Remodeling of the Dermal Extracellular Matrix in a Tissue-Engineered Psoriatic Skin Model by n-3 Polyunsaturated Fatty Acids
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture and Production of Tissue-Engineered Skin Substitutes
2.2. Histological Analysis
2.3. Immunofluorescence
2.4. Profiling Gene Expression
2.5. Protein Extract Preparation
2.6. Dot Blots
2.7. LC-MS/MS
2.8. Statistics
3. Results
3.1. Characterization of the Skin Substitute Morphology
3.2. Dual Effects of ALA Treatment on Lipid Mediator Levels in Psoriatic Skin Substitute Dermis
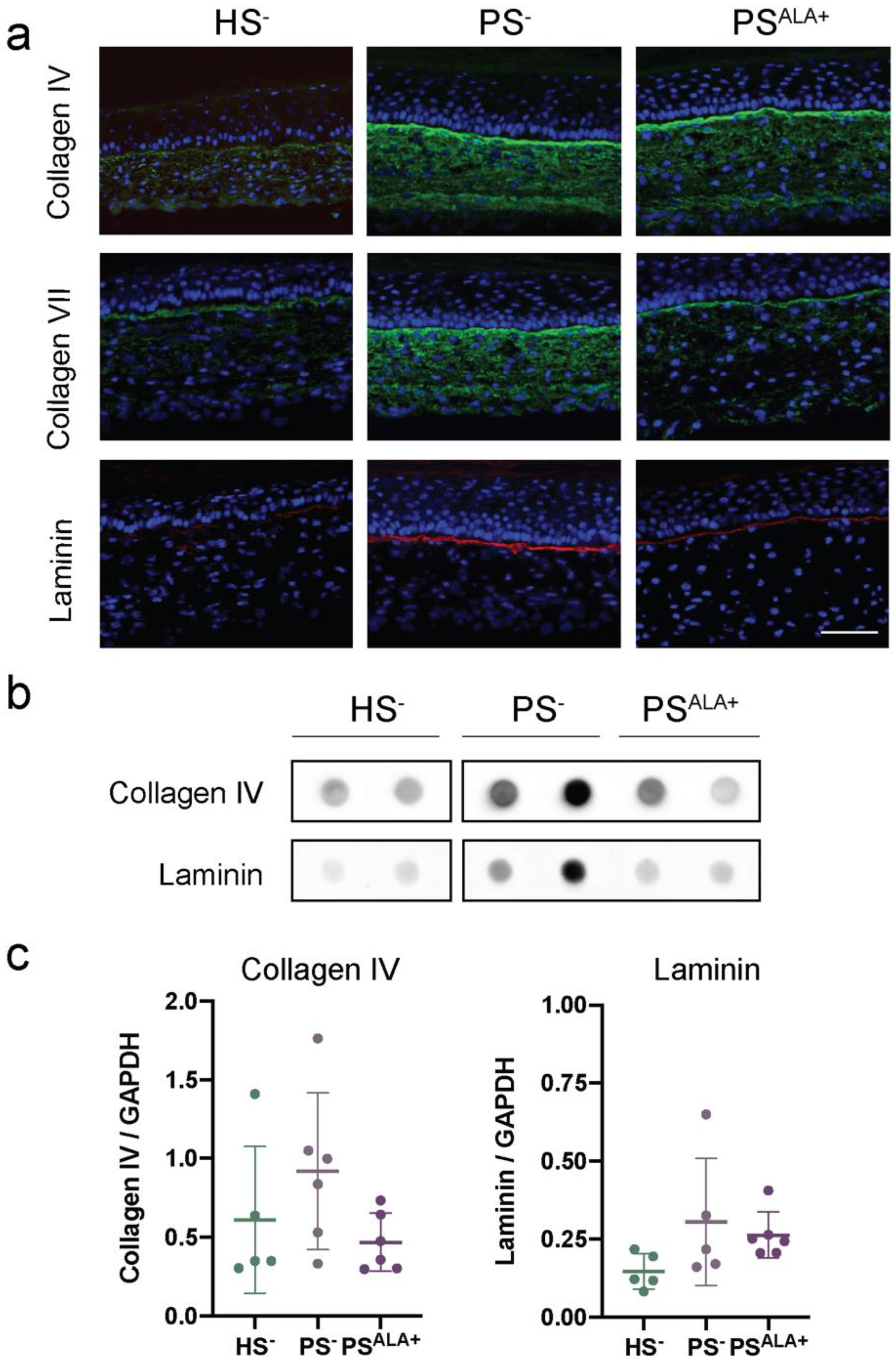
3.3. Expression of the Extracellular Matrix Proteins in Healthy and Psoriatic Skin Substitutes
3.4. Impact of ALA Treatment on the Expression of Extracellular Matrix Proteins in the Skin Substitutes
3.5. Impact of ALA Supplementation on the Expression of the Dermo-Epidermal Junction Proteins in the Skin Substitutes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lowes, M.A.; Bowcock, A.M.; Krueger, J.G. Pathogenesis and therapy of psoriasis. Nature 2007, 445, 866–873. [Google Scholar] [CrossRef] [PubMed]
- Schon, M.P.; Boehncke, W.H. Psoriasis. N. Engl. J. Med. 2005, 352, 1899–1912. [Google Scholar] [CrossRef] [PubMed]
- Krueger, J.G.; Bowcock, A. Psoriasis pathophysiology: Current concepts of pathogenesis. Ann. Rheum. Dis. 2005, 64 (Suppl 2), ii30–ii36. [Google Scholar] [CrossRef] [PubMed]
- Young, M.; Aldredge, L.; Parker, P. Psoriasis for the primary care practitioner. J. Am. Assoc. Nurse Pract. 2017, 29, 157–178. [Google Scholar] [CrossRef]
- Garcia-Perez, M.E.; Jean, J.; Pouliot, R. Antipsoriatic drug development: Challenges and new emerging therapies. Recent Pat. Inflamm. Allergy Drug Discov. 2012, 6, 3–21. [Google Scholar] [CrossRef]
- Fluhr, J.W.; Cavallotti, C.; Berardesca, E. Emollients, moisturizers, and keratolytic agents in psoriasis. Clin. Dermatol. 2008, 26, 380–386. [Google Scholar] [CrossRef]
- Dattola, A.; Silvestri, M.; Bennardo, L.; Passante, M.; Rizzuto, F.; Dastoli, S.; Patruno, C.; Bianchi, L.; Nisticò, S.P. A novel vehicle for the treatment of psoriasis. Br. J. Dermatol. 2019, 33, e13185. [Google Scholar] [CrossRef] [Green Version]
- Reid, C.; Griffiths, C.E.M. Psoriasis and Treatment: Past, Present and Future Aspects. Acta Derm.-Venereol. 2020, 100, adv00032. [Google Scholar] [CrossRef] [Green Version]
- Armstrong, A.W.; Read, C. Pathophysiology, Clinical Presentation, and Treatment of Psoriasis: A Review. JAMA 2020, 323, 1945–1960. [Google Scholar] [CrossRef]
- Greb, J.E.; Goldminz, A.M.; Elder, J.T.; Lebwohl, M.G.; Gladman, D.D.; Wu, J.J.; Mehta, N.N.; Finlay, A.Y.; Gottlieb, A.B. Psoriasis. Nat. Rev. Dis. Primers 2016, 2, 16082. [Google Scholar] [CrossRef]
- Ziboh, V.A.; Chapkin, R.S. Metabolism and function of skin lipids. Prog. Lipid Res. 1988, 27, 81–105. [Google Scholar] [CrossRef]
- Brown, T.; Krishnamurthy, K. Histology, Dermis; StatPearls, 2018. Available online: https://pubmed.ncbi.nlm.nih.gov/30570967/ (accessed on 18 August 2021).
