Amyloid Beta Oligomers-Induced Ca2+ Entry Pathways: Role of Neuronal Networks, NMDA Receptors and Amyloid Channel Formation
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Reagents
2.2. Cell Lines
2.3. Mouse Anterior Pituitary Cells
2.4. Primary Rat Cerebellar or Hippocampal Neuron Cultures
2.5. Preparation of Amyloid β Peptide1–42 Oligomers
2.6. Fluorescence Imaging of Cytosolic Ca2+ Concentration
2.7. Statistics
3. Results
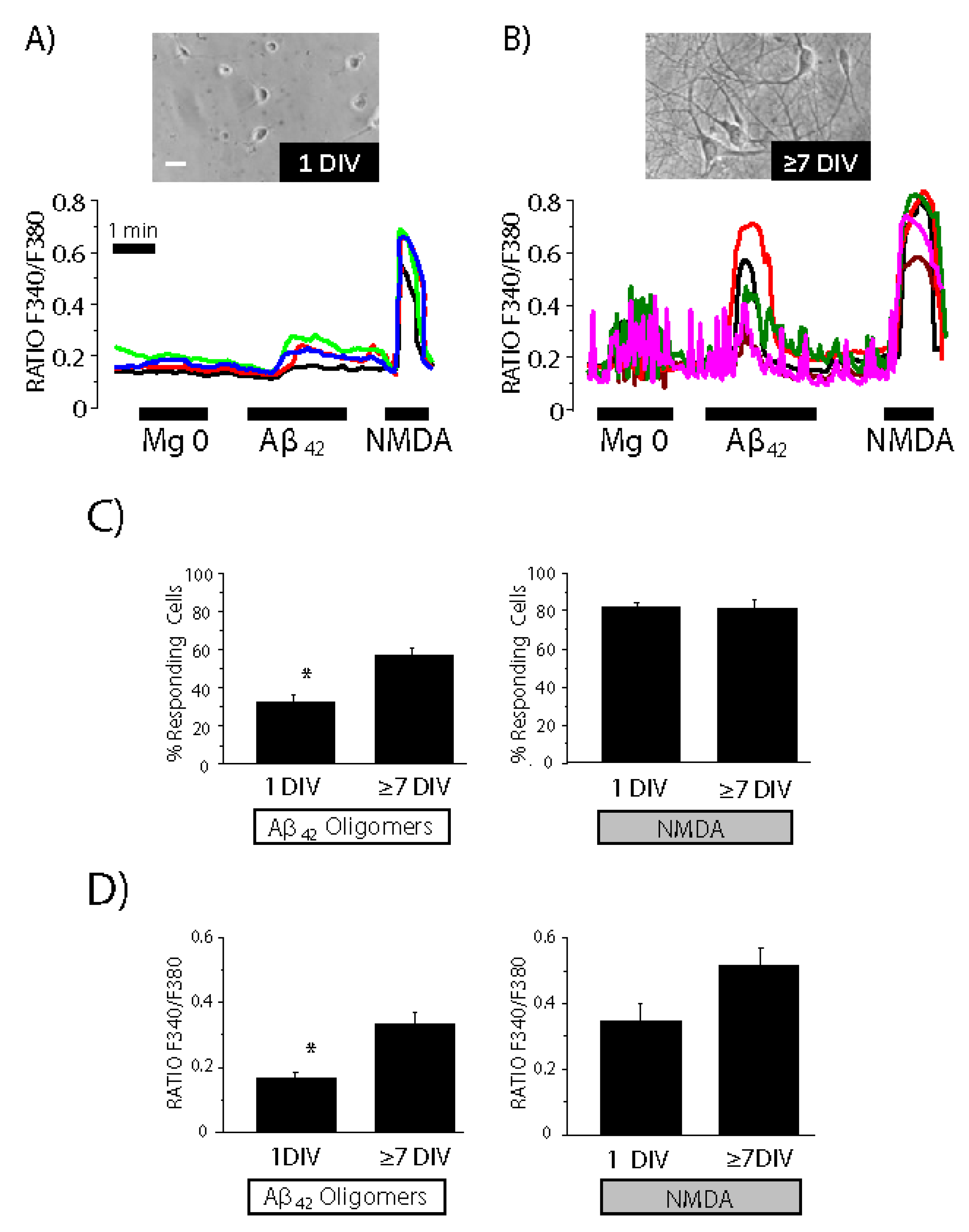
3.1. Ca2+ Entry Pathways Activated by Amyloid β Oligomers in Rat Neurons
3.2. Cultured Neurons Develop Neural Networks as Shown by Synchronous Oscillations of Cytosolic Ca2+ Concentration That Are Susceptible to Activation by Aβ1–42 Oligomers
