Vascularization of Patient-Derived Tumoroid from Non-Small-Cell Lung Cancer and Its Microenvironment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Human Specimens, Tissue Preparation and PDT Formation
2.2. Vascularization of Fibrin Matrix and PDTs
2.3. Combination of Vascularized Fibrin Matrix and Vascularized PDTs
2.4. Histologic Examination
2.5. Immunofluorescence Staining
3. Results
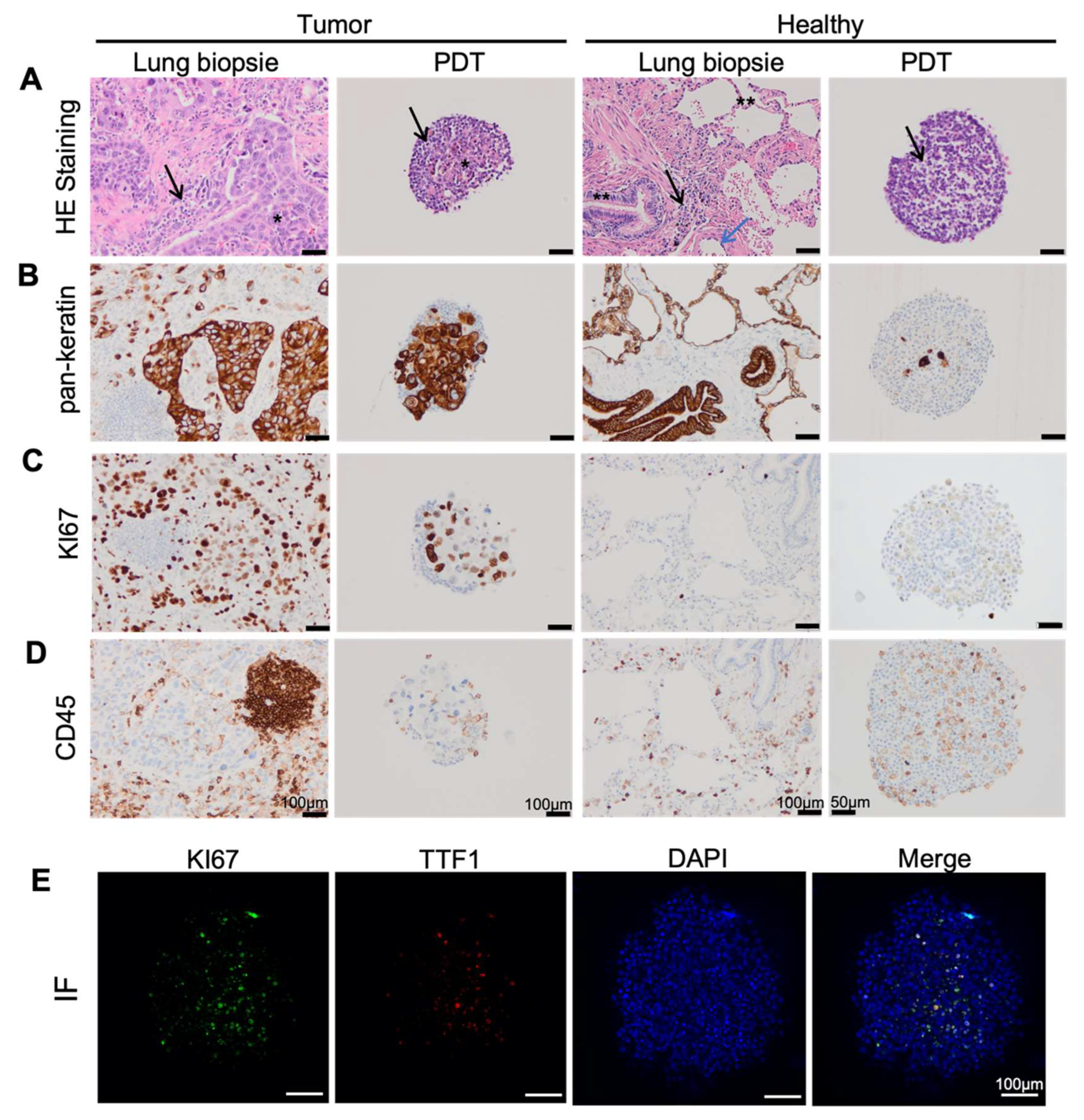
3.1. Lung Cancer PDTs Maintain Histopathological Features of Original Cancer Tissue
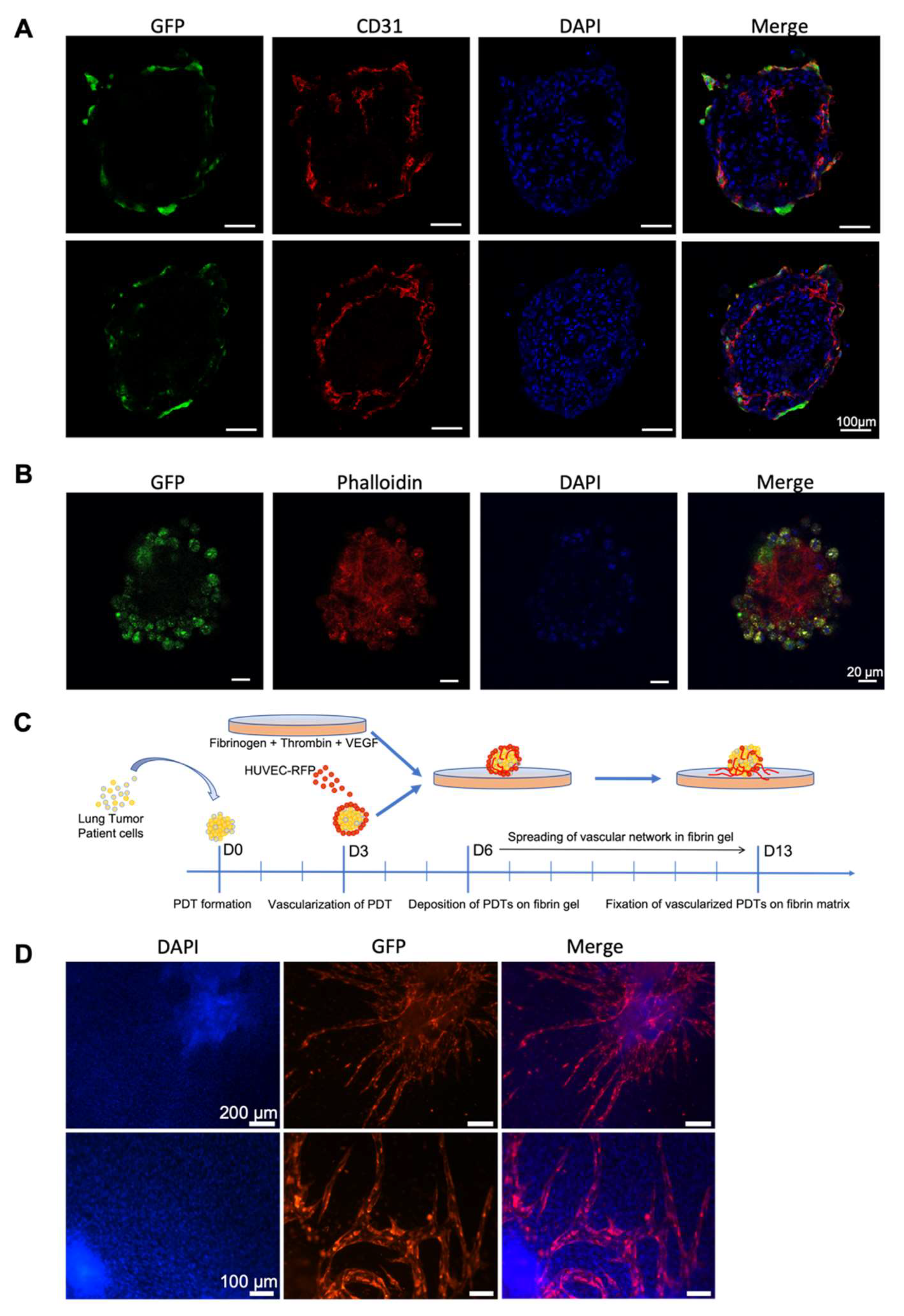
3.2. Endothelial Cells Infiltrate PDT from Normal Vascular Microenvironment
3.3. Endothelial Cells Infiltrate Normal Surrounding Microenvironment from Vascularized PDT
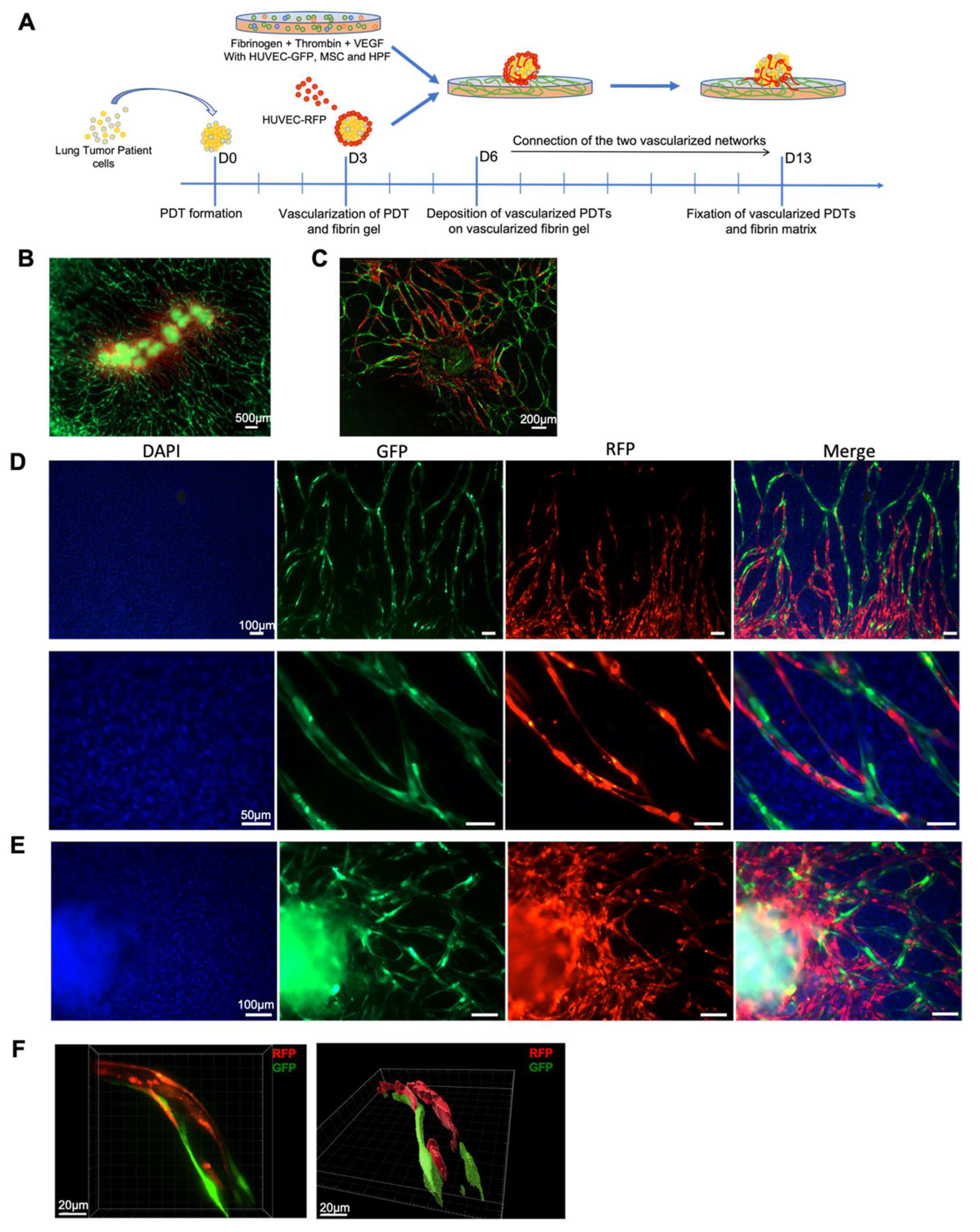
3.4. Vascular Network Connection between Vascularized Microenvironment and Vascularized PDT
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Takebe, T.; Imai, R.; Ono, S. The Current Status of Drug Discovery and Development as Originated in United States Academia: The Influence of Industrial and Academic Collaboration on Drug Discovery and Development. Clin. Transl. Sci. 2018, 11, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Khatami, M. Analyses of Repeated Failures in Cancer Therapy for Solid Tumors: Poor Tumor-Selective Drug Delivery, Low Therapeutic Efficacy and Unsustainable Costs. Clin. Transl. Med. 2018, 7, 11. [Google Scholar] [CrossRef] [PubMed]
- Cukierman, E.; Pankov, R.; Stevens, D.R.; Yamada, K.M. Taking Cell-Matrix Adhesions to the Third Dimension. Science 2001, 294, 1708–1712. [Google Scholar] [CrossRef] [PubMed]