- Haydont, V.; Bernard, B.A.; Fortunel, N.O. Age-related evolutions of the dermis: Clinical signs, fibroblast and extracellular matrix dynamics. Mech. Ageing Dev. 2019, 177, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Poschl, E.; Schlotzer-Schrehardt, U.; Brachvogel, B.; Saito, K.; Ninomiya, Y.; Mayer, U. Collagen IV is essential for basement membrane stability but dispensable for initiation of its assembly during early development. Development 2004, 131, 1619–1628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abreu-Velez, A.M.; Howard, M.S. Collagen IV in Normal Skin and in Pathological Processes. N. Am. J. Med. Sci. 2012, 4, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Breitkreutz, D.; Koxholt, I.; Thiemann, K.; Nischt, R. Skin basement membrane: The foundation of epidermal integrity--BM functions and diverse roles of bridging molecules nidogen and perlecan. BioMed Res. Int. 2013, 2013, 179784. [Google Scholar] [CrossRef] [Green Version]
- Chowdhari, S.; Sardana, K.; Saini, N. miR-4516, a microRNA downregulated in psoriasis inhibits keratinocyte motility by targeting fibronectin/integrin alpha9 signaling. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 3142–3152. [Google Scholar] [CrossRef]
- Fyrand, O. Studies on fibronectin in the skin. II. Indirect immunofluorescence studies in psoriasis vulgaris. Arch. Dermatol. Res. 1979, 266, 33–41. [Google Scholar] [CrossRef]
- Gal, B.; Dulic, S.; Kiss, M.; Groma, G.; Kovacs, L.; Kemeny, L.; Bata-Csorgo, Z. Increased circulating anti-alpha6-integrin autoantibodies in psoriasis and psoriatic arthritis but not in rheumatoid arthritis. J. Dermatol. 2017, 44, 370–374. [Google Scholar] [CrossRef] [Green Version]
- Vaccaro, M.; Magaudda, L.; Cutroneo, G.; Trimarchi, F.; Barbuzza, O.; Guarneri, F.; Guarneri, B. Changes in the distribution of laminin alpha1 chain in psoriatic skin: Immunohistochemical study using confocal laser scanning microscopy. Br. J. Dermatol. 2002, 146, 392–398. [Google Scholar] [CrossRef]
- Ho, T.C.; Yeh, S.I.; Chen, S.L.; Tsao, Y.P. The Psoriasis Therapeutic Potential of a Novel Short Laminin Peptide C16. Int. J. Mol. Sci. 2019, 20, 3144. [Google Scholar] [CrossRef] [Green Version]
- Natsumi, A.; Sugawara, K.; Yasumizu, M.; Mizukami, Y.; Sano, S.; Morita, A.; Paus, R.; Tsuruta, D. Re-investigating the Basement Membrane Zone of Psoriatic Epidermal Lesions: Is Laminin-511 a New Player in Psoriasis Pathogenesis? J. Histochem. Cytochem. 2018, 66, 847–862. [Google Scholar] [CrossRef] [PubMed]
- Roy, B.; Simard, M.; Lorthois, I.; Bélanger, A.; Maheux, M.; Duque-Fernandez, A.; Rioux, G.; Simard, P.; Deslauriers, M.; Masson, L.-C.; et al. In vitro models of psoriasis. In Skin Tissue Models for Regenerative Medicine; Elsevier: Amsterdam, The Netherlands, 2018; pp. 103–128. [Google Scholar]
