP1 in human pancreatic cancer. J. Cell. Biochem. 2014, 115, 1724–1728. [Google Scholar] [CrossRef] [PubMed]
- Momiyama, M.; Suetsugu, A.; Kimura, H.; Kishimoto, H.; Aki, R.; Yamada, A.; Sakurada, H.; Chishima, T.; Bouvet, M.; Endo, I.; et al. Imaging the efficacy of UVC irradiation on superficial brain tumors and metastasis in live mice at the subcellular level. J. Cell. Biochem. 2013, 114, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Kimura, H.; Lee, C.; Hayashi, K.; Yamauchi, K.; Yamamoto, N.; Tsuchiya, H.; Tomita, K.; Bouvet, M.; Hoffman, R.M. UV light killing efficacy of fluorescent protein-expressing cancer cells in vitro and in vivo. J. Cell. Biochem. 2010, 110, 1439–1446. [Google Scholar] [CrossRef] [PubMed]
- Chun, K.-S.; Kim, D.-H.; Surh, Y.-J. Role of reductive versus oxidative stress in tumor progression and anticancer drug resistance. Cells 2021, 10, 758. [Google Scholar] [CrossRef]
- Marullo, R.; Werner, E.; Degtyareva, N.; Moore, B.; Altavilla, G.; Ramalingam, S.S.; Doetsch, P.W. Cisplatin induces a mitochondrial-ROS response that contributes to cytotoxicity depending on mitochondrial redox status and bioenergetic functions. PLoS ONE 2013, 8, e81162. [Google Scholar] [CrossRef] [PubMed]
- Espinosa-Diez, C.; Miguel, V.; Mennerich, D.; Kietzmann, T.; Pérez, P.S.; Cadenas, S.; Lamas, S. Antioxidant responses and cellular adjustments to oxidative stress. Redox Biol. 2015, 6, 183–197. [Google Scholar] [CrossRef]
- Shiau, J.-P.; Chuang, Y.-T.; Yang, K.-H.; Chang, F.-R.; Sheu, J.-H.; Hou, M.-F.; Jeng, J.-H.; Tang, J.-Y.; Chang, H.-W. Brown algae-derived fucoidan exerts oxidative stress-dependent antiproliferation on oral cancer cells. Antioxidants 2022, 11, 841. [Google Scholar] [CrossRef]
- Perillo, B.; Di Donato, M.; Pezone, A.; Di Zazzo, E.; Giovannelli, P.; Galasso, G.; Castoria, G.; Migliaccio, A. ROS in cancer therapy: The bright side of the moon. Exp. Mol. Med. 2020, 52, 192–203. [Google Scholar] [CrossRef]
- de Jager, T.L.; Cockrell, A.E.; Du Plessis, S.S. Ultraviolet light induced generation of reactive oxygen species. Adv. Exp. Med. Biol. 2017, 996, 15–23. [Google Scholar]
- Chan, W.-H.; Wu, C.-C.; Yu, J.-S. Curcumin inhibits UV irradiation-induced oxidative stress and apoptotic biochemical changes in human epidermoid carcinoma A431 cells. J. Cell. Biochem. 2003, 90, 327–338. [Google Scholar] [CrossRef]
- Chan, W.-H.; Yu, J.-S. Inhibition of UV irradiation-induced oxidative stress and apoptotic biochemical changes in human epidermal carcinoma A431 cells by genistein. J. Cell. Biochem. 2000, 78, 73–84. [Google Scholar] [CrossRef]
- McAdam, E.; Brem, R.; Karran, P. Oxidative stress–induced protein damage inhibits DNA repair and determines mutation risk and therapeutic efficacy. Mol. Cancer Res. 2016, 14, 612–622. [Google Scholar] [CrossRef] [PubMed]
- Van Houten, B.; Santa-Gonzalez, G.A.; Camargo, M. DNA repair after oxidative stress: Current challenges. Curr. Opin. Toxicol. 2018, 7, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Torres-Gonzalez, M.; Gawlowski, T.; Kocalis, H.; Scott, B.T.; Dillmann, W.H. Mitochondrial 8-oxoguanine glycosylase decreases mitochondrial fragmentation and improves mitochondrial function in H9C2 cells under oxidative stress conditions. Am. J. Physiol. Cell Physiol. 2014, 306, C221–C229. [Google Scholar] [CrossRef]
- Brand, R.M.; Wipf, P.; Durham, A.; Epperly, M.W.; Greenberger, J.S.; Falo, L.D., Jr. Targeting mitochondrial oxidative stress to mitigate UV-induced skin damage. Front. Pharmacol. 2018, 9, 920. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.-C.; Yen, C.-Y.; Shiau, J.-P.; Chang, M.-Y.; Hou, M.-F.; Tang, J.-Y.; Chang, H.-W. Combined Treatment of Nitrated [6,6,6]Tricycles Derivative (SK2)/Ultraviolet C Highly Inhibits Proliferation in Oral Cancer Cells In Vitro. Biomedicines 2022, 10, 1196. https://doi.org/10.3390/biomedicines10051196
Wang S-C, Yen C-Y, Shiau J-P, Chang M-Y, Hou M-F, Tang J-Y, Chang H-W. Combined Treatment of Nitrated [6,6,6]Tricycles Derivative (SK2)/Ultraviolet C Highly Inhibits Proliferation in Oral Cancer Cells In Vitro. Biomedicines. 2022; 10(5):1196. https://doi.org/10.3390/biomedicines10051196
Chicago/Turabian StyleWang, Sheng-Chieh, Ching-Yu Yen, Jun-Ping Shiau, Meng-Yang Chang, Ming-Feng Hou, Jen-Yang Tang, and Hsueh-Wei Chang. 2022. "Combined Treatment of Nitrated [6,6,6]Tricycles Derivative (SK2)/Ultraviolet C Highly Inhibits Proliferation in Oral Cancer Cells In Vitro" Biomedicines 10, no. 5: 1196. https://doi.org/10.3390/biomedicines10051196
APA StyleWang, S.-C., Yen, C.-Y., Shiau, J.-P., Chang, M.-Y., Hou, M.-F., Tang, J.-Y., & Chang, H.-W. (2022). Combined Treatment of Nitrated [6,6,6]Tricycles Derivative (SK2)/Ultraviolet C Highly Inhibits Proliferation in Oral Cancer Cells In Vitro. Biomedicines, 10(5), 1196. https://doi.org/10.3390/biomedicines10051196