- Thoma, C.R.; Zimmermann, M.; Agarkova, I.; Kelm, J.M.; Krek, W. 3D Cell Culture Systems Modeling Tumor Growth Determinants in Cancer Target Discovery. Adv. Drug Deliv. Rev. 2014, 69–70, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Langhans, S.A. Three-Dimensional in Vitro Cell Culture Models in Drug Discovery and Drug Repositioning. Front. Pharmacol. 2018, 9, 6. [Google Scholar] [CrossRef]
- Hickman, J.A.; Graeser, R.; de Hoogt, R.; Vidic, S.; Brito, C.; Gutekunst, M.; van der Kuip, H. IMI PREDECT Consortium Three-Dimensional Models of Cancer for Pharmacology and Cancer Cell Biology: Capturing Tumor Complexity in Vitro/Ex Vivo. Biotechnol. J. 2014, 9, 1115–1128. [Google Scholar] [CrossRef]
- Whiteside, T. The Tumor Microenvironment and Its Role in Promoting Tumor Growth. Oncogene 2008, 27, 5904–5912. [Google Scholar] [CrossRef] [Green Version]
- Balkwill, F.R.; Capasso, M.; Hagemann, T. The Tumor Microenvironment at a Glance. J. Cell Sci. 2012, 125, 5591–5596. [Google Scholar] [CrossRef] [Green Version]
- Mroue, R.; Bissell, M.J. Three-Dimensional Cultures of Mouse Mammary Epithelial Cells. Methods Mol. Biol. 2013, 945, 221–250. [Google Scholar] [CrossRef] [Green Version]
- Klemm, F.; Joyce, J.A. Microenvironmental Regulation of Therapeutic Response in Cancer. Trends Cell Biol. 2015, 25, 198–213. [Google Scholar] [CrossRef] [Green Version]
- Jin, M.-Z.; Jin, W.-L. The Updated Landscape of Tumor Microenvironment and Drug Repurposing. Signal Transduct. Target. Ther. 2020, 5, 166. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Mun, H.; Sung, C.O.; Cho, E.J.; Jeon, H.-J.; Chun, S.-M.; Jung, D.J.; Shin, T.H.; Jeong, G.S.; Kim, D.K.; et al. Patient-Derived Lung Cancer Organoids as in Vitro Cancer Models for Therapeutic Screening. Nat. Commun. 2019, 10, 3991. [Google Scholar] [CrossRef] [PubMed]