- Desmet, E.; Ramadhas, A.; Lambert, J.; Van Gele, M. In vitro psoriasis models with focus on reconstructed skin models as promising tools in psoriasis research. Exp. Biol. Med. 2017, 242, 1158–1169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niehues, H.; van den Bogaard, E.H. Past, present and future of in vitro 3D reconstructed inflammatory skin models to study psoriasis. Exp. Dermatol. 2018, 27, 512–519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rioux, G.; Pouliot-Berube, C.; Simard, M.; Benhassine, M.; Soucy, J.; Guerin, S.L.; Pouliot, R. The Tissue-Engineered Human Psoriatic Skin Substitute: A Valuable In Vitro Model to Identify Genes with Altered Expression in Lesional Psoriasis. Int. J. Mol. Sci. 2018, 19, 2923. [Google Scholar] [CrossRef] [Green Version]
- Jean, J.; Lapointe, M.; Soucy, J.; Pouliot, R. Development of an in vitro psoriatic skin model by tissue engineering. J. Dermatol. Sci. 2009, 53, 19–25. [Google Scholar] [CrossRef]
- Simard, M.; Rioux, G.; Morin, S.; Martin, C.; Guerin, S.L.; Flamand, N.; Julien, P.; Fradette, J.; Pouliot, R. Investigation of Omega-3 Polyunsaturated Fatty Acid Biological Activity in a Tissue-Engineered Skin Model Involving Psoriatic Cells. J. Investig. Dermatol. 2021, 141, 2391–2401.e13. [Google Scholar] [CrossRef]
- Germain, L.; Rouabhia, M.; Guignard, R.; Carrier, L.; Bouvard, V.; Auger, F.A. Improvement of human keratinocyte isolation and culture using thermolysin. Burns 1993, 19, 99–104. [Google Scholar] [CrossRef]
- Simard, M.; Julien, P.; Fradette, J.; Pouliot, R. Modulation of the Lipid Profile of Reconstructed Skin Substitutes after Essential Fatty Acid Supplementation Affects Testosterone Permeability. Cells 2019, 8, 1142. [Google Scholar] [CrossRef] [Green Version]
- Duque-Fernandez, A.; Gauthier, L.; Simard, M.; Jean, J.; Gendreau, I.; Morin, A.; Soucy, J.; Auger, M.; Pouliot, R. A 3D-psoriatic skin model for dermatological testing: The impact of culture conditions. Biochem. Biophys. Rep. 2016, 8, 268–276. [Google Scholar] [CrossRef] [Green Version]
- Ouellette, M.E.; Berube, J.C.; Bourget, J.M.; Vallee, M.; Bosse, Y.; Fradette, J. Linoleic acid supplementation of cell culture media influences the phospholipid and lipid profiles of human reconstructed adipose tissue. PLoS ONE 2019, 14, e0224228. [Google Scholar] [CrossRef] [Green Version]
- Simard, M.; Tremblay, A.; Morin, S.; Martin, C.; Julien, P.; Fradette, J.; Flamand, N.; Pouliot, R. alpha-Linolenic acid and linoleic acid modulate the lipidome and the skin barrier of a tissue-engineered skin model. Acta Biomater. 2021, 140, 261–274. [Google Scholar] [CrossRef] [PubMed]
- Manca, C.; Boubertakh, B.; Leblanc, N.; Deschenes, T.; Lacroix, S.; Martin, C.; Houde, A.; Veilleux, A.; Flamand, N.; Muccioli, G.G.; et al. Germ-free mice exhibit profound gut microbiota-dependent alterations of intestinal endocannabinoidome signaling. J. Lipid Res. 2020, 61, 70–85. [Google Scholar] [CrossRef]
- Everard, A.; Plovier, H.; Rastelli, M.; Van Hul, M.; de Wouters d’Oplinter, A.; Geurts, L.; Druart, C.; Robine, S.; Delzenne, N.M.; Muccioli, G.G.; et al. Intestinal epithelial N-acylphosphatidylethanolamine phospholipase D links dietary fat to metabolic adaptations in obesity and steatosis. Nat. Commun. 2019, 10, 457. [Google Scholar] [CrossRef] [PubMed]