3.3. Contribution of the Neuronal Networking Activity to Ca2+ Responses Induced by Aβ1–42 Oligomers
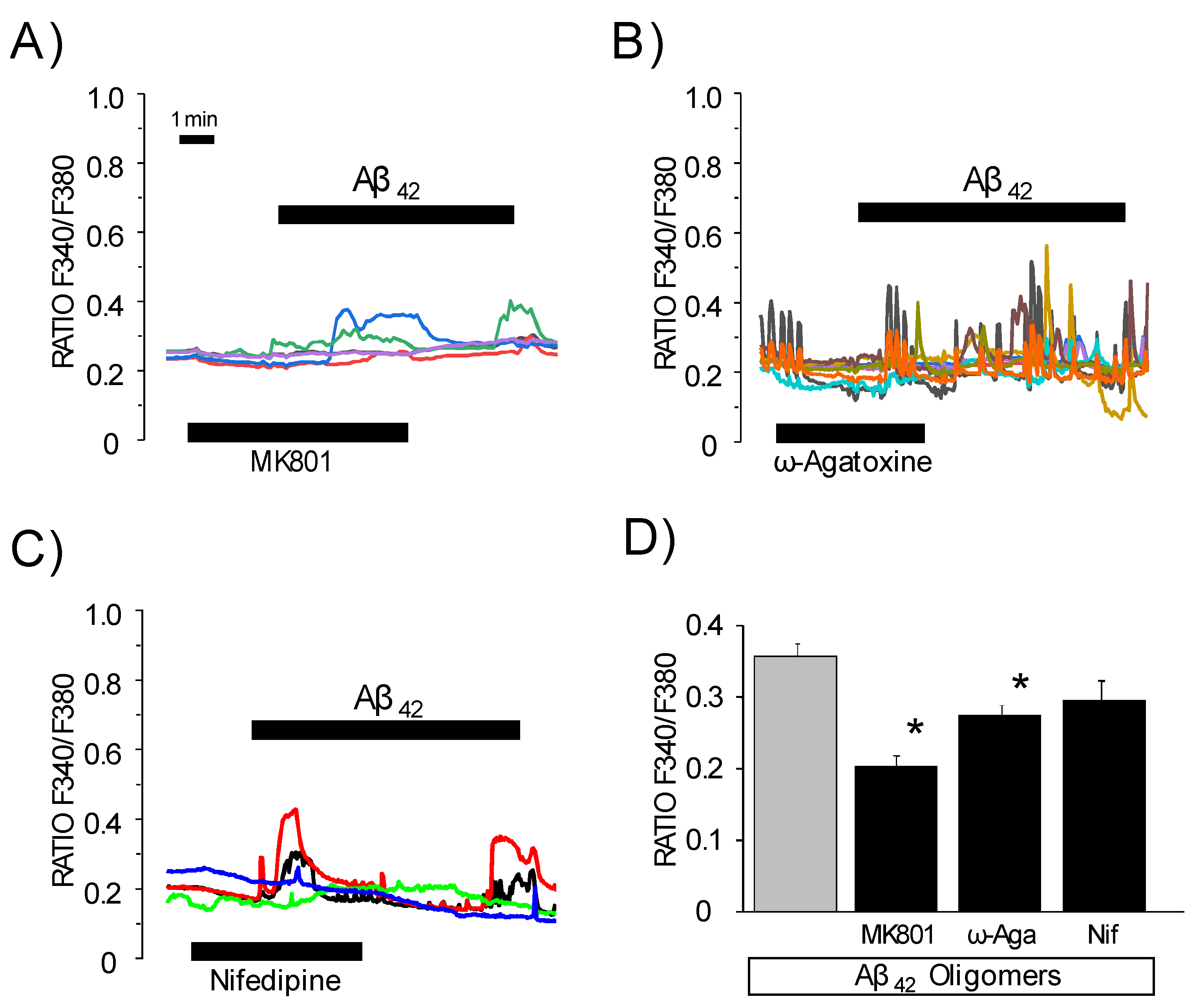
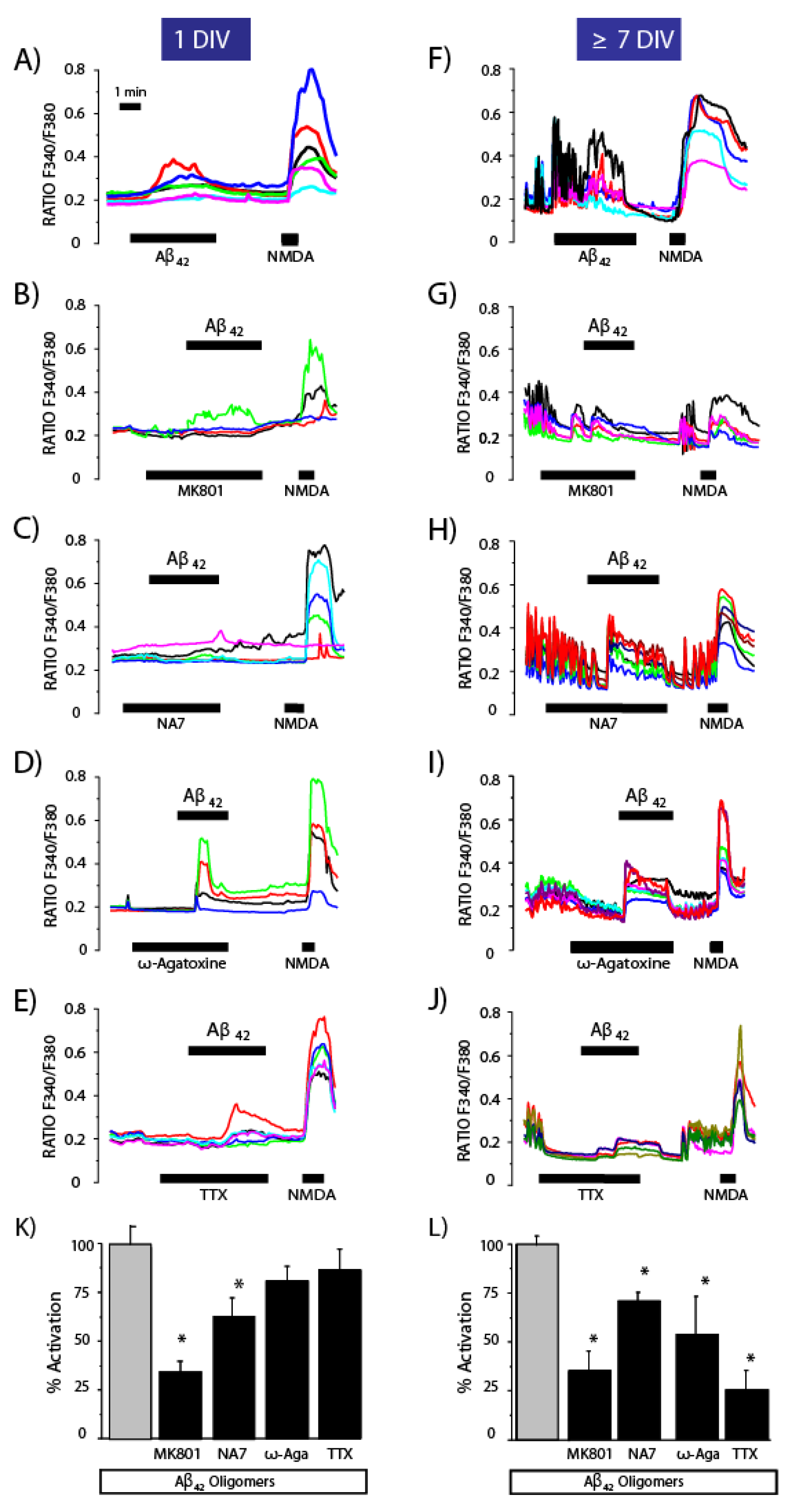
3.4. Effects of Channel Antagonists on Ca2+ Responses Induced by Aβ1–42 Oligomers with or without Networking Activity