- Driehuis, E.; Kretzschmar, K.; Clevers, H. Establishment of Patient-Derived Cancer Organoids for Drug-Screening Applications. Nat. Protoc. 2020, 15, 3380–3409. [Google Scholar] [CrossRef] [PubMed]
- Senapati, S.; Mahanta, A.K.; Kumar, S.; Maiti, P. Controlled Drug Delivery Vehicles for Cancer Treatment and Their Performance. Signal Transduct. Target. Ther. 2018, 3, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dewhirst, M.W.; Secomb, T.W. Transport of Drugs from Blood Vessels to Tumour Tissue. Nat. Rev. Cancer 2017, 17, 738–750. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Herbst, R.S.; Heymach, J.V.; Lippman, S.M. Lung Cancer. N. Engl. J. Med. 2008, 359, 1367–1380. [Google Scholar] [CrossRef] [Green Version]
- Samet, J.M.; Avila-Tang, E.; Boffetta, P.; Hannan, L.M.; Olivo-Marston, S.; Thun, M.J.; Rudin, C.M. Lung Cancer in Never Smokers: Clinical Epidemiology and Environmental Risk Factors. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2009, 15, 5626–5645. [Google Scholar] [CrossRef] [Green Version]
- Brambilla, E.; Le Teuff, G.; Marguet, S.; Lantuejoul, S.; Dunant, A.; Graziano, S.; Pirker, R.; Douillard, J.-Y.; Le Chevalier, T.; Filipits, M.; et al. Prognostic Effect of Tumor Lymphocytic Infiltration in Resectable Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2016, 34, 1223–1230. [Google Scholar] [CrossRef]