- Brizzi, M.F.; Tarone, G.; Defilippi, P. Extracellular matrix, integrins, and growth factors as tailors of the stem cell niche. Curr. Opin. Cell Biol. 2012, 24, 645–651. [Google Scholar] [CrossRef]
- Morgner, J.; Ghatak, S.; Jakobi, T.; Dieterich, C.; Aumailley, M.; Wickstrom, S.A. Integrin-linked kinase regulates the niche of quiescent epidermal stem cells. Nat. Commun. 2015, 6, 8198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, P.; Cescon, M.; Bonaldo, P. Lack of Collagen VI Promotes Wound-Induced Hair Growth. J. Investig. Dermatol. 2015, 135, 2358–2367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morin, S.; Simard, M.; Flamand, N.; Pouliot, R. Biological action of docosahexaenoic acid in a 3D tissue-engineered psoriatic skin model: Focus on the PPAR signaling pathway. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2021, 1866, 159032. [Google Scholar] [CrossRef]
- Tremblay, A.; Simard, M.; Morin, S.; Pouliot, R. Docosahexaenoic Acid Modulates Paracellular Absorption of Testosterone and Claudin-1 Expression in a Tissue-Engineered Skin Model. Int. J. Mol. Sci. 2021, 22, 13091. [Google Scholar] [CrossRef]
- Henno, A.; Blacher, S.; Lambert, C.; Colige, A.; Seidel, L.; Noel, A.; Lapiere, C.; de la Brassinne, M.; Nusgens, B.V. Altered expression of angiogenesis and lymphangiogenesis markers in the uninvolved skin of plaque-type psoriasis. Br. J. Dermatol. 2009, 160, 581–590. [Google Scholar] [CrossRef]
- Wagner, M.; Theodoro, T.R.; Filho, C.; Oyafuso, L.K.M.; Pinhal, M.A.S. Extracellular matrix alterations in the skin of patients affected by psoriasis. BMC Mol. Cell Biol. 2021, 22, 55. [Google Scholar] [CrossRef]
- Fleischmajer, R.; Kuroda, K.; Hazan, R.; Gordon, R.E.; Lebwohl, M.G.; Sapadin, A.N.; Unda, F.; Iehara, N.; Yamada, Y. Basement membrane alterations in psoriasis are accompanied by epidermal overexpression of MMP-2 and its inhibitor TIMP-2. J. Investig. Dermatol. 2000, 115, 771–777. [Google Scholar] [CrossRef] [Green Version]
- Swindell, W.R.; Remmer, H.A.; Sarkar, M.K.; Xing, X.; Barnes, D.H.; Wolterink, L.; Voorhees, J.J.; Nair, R.P.; Johnston, A.; Elder, J.T.; et al. Proteogenomic analysis of psoriasis reveals discordant and concordant changes in mRNA and protein abundance. Genome Med. 2015, 7, 86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rioux, G.; Ridha, Z.; Simard, M.; Turgeon, F.; Guerin, S.L.; Pouliot, R. Transcriptome Profiling Analyses in Psoriasis: A Dynamic Contribution of Keratinocytes to the Pathogenesis. Genes 2020, 11, 1155. [Google Scholar] [CrossRef] [PubMed]