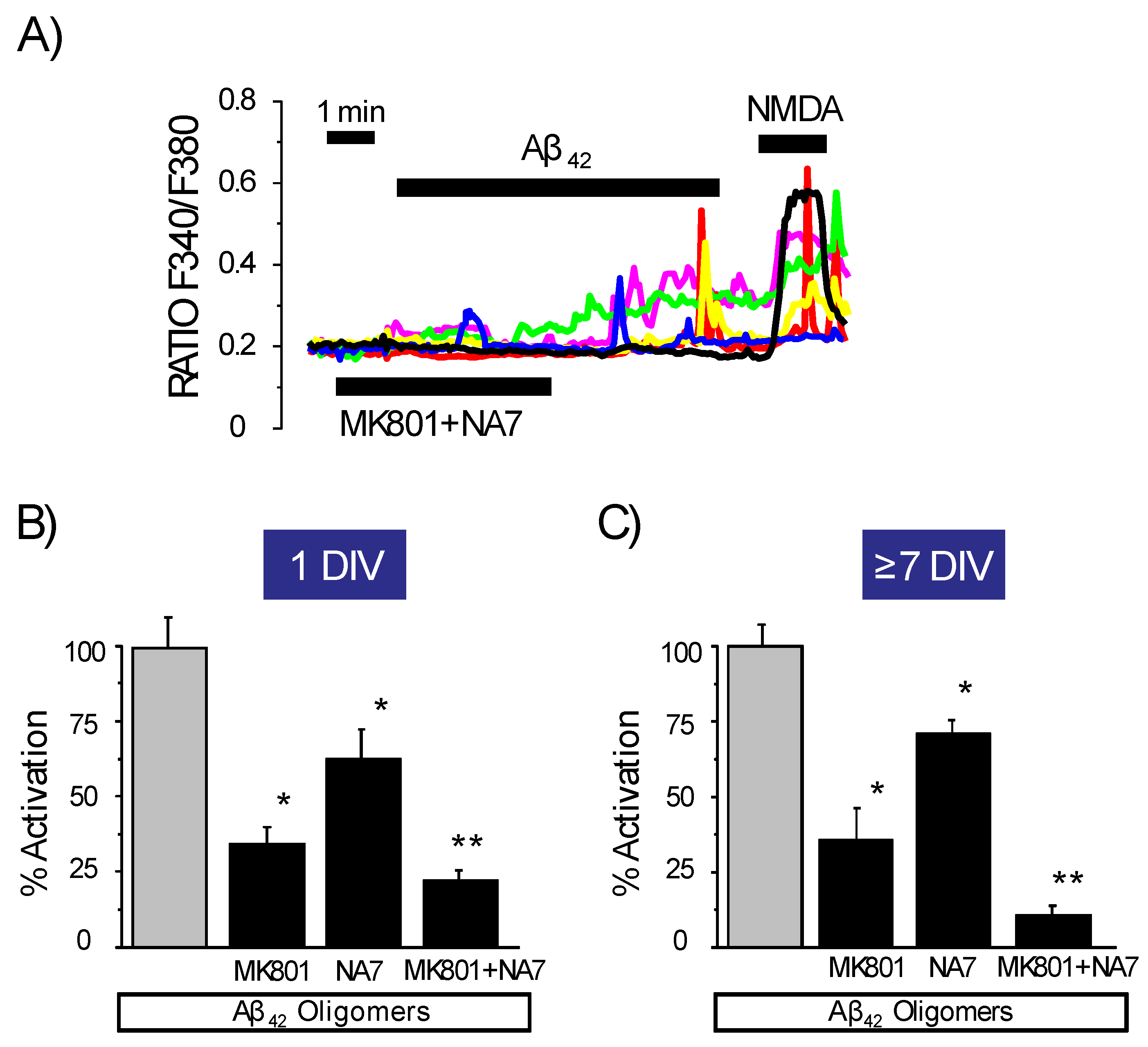
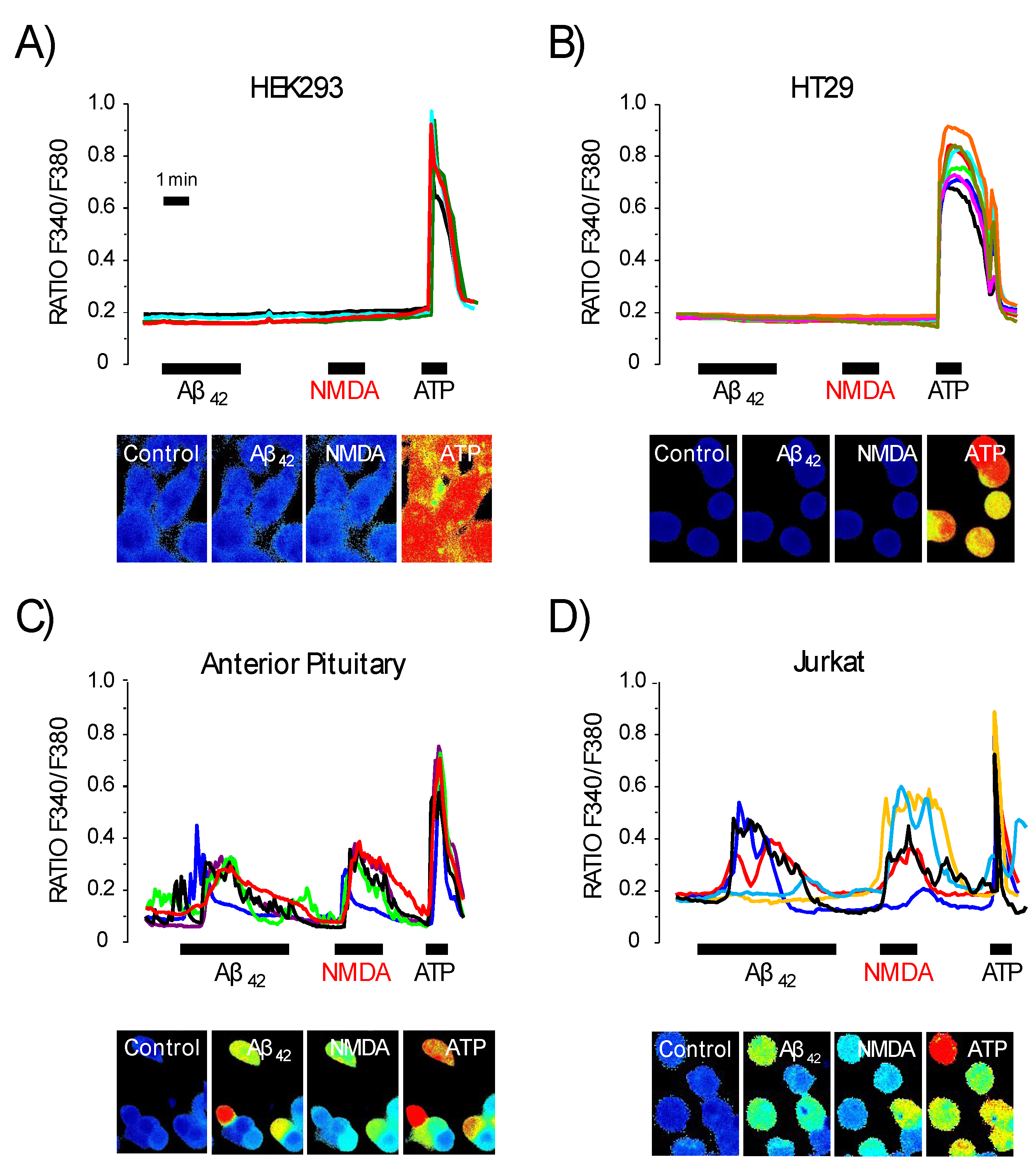
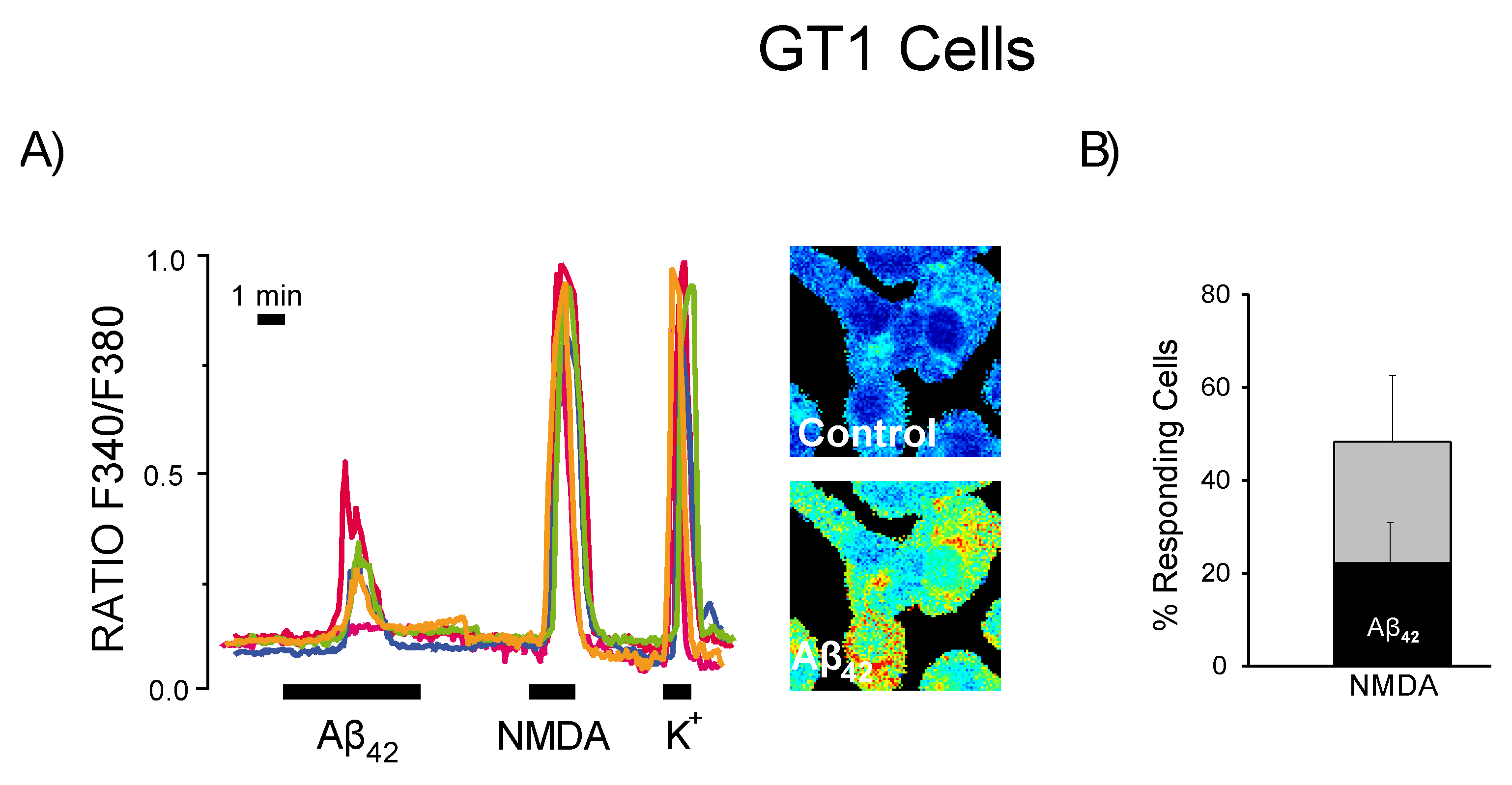
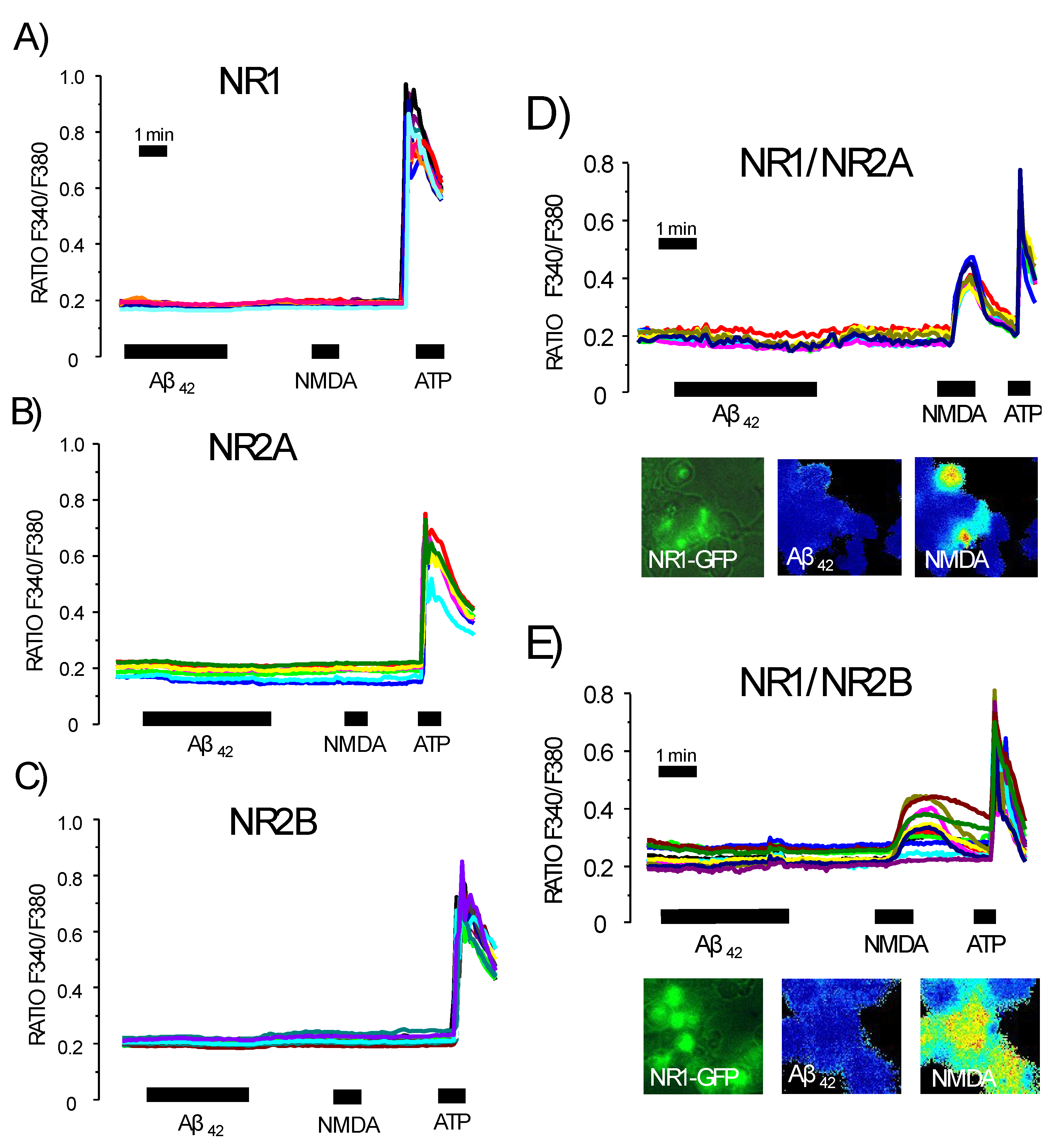
3.5. Expression of NMDA Receptors Is Mandatory for Ca2+ Responses to Aβ1–42 Oligomers
3.6. Expression of Functional NMDA Receptors Is Not Sufficient for Ca2+ Responses Induced by Aβ1–42 Oligomers
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alzheimer, A. Uber eine eigenartige Erkrankung der Hirnrinde. Allg. Z. Psychiatr. Phychish Gerichtl. Med. 1907, 64, 146–148. [Google Scholar]
- Tahami Monfared, A.A.; Byrnes, M.J.; White, L.A.; Zhang, Q. The Humanistic and Economic Burden of Alzheimer’s Disease. Neurol. Ther. 2022, ahead of print.