- Buisseret, L.; Garaud, S.; de Wind, A.; Van den Eynden, G.; Boisson, A.; Solinas, C.; Gu-Trantien, C.; Naveaux, C.; Lodewyckx, J.-N.; Duvillier, H.; et al. Tumor-Infiltrating Lymphocyte Composition, Organization and PD-1/ PD-L1 Expression Are Linked in Breast Cancer. Oncoimmunology 2016, 6, e1257452. [Google Scholar] [CrossRef]
- Salgueiro, L.; Kummer, S.; Sonntag-Buck, V.; Weiß, A.; Schneider, M.A.; Kräusslich, H.-G.; Sotillo, R. Generation of Human Lung Organoid Cultures from Healthy and Tumor Tissue to Study Infectious Diseases. J. Virol. 2022, 96, e0009822. [Google Scholar] [CrossRef] [PubMed]
- Sandhya, S.; Hogenson, T.L.; Fernandez-Zapico, M.E. Patient-Derived Organoids, Creating a New Window of Opportunities for Pancreatic Cancer Patients. EMBO Mol. Med. 2022, 14, e15707. [Google Scholar] [CrossRef] [PubMed]
- Tuveson, D.; Clevers, H. Cancer Modeling Meets Human Organoid Technology. Science 2019, 364, 952–955. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Hu, W.; Matulay, J.T.; Silva, M.V.; Owczarek, T.B.; Kim, K.; Chua, C.W.; Barlow, L.J.; Kandoth, C.; Williams, A.B.; et al. Tumor Evolution and Drug Response in Patient-Derived Organoid Models of Bladder Cancer. Cell 2018, 173, 515–528.e17. [Google Scholar] [CrossRef] [Green Version]
- Fatehullah, A.; Tan, S.H.; Barker, N. Organoids as an in Vitro Model of Human Development and Disease. Nat. Cell Biol. 2016, 18, 246–254. [Google Scholar] [CrossRef] [Green Version]
- Yao, Y.; Xu, X.; Yang, L.; Zhu, J.; Wan, J.; Shen, L.; Xia, F.; Fu, G.; Deng, Y.; Pan, M.; et al. Patient-Derived Organoids Predict Chemoradiation Responses of Locally Advanced Rectal Cancer. Cell Stem Cell 2020, 26, 17–26.e6. [Google Scholar] [CrossRef]