- Koivukangas, V.; Kallionen, M.; Karvonen, J.; Autio-Harmainen, H.; Risteli, J.; Risteli, L.; Oikarinen, A. Increased collagen synthesis in psoriasis in vivo. Arch. Dermatol. Res. 1995, 287, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, N.R.; Dowlatshahi Pour, M.; Vandikas, M.S.; Neittaanmaki, N.; Osmancevic, A.; Malmberg, P. Investigation of psoriasis skin tissue by label-free multi-modal imaging: A case study on a phototherapy-treated patient. Psoriasis 2019, 9, 43–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robins, S.P.; Milne, G.; Duncan, A.; Davies, C.; Butt, R.; Greiling, D.; James, I.T. Increased skin collagen extractability and proportions of collagen type III are not normalized after 6 months healing of human excisional wounds. J. Investig. Dermatol. 2003, 121, 267–272. [Google Scholar] [CrossRef] [Green Version]
- Hance, A.J.; Crystal, R.G. Rigid control of synthesis of collagen types I and III by cells in culture. Nature 1977, 268, 152–154. [Google Scholar] [CrossRef]
- Cheng, W.; Yan-hua, R.; Fang-gang, N.; Guo-an, Z. The content and ratio of type I and III collagen in skin differ with age and injury. Afr. J. Biotechnol. 2011, 10, 2524–2529. [Google Scholar] [CrossRef]
- Pauschinger, M.; Knopf, D.; Petschauer, S.; Doerner, A.; Poller, W.; Schwimmbeck, P.L.; Kuhl, U.; Schultheiss, H.P. Dilated cardiomyopathy is associated with significant changes in collagen type I/III ratio. Circulation 1999, 99, 2750–2756. [Google Scholar] [CrossRef] [Green Version]
- McFadden, J.P.; Kimber, I. A Review on the Potential Role of Basement Membrane Laminin in the Pathogenesis of Psoriasis. Scand. J. Immunol. 2016, 83, 3–9. [Google Scholar] [CrossRef]
- Guban, B.; Vas, K.; Balog, Z.; Manczinger, M.; Bebes, A.; Groma, G.; Szell, M.; Kemeny, L.; Bata-Csorgo, Z. Abnormal regulation of fibronectin production by fibroblasts in psoriasis. Br. J. Dermatol. 2016, 174, 533–541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pellegrini, G.; De Luca, M.; Orecchia, G.; Balzac, F.; Cremona, O.; Savoia, P.; Cancedda, R.; Marchisio, P.C. Expression, topography, and function of integrin receptors are severely altered in keratinocytes from involved and uninvolved psoriatic skin. J. Clin. Investig. 1992, 89, 1783–1795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toti, P.; Pellegrino, M.; Villanova, M.; Flori, M.L.; Miracco, C.; Bartolommei, S.; Andreassi, L. Altered expression of the alpha2 laminin chain in psoriatic skin: The effect of treatment with cyclosporin. Br. J. Dermatol. 1998, 139, 375–379. [Google Scholar] [CrossRef]
- Chen, W.Y.; Lin, S.Y.; Pan, H.C.; Liao, S.L.; Chuang, Y.H.; Yen, Y.J.; Lin, S.Y.; Chen, C.J. Beneficial effect of docosahexaenoic acid on cholestatic liver injury in rats. J. Nutr. Biochem. 2012, 23, 252–264. [Google Scholar] [CrossRef] [PubMed]
- Laurent, G.; Moe, G.; Hu, X.; Holub, B.; Leong-Poi, H.; Trogadis, J.; Connelly, K.; Courtman, D.; Strauss, B.H.; Dorian, P. Long chain n-3 polyunsaturated fatty acids reduce atrial vulnerability in a novel canine pacing model. Cardiovasc. Res. 2008, 77, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Huber, J.; Löffler, M.; Bilban, M.; Reimers, M.; Kadl, A.; Todoric, J.; Zeyda, M.; Geyeregger, R.; Schreiner, M.; Weichhart, T.; et al. Prevention of high-fat diet-induced adipose tissue remodeling in obese diabetic mice by n-3 polyunsaturated fatty acids. Int. J. Obes. 2006, 31, 1004–1013. [Google Scholar] [CrossRef] [Green Version]