- Kim, C.K.; Lee, Y.R.; Ong, L.; Gold, M.; Kalali, A.; Sarkar, J. Alzheimer’s Disease: Key Insights from Two Decades of Clinical Trial Failures. J. Alzheimer’s Dis. 2022, 87, 83–100. [Google Scholar] [CrossRef] [PubMed]
- Haass, C.; Selkoe, D.J. Soluble protein oligomers in neurodegeneration: Lessons from the Alzheimer’s amyloid beta peptide. Nat. Rev. Mol. Cell. Biol. 2007, 8, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Jellinger, K.A. Recent update on the heterogeneity of the Alzheimer’s disease spectrum. J. Neural Transm. 2022, 129, 1–24. [Google Scholar] [CrossRef]
- Tanzi, R.E.; Kovacs, D.M.; Kim, T.W.; Moir, R.D.; Guenette, S.Y.; Wasco, W. The gene defects responsible for familial Alzheimer’s disease. Neurobiol. Dis. 1996, 3, 159–168. [Google Scholar] [CrossRef]
- Sanz-Blasco, S.; Valero, R.A.; Rodríguez-Crespo, I.; Villalobos, C.; Núñez, L. Mitochondrial Ca2+ overload underlies Aβ oligomers neurotoxicity providing an unexpected mechanism of neuroprotection by NSAIDs. PLoS ONE 2008, 3, e2718. [Google Scholar] [CrossRef]
- Calvo, M.; Sanz-Blasco, S.; Caballero, E.; Villalobos, C.; Núñez, L. Susceptibility to excitotoxicity in aged hippocampal cultures and neuroprotection by non-steroidal anti-inflammatory drugs: Role of mitochondrial calcium. J. Neurochem. 2015, 132, 403–417. [Google Scholar] [CrossRef]
- Caballero, E.; Calvo-Rodriguez, M.; Gonzalo-Ruiz, A.; Villalobos, C.; Núñez, L. A new procedure for amyloid β oligomers preparation enables the unambiguous testing of their effects on cytosolic and mitochondrial Ca2+ entry and cell death in primary neurons. Neurosci. Lett. 2016, 612, 66–73. [Google Scholar] [CrossRef]
- Calvo-Rodríguez, M.; Núñez, L.; Villalobos, C. Non-steroidal anti-inflammatory drugs (NSAIDs) and neuroprotection in the elderly: A view from the mitochondria. Neural Regen. Res. 2015, 10, 1371–1372. [Google Scholar]
- Calvo-Rodríguez, M.; García-Durillo, M.; Villalobos, C.; Núñez, L. Aging enables Ca2+ overload and apoptosis induced by amyloid-β oligomers in rat hippocampal neurons: Neuroprotection by non-steroidal anti-inflammatory drugs and R-flurbiprofen in aging neurons. J. Alzheimer’s Dis. 2016, 54, 207–221. [Google Scholar] [CrossRef]
- Sanz-Blasco, S.; Calvo-Rodríguez, M.; Caballero, E.; García-Durillo, M.; Núñez, L.; Villalobos, C. Is it all said for NSAIDs in Alzheimer’s disease? Role of mitochondrial calcium uptake. Curr. Alzheimer Res. 2018, 15, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Calvo-Rodríguez, M.; García-Durillo, M.; Villalobos, C.; Núñez, L. In vitro aging promotes endoplasmic reticulum (ER)-mitochondria Ca2+ cross talk and loss of store-operated Ca2+ entry (SOCE) in rat hippocampal neurons. Biochim. Biophys. Acta Mol. Cell Res. 2016, 863, 2637–2649. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wu, L.; Pchitskaya, E.; Zakharova, O.; Saito, T.; Saido, T.; Bezprozvanny, I. Neuronal Store-Operated Calcium Entry and Mushroom Spine Loss in Amyloid Precursor Protein Knock-In Mouse Model of Alzheimer’s Disease. J. Neurosci. 2015, 35, 13275–13286. [Google Scholar] [CrossRef] [PubMed]
- Calvo-Rodríguez, M.; de la Fuente, C.; García-Durillo, M.; García-Rodríguez, C.; Villalobos, C.; Núñez, L. Aging and amyloid β oligomers enhance TLR4 expression, LPS-induced Ca2+ responses and neuron cell death in cultured rat hippocampal neurons. J. Neuroinflamm. 2017, 14, 24. [Google Scholar] [CrossRef]
- Calvo-Rodriguez, M.; Hernando-Pérez, E.; Núñez, L.; Villalobos, C. Amyloid β oligomers increase ER-mitochondria Ca2+ cross talk in young hippocampal neurons and exacerbate aging-induced intracellular Ca2+ remodeling. Front. Cell. Neurosci. 2019, 13, 22. [Google Scholar] [CrossRef] [PubMed]
- Calvo-Rodriguez, M.; García-Rodríguez, C.; Villalobos, C.; Núñez, L. Role of Toll like receptor 4 in Alzheimer´s disease. Front. Immunol. 2020, 11, 1588. [Google Scholar] [CrossRef]