- Drost, J.; Clevers, H. Organoids in Cancer Research. Nat. Rev. Cancer 2018, 18, 407–418. [Google Scholar] [CrossRef]
- Kozlowski, M.T.; Crook, C.J.; Ku, H.T. Towards Organoid Culture without Matrigel. Commun. Biol. 2021, 4, 1387. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, H.; Ding, Q.; Xing, Y.; Xu, Z.; Lu, C.; Luo, D.; Xu, L.; Xia, W.; Zhou, C.; et al. Establishment of Patient-Derived Tumor Spheroids for Non-Small Cell Lung Cancer. PLoS ONE 2018, 13, e0194016. [Google Scholar] [CrossRef]
- Aboulkheyr Es, H.; Montazeri, L.; Aref, A.R.; Vosough, M.; Baharvand, H. Personalized Cancer Medicine: An Organoid Approach. Trends Biotechnol. 2018, 36, 358–371. [Google Scholar] [CrossRef]
- Han, S.; Kim, S.; Chen, Z.; Shin, H.K.; Lee, S.-Y.; Moon, H.E.; Paek, S.H.; Park, S. 3D Bioprinted Vascularized Tumour for Drug Testing. Int. J. Mol. Sci. 2020, 21, 2993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, C.; Gao, D.; Yuan, T.; Chen, Y.; Liu, L.; Chen, X.; Jiang, Y. Microfluidic Three-Dimensional Biomimetic Tumor Model for Studying Breast Cancer Cell Migration and Invasion in the Presence of Interstitial Flow. Chin. Chem. Lett. 2019, 30, 1038–1042. [Google Scholar] [CrossRef]
- Li, X.; Valadez, A.V.; Zuo, P.; Nie, Z. Microfluidic 3D Cell Culture: Potential Application for Tissue-Based Bioassays. Bioanalysis 2012, 4, 1509–1525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Duinen, V.; Trietsch, S.J.; Joore, J.; Vulto, P.; Hankemeier, T. Microfluidic 3D Cell Culture: From Tools to Tissue Models. Curr. Opin. Biotechnol. 