- Qu, X.; Zhang, X.; Yao, J.; Song, J.; Nikolic-Paterson, D.J.; Li, J. Resolvins E1 and D1 inhibit interstitial fibrosis in the obstructed kidney via inhibition of local fibroblast proliferation. J. Pathol. 2012, 228, 506–519. [Google Scholar] [CrossRef]
- Tourtas, T.; Birke, M.T.; Kruse, F.E.; Welge-Lussen, U.C.; Birke, K. Preventive effects of omega-3 and omega-6 Fatty acids on peroxide mediated oxidative stress responses in primary human trabecular meshwork cells. PLoS ONE 2012, 7, e31340. [Google Scholar] [CrossRef] [Green Version]
- Hellmann, J.; Sansbury, B.E.; Wong, B.; Li, X.; Singh, M.; Nuutila, K.; Chiang, N.; Eriksson, E.; Serhan, C.N.; Spite, M. Biosynthesis of D-Series Resolvins in Skin Provides Insights into their Role in Tissue Repair. J. Investig. Dermatol. 2018, 138, 2051–2060. [Google Scholar] [CrossRef] [Green Version]
- Candreva, T.; Kuhl, C.M.C.; Burger, B.; Dos Anjos, M.B.P.; Torsoni, M.A.; Consonni, S.R.; Crisma, A.R.; Fisk, H.L.; Calder, P.C.; de Mato, F.C.P.; et al. Docosahexaenoic acid slows inflammation resolution and impairs the quality of healed skin tissue. Clin. Sci. 2019, 133, 2345–2360. [Google Scholar] [CrossRef] [Green Version]
- Komprda, T.; Sladek, Z.; Sevcikova, Z.; Svehlova, V.; Wijacki, J.; Guran, R.; Do, T.; Lackova, Z.; Polanska, H.; Vrlikova, L.; et al. Comparison of Dietary Oils with Different Polyunsaturated Fatty Acid n-3 and n-6 Content in the Rat Model of Cutaneous Wound Healing. Int. J. Mol. Sci. 2020, 21, 7911. [Google Scholar] [CrossRef] [PubMed]
- Nickoloff, B.J.; Bonish, B.K.; Marble, D.J.; Schriedel, K.A.; DiPietro, L.A.; Gordon, K.B.; Lingen, M.W. Lessons learned from psoriatic plaques concerning mechanisms of tissue repair, remodeling, and inflammation. J. Investig. Derm. Symp. Proc. 2006, 11, 16–29. [Google Scholar] [CrossRef] [PubMed]
- Alexander, L.D.; Ding, Y.; Alagarsamy, S.; Cui, X. Angiotensin II stimulates fibronectin protein synthesis via a Gbetagamma/arachidonic acid-dependent pathway. Am. J. Physiol. Ren. Physiol. 2014, 307, F287–F302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Priante, G.; Musacchio, E.; Valvason, C.; Baggio, B. EPA and DHA suppress AngII- and arachidonic acid-induced expression of profibrotic genes in human mesangial cells. J. Nephrol. 2009, 22, 137–143. [Google Scholar] [PubMed]
- Reddy, M.A.; Thimmalapura, P.R.; Lanting, L.; Nadler, J.L.; Fatima, S.; Natarajan, R. The oxidized lipid and lipoxygenase product 12(S)-hydroxyeicosatetraenoic acid induces hypertrophy and fibronectin transcription in vascular smooth muscle cells via p38 MAPK and cAMP response element-binding protein activation. Mediation of angiotensin II effects. J. Biol. Chem. 2002, 277, 9920–9928. [Google Scholar] [CrossRef] [Green Version]
- Wen, Y. Overexpression of 12-Lipoxygenase and Cardiac Fibroblast Hypertrophy. Trends Cardiovasc. Med. 2003, 13, 129–136. [Google Scholar] [CrossRef]