- Calvo-Rodriguez, M.; Hernando-Pérez, E.; López-Vázquez, S.; Núñez, J.; Villalobos, C.; Núñez, L. Remodeling of intracellular Ca2+ homeostasis in rat hippocampal neurons aged in vitro. Int. J. Mol. Sci. 2020, 21, 1549. [Google Scholar] [CrossRef]
- Pascual, M.; Calvo-Rodríguez, M.; Núñez, L.; Villalobos, C.; Ureña, J.; Guerri, C. Toll-like receptors in neuroinflammation, neurodegeneration and alcohol-induced brain damage. IUBMB Life 2021, 73, 900–915. [Google Scholar] [CrossRef]
- Arispe, N.; Rojas, E.; Pollard, H.B. Alzheimer disease amyloid beta protein forms calcium channels in bilayer membranes: Blockade by tromethamine and aluminum. Proc. Natl. Acad. Sci. USA 1993, 90, 567–571. [Google Scholar] [CrossRef]
- Lin, H.; Arispe, N.J. Single-cell screening of cytosolic [Ca2+] reveals cell-selective action by the Alzheimer’s Aβ peptide ion channel. Cell Stress Chaperones 2015, 20, 333–342. [Google Scholar] [CrossRef]
- De Felice, F.G.; Velasco, P.T.; Lambert, M.P.; Viola, K.; Fernandez, S.J.; Ferreira, S.T.; Klein, W.L. Abeta oligomers induce neuronal oxidative stress through an N-methyl-D-aspartate receptor-dependent mechanism that is blocked by the Alzheimer drug memantine. J. Biol. Chem. 2007, 282, 11590–11601. [Google Scholar] [CrossRef] [PubMed]
- Alberdi, E.; Sánchez-Gómez, M.V.; Cavaliere, F.; Pérez-Samartín, A.; Zugaza, J.L.; Trullas, R.; Domercq, M.; Matute, C. Amyloid beta oligomers induce Ca2+ dysregulation and neuronal death through activation of ionotropic glutamate receptors. Cell Calcium 2010, 47, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Texidó, L.; Martín-Satué, M.; Alberdi, E.; Solsona, C.; Matute, C. Amyloid beta peptide oligomers directly activate NMDA receptors. Cell Calcium 2011, 49, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Sivanesan, S.; Tan, A.; Rajadas, J. Pathogenesis of Aβ oligomers in synaptic failure. Curr. Alzheimer Res. 2013, 10, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Núñez, L.; Sánchez, A.; Fonteriz, R.I.; Garcia-Sancho, J. Mechanisms for synchronous calcium oscillations in cultured rat cerebellar neurons. Eur. J. Neurosci. 1996, 8, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Alonso, M.T.; Chamero, P.; Villalobos, C.; García-Sancho, J. Fura-2 antagonises calcium-induced calcium release. Cell Calcium 2003, 33, 27–35. [Google Scholar] [CrossRef]
- Sobradillo, D.; Hernández-Morales, M.; Ubierna, D.; Moyer, M.P.; Núñez, L.; Villalobos, C. A reciprocal shift in transient receptor potential channel 1 (TRPC1) and stromal interaction molecule 2 (STIM2) contributes to Ca2+ remodeling and cancer hallmarks in colorectal carcinoma cells. J. Biol. Chem. 2014, 289, 28765–28782. [Google Scholar] [CrossRef]
- Jara, E.; Hidalgo, M.A.; Hancke, J.L.; Hidalgo, A.I.; Brauchi, S.; Nuñez, L.; Villalobos, C.; Burgos, R.A. Delphinidin activates NFAT and induces IL-2 production through SOCE in T cells. Cell Biochem. Biophys. 2014, 68, 497–509. [Google Scholar] [CrossRef]
- Nuñez, L.; Faught, W.J.; Frawley, L.S. Episodic gonadotropin-releasing hormone gene expression revealed by dynamic monitoring of luciferase reporter activity in single, living neurons. Proc. Natl. Acad. Sci. USA 1998, 95, 9648–9653. [Google Scholar] [CrossRef]
- Núñez, L.; Bird, G.S.; Hernando-Pérez, E.; Pérez-Riesgo, E.; Putney, J.W., Jr.; Villalobos, C. Store-operated Ca2+ entry and Ca2+ responses to hypothalamic releasing hormones in anterior pituitary cells from Orai1-/- and heptaTRPC knockout mice. Biochim. Biophys. Acta Mol. Cell. Res. 2019, 1866, 1124–1136. [Google Scholar] [CrossRef]
- Penn, Y.; Segal, M.; Moses, E. Network synchronization in hippocampal neurons. Proc. Natl. Acad. Sci. USA 2016, 113, 3341–3346. [Google Scholar] [CrossRef] [PubMed]