2015, 35, 118–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schaaf, M.B.; Garg, A.D.; Agostinis, P. Defining the Role of the Tumor Vasculature in Antitumor Immunity and Immunotherapy. Cell Death Dis. 2018, 9, 115. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seitlinger, J.; Nounsi, A.; Idoux-Gillet, Y.; Santos Pujol, E.; Lê, H.; Grandgirard, E.; Olland, A.; Lindner, V.; Zaupa, C.; Balloul, J.-M.; et al. Vascularization of Patient-Derived Tumoroid from Non-Small-Cell Lung Cancer and Its Microenvironment. Biomedicines 2022, 10, 1103. https://doi.org/10.3390/biomedicines10051103
Seitlinger J, Nounsi A, Idoux-Gillet Y, Santos Pujol E, Lê H, Grandgirard E, Olland A, Lindner V, Zaupa C, Balloul J-M, et al. Vascularization of Patient-Derived Tumoroid from Non-Small-Cell Lung Cancer and Its Microenvironment. Biomedicines. 2022; 10(5):1103. https://doi.org/10.3390/biomedicines10051103
Chicago/Turabian StyleSeitlinger, Joseph, Anasse Nounsi, Ysia Idoux-Gillet, Eloy Santos Pujol, Hélène Lê, Erwan Grandgirard, Anne Olland, Véronique Lindner, Cécile Zaupa, Jean-Marc Balloul, and et al. 2022. "Vascularization of Patient-Derived Tumoroid from Non-Small-Cell Lung Cancer and Its Microenvironment" Biomedicines 10, no. 5: 1103. https://doi.org/10.3390/biomedicines10051103
APA StyleSeitlinger, J., Nounsi, A., Idoux-Gillet, Y., Santos Pujol, E., Lê, H., Grandgirard, E., Olland, A., Lindner, V., Zaupa, C., Balloul, J.-M., Quemeneur, E., Massard, G., Falcoz, P.-E., Hua, G., & Benkirane-Jessel, N. (2022). Vascularization of Patient-Derived Tumoroid from Non-Small-Cell Lung Cancer and Its Microenvironment. Biomedicines, 10(5), 1103. https://doi.org/10.3390/biomedicines10051103