- Oh, S.Y.; Lee, S.J.; Jung, Y.H.; Lee, H.J.; Han, H.J. Arachidonic acid promotes skin wound healing through induction of human MSC migration by MT3-MMP-mediated fibronectin degradation. Cell Death Dis. 2015, 6, e1750. [Google Scholar] [CrossRef] [Green Version]
- Rieger, G.M.; Hein, R.; Adelmann-Grill, B.C.; Ruzicka, T.; Krieg, T. Influence of eicosanoids on fibroblast chemotaxis and protein synthesis in vitro. J. Dermatol. Sci. 1990, 1, 347–354. [Google Scholar] [CrossRef]
- Shim, J.H. Prostaglandin E2 Induces Skin Aging via E-Prostanoid 1 in Normal Human Dermal Fibroblasts. Int. J. Mol. Sci. 2019, 20, 5555. [Google Scholar] [CrossRef] [Green Version]
- Kuivaniemi, H.; Tromp, G. Type III collagen (COL3A1): Gene and protein structure, tissue distribution, and associated diseases. Gene 2019, 707, 151–171. [Google Scholar] [CrossRef]

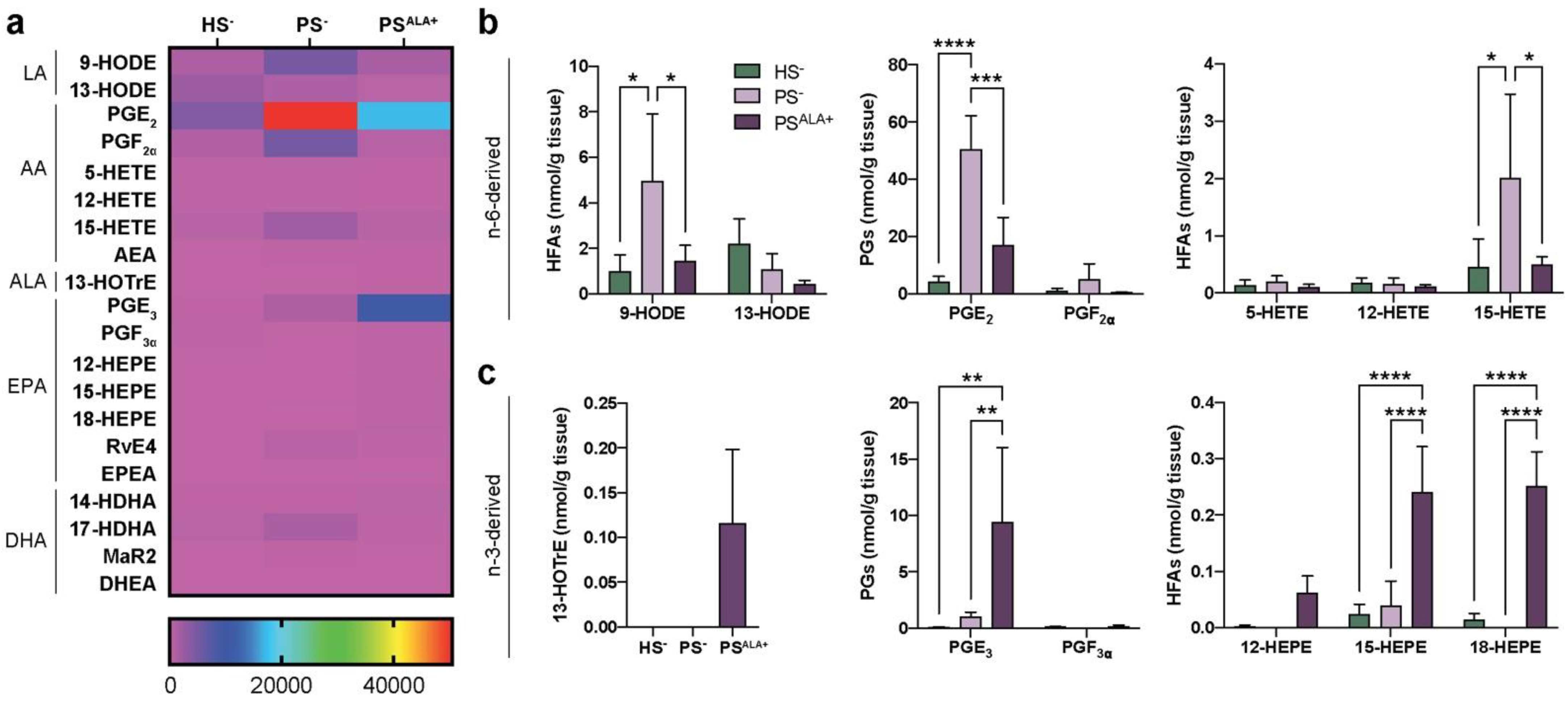

| Gene Symbol | Gene Name | Linear Signal HS− | Linear Signal PS− | Fold Change PS−/HS− |
|---|---|---|---|---|
| COL1A1 | Type I collagen, alpha-1 chain | 18,221 | 76,915 | 4.2 * |
| COL1A2 | Type I collagen, alpha-2 chain | 24,079 | 73,190 | 3.0 * |
| COL3A1 | Type III collagen, alpha-1 chain | 2759 | 12,201 | 4.4 * |
| COL4A1 | Type IV collagen, alpha-1 chain | 360 | 2229 | 2.3 * |
| COL4A2 | Type IV collagen, alpha-2 chain | 2279 | 14,503 | 6.3 * |
| COL4A5 | Type IV collagen, alpha-5 chain | 750 | 608 | 0.8 |
| COL4A6 | Type IV collagen, alpha-6 chain | 505 | 332 | 0.7 |
| COL5A1 | Type V collagen, alpha-1 chain | 1248 | 4116 | 3.3 * |
| COL5A2 | Type V collagen, alpha-2 chain | 1909 | 9909 | 5.2 * |
| COL5A3 | Type V collagen, alpha-3 chain | 100 | 460 | 4.6 * |
| COL7A1 | Type VII collagen, alpha-1 chain | 1118 | 1358 | 1.2 |
| LAMA1 | Laminin subunit alpha-1 | 105 | 118 | 1.1 |
| LAMA2 | Laminin subunit alpha-2 | 718 | 1586 | 2.2 |
| LAMA3 | Laminin subunit alpha-3 | 3650 | 1606 | 0.4 |
| LAMA4 | Laminin subunit alpha-4 | 187 | 225 | 1.2 |
| LAMA5 | Laminin subunit alpha-5 | 231 | 213 | 0.9 |
| LAMB1 | Laminin subunit beta-1 | 576 | 1357 | 2.4 |
| LAMB2 | Laminin subunit beta-2 | 2566 | 5487 | 2.1 |
| LAMB3 | Laminin subunit beta-3 | 9875 | 6013 | 0.6 |
| LAMB4 | Laminin subunit beta-4 | 119 | 120 | 1.0 |
| LAMC1 | Laminin subunit gamma-1 | 1669 | 4506 | 2.7 |
| LAMC2 | Laminin subunit gamma-2 | 1580 | 977 | 0.6 |
| LAMC3 | Laminin subunit gamma-3 | 59 | 56 | 0.9 |
| Lipid Mediators | Collagen I | Collagen III | Col I/Col III | Elastin | Fibronectin | Collagen IV | Laminin | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | r | p | r | p | r | p | r | p | |
| PGE3 | 0.384 | 0.452 | 0.410 | 0.419 | −0.259 | 0.621 | −0.322 | 0.941 | −0.149 | 0.777 | −0.540 | 0.269 | −0.149 | 0.534 |
| PGF3α | 0.803 | 0.055 | 0.908 | 0.012 | −0.360 | 0.483 | −0.244 | 0.570 | 0.009 | 0.987 | −0.279 | 0.593 | 0.009 | 0.641 |
| 13-HOTrE | 0.585 | 0.223 | 0.807 | 0.052 | −0.637 | 0.174 | −0.191 | 0.875 | −0.086 | 0.871 | −0.314 | 0.544 | −0.086 | 0.717 |