- Villalobos, C.; Núñez, L.; Garcia-Sancho, J. Functional glutamate receptors in a subpopulation of anterior pituitary cells. FASEB J. 1996, 10, 654–660. [Google Scholar] [CrossRef] [PubMed]
- Miglio, G.; Dianzani, C.; Fallarini, S.; Fantozzi, R.; Lombardi, G. Stimulation of N-methyl-D-aspartate receptors modulates Jurkat T cell growth and adhesion to fibronectin. Biochem. Biophys. Res. Commun. 2007, 361, 404–409. [Google Scholar] [CrossRef] [PubMed]
- Mahesh, V.B.; Zamorano, P.; De Sevilla, L.; Lewis, D.; Brann, D.W. Characterization of ionotropic glutamate receptors in rat hypothalamus, pituitary and immortalized gonadotropin-releasing hormone (GnRH) neurons (GT1-7 cells). Neuroendocrinology 1999, 69, 397–407. [Google Scholar] [CrossRef]
- Kloda, A.; Martinac, B.; Adams, D.J. Polymodal regulation of NMDA receptor channels. Channels 2007, 1, 334–343. [Google Scholar] [CrossRef][Green Version]
- Atwood, C.; Bacskai, B.; Kuchibhotla, K.; Bezprozvanny, I.; Chakroborty, S.; Fagan, T.; Foskett, K.; Green, K.; Goussakov, I.; Moyer, J.; et al. Alzheimer research forum live discussion: Calcium in AD pathogenesis. J. Alzheimer’s Dis. 2009, 16, 909–917. [Google Scholar]
- Berridge, M.J. Calcium signalling and Alzheimer’s disease. Neurochem. Res. 2011, 36, 1149–1156. [Google Scholar] [CrossRef]
- Cascella, R.; Cecchi, C. Calcium Dyshomeostasis in Alzheimer’s Disease Pathogenesis. Int. J. Mol. Sci. 2021, 22, 4914. [Google Scholar] [CrossRef]
- Arbel-Ornath, M.; Hudry, E.; Boivin, J.R.; Hashimoto, T.; Takeda, S.; Kuchibhotla, K.V.; Hou, S.; Lattarulo, C.R.; Belcher, A.M.; Shakerdge, N.; et al. Soluble oligomeric amyloid-β induces calcium dyshomeostasis that precedes synapse loss in the living mouse brain. Mol. Neurodegener. 2017, 12, 27. [Google Scholar] [CrossRef]
- Calvo-Rodriguez, M.; Hou, S.S.; Snyder, A.C.; Kharitonova, E.K.; Russ, A.N.; Das, S.; Fan, Z.; Muzikansky, A.; Garcia-Alloza, M.; Serrano-Pozo, A.; et al. Increased mitochondrial calcium levels associated with neuronal death in a mouse model of Alzheimer’s disease. Nat. Commun. 2020, 11, 2146. [Google Scholar] [CrossRef]
- Ferreira, I.L.; Ferreiro, E.; Schmidt, J.; Cardoso, J.M.; Pereira, C.M.; Carvalho, A.L.; Oliveira, C.R.; Rego, A.C. Aβ and NMDAR activation cause mitochondrial dysfunction involving ER calcium release. Neurobiol. Aging 2015, 36, 680–692. [Google Scholar] [CrossRef] [PubMed]
- Zempel, H.; Thies, E.; Mandelkow, E.; Mandelkow, E.M. Aβ oligomers cause localized Ca2+ elevation, missorting of endogenous Tau into dendrites, Tau phosphorylation, and destruction of microtubules and spines. J. Neurosci. 2010, 30, 11938–11950. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caballero, E.; Hernando-Pérez, E.; Tapias, V.; Calvo-Rodríguez, M.; Villalobos, C.; Núñez, L. Amyloid Beta Oligomers-Induced Ca2+ Entry Pathways: Role of Neuronal Networks, NMDA Receptors and Amyloid Channel Formation. Biomedicines 2022, 10, 1153. https://doi.org/10.3390/biomedicines10051153
Caballero E, Hernando-Pérez E, Tapias V, Calvo-Rodríguez M, Villalobos C, Núñez L. Amyloid Beta Oligomers-Induced Ca2+ Entry Pathways: Role of Neuronal Networks, NMDA Receptors and Amyloid Channel Formation. Biomedicines. 2022; 10(5):1153. https://doi.org/10.3390/biomedicines10051153
Chicago/Turabian StyleCaballero, Erica, Elena Hernando-Pérez, Victor Tapias, María Calvo-Rodríguez, Carlos Villalobos, and Lucía Núñez. 2022. "Amyloid Beta Oligomers-Induced Ca2+ Entry Pathways: Role of Neuronal Networks, NMDA Receptors and Amyloid Channel Formation" Biomedicines 10, no. 5: 1153. https://doi.org/10.3390/biomedicines10051153
APA StyleCaballero, E., Hernando-Pérez, E., Tapias, V., Calvo-Rodríguez, M., Villalobos, C., & Núñez, L. (2022). Amyloid Beta Oligomers-Induced Ca2+ Entry Pathways: Role of Neuronal Networks, NMDA Receptors and Amyloid Channel Formation. Biomedicines, 10(5), 1153. https://doi.org/10.3390/biomedicines10051153