| 12-HEPE | 0.562 | 0.246 | 0.668 | 0.147 | −0.450 | 0.371 | −0.339 | 0.952 | −0.164 | 0.756 | −0.537 | 0.272 | −0.164 | 0.511 |
| 15-HEPE | 0.518 | 0.293 | 0.717 | 0.109 | −0.470 | 0.346 | −0.097 | 0.808 | −0.038 | 0.943 | −0.309 | 0.551 | −0.038 | 0.855 |
| 18-HEPE | 0.345 | 0.503 | 0.483 | 0.332 | −0.555 | 0.253 | −0.343 | 0.681 | −0.264 | 0.614 | −0.632 | 0.178 | −0.264 | 0.506 |
| 14-HDHA | 0.639 | 0.172 | 0.815 | 0.048 | −0.578 | 0.230 | −0.251 | 0.973 | −0.024 | 0.964 | −0.449 | 0.371 | −0.024 | 0.631 |
| 17-HDHA | 0.256 | 0.625 | −0.065 | 0.902 | 0.495 | 0.319 | −0.225 | 0.098 | 0.450 | 0.371 | −0.159 | 0.764 | 0.450 | 0.668 |
| RvE4 | −0.174 | 0.742 | −0.471 | 0.346 | 0.843 | 0.035 | 0.239 | 0.294 | 0.526 | 0.283 | 0.396 | 0.436 | 0.526 | 0.649 |
| MaR2 | −0.402 | 0.430 | −0.664 | 0.151 | 0.580 | 0.227 | 0.035 | 0.559 | 0.098 | 0.854 | 0.248 | 0.635 | 0.098 | 0.948 |
| PGE2 | −0.342 | 0.507 | −0.553 | 0.255 | 0.911 | 0.011 | 0.529 | 0.572 | 0.556 | 0.252 | 0.681 | 0.136 | 0.556 | 0.281 |
| PGF2α | −0.483 | 0.332 | −0.544 | 0.265 | 0.877 | 0.022 | 0.910 | 0.835 | 0.782 | 0.066 | 0.856 | 0.030 | 0.782 | 0.012 |
| 9-HODE | −0.424 | 0.402 | −0.489 | 0.325 | 0.842 | 0.035 | 0.859 | 0.974 | 0.727 | 0.101 | 0.906 | 0.013 | 0.727 | 0.028 |
| 13-HODE | −0.406 | 0.424 | −0.446 | 0.375 | 0.823 | 0.044 | 0.901 | 0.901 | 0.770 | 0.073 | 0.903 | 0.014 | 0.770 | 0.014 |
| 5-HETE | −0.351 | 0.495 | −0.400 | 0.432 | 0.757 | 0.081 | 0.826 | 0.942 | 0.807 | 0.052 | 0.795 | 0.059 | 0.807 | 0.043 |
| 12-HETE | −0.116 | 0.827 | −0.170 | 0.748 | 0.813 | 0.049 | 0.829 | 0.876 | 0.931 | 0.007 | 0.724 | 0.104 | 0.931 | 0.041 |
| 15-HETE | −0.387 | 0.448 | −0.501 | 0.312 | 0.917 | 0.010 | 0.823 | 0.925 | 0.829 | 0.041 | 0.802 | 0.055 | 0.829 | 0.044 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simard, M.; Grenier, A.; Rioux, G.; Tremblay, A.; Blais, I.; Flamand, N.; Pouliot, R. Remodeling of the Dermal Extracellular Matrix in a Tissue-Engineered Psoriatic Skin Model by n-3 Polyunsaturated Fatty Acids. Biomedicines 2022, 10, 1078. https://doi.org/10.3390/biomedicines10051078
Simard M, Grenier A, Rioux G, Tremblay A, Blais I, Flamand N, Pouliot R. Remodeling of the Dermal Extracellular Matrix in a Tissue-Engineered Psoriatic Skin Model by n-3 Polyunsaturated Fatty Acids. Biomedicines. 2022; 10(5):1078. https://doi.org/10.3390/biomedicines10051078
Chicago/Turabian StyleSimard, Mélissa, Alexe Grenier, Geneviève Rioux, Andréa Tremblay, Isalie Blais, Nicolas Flamand, and Roxane Pouliot. 2022. "Remodeling of the Dermal Extracellular Matrix in a Tissue-Engineered Psoriatic Skin Model by n-3 Polyunsaturated Fatty Acids" Biomedicines 10, no. 5: 1078. https://doi.org/10.3390/biomedicines10051078
APA StyleSimard, M., Grenier, A., Rioux, G., Tremblay, A., Blais, I., Flamand, N., & Pouliot, R. (2022). Remodeling of the Dermal Extracellular Matrix in a Tissue-Engineered Psoriatic Skin Model by n-3 Polyunsaturated Fatty Acids. Biomedicines, 10(5), 1078. https://doi.org/10.3390/biomedicines10051078










