Physical Activity and Sedentary Time in Pregnancy: An Exploratory Study on Oxidative Stress Markers in the Placenta of Women with Obesity
Abstract
:1. Introduction
2. Materials and Methods
2.1. DALI Study
2.2. Data Collection
2.3. Physical Activity and Sedentary Time
2.4. Placental Tissue Collection and RNA Isolation
2.5. Statistical Methods
3. Results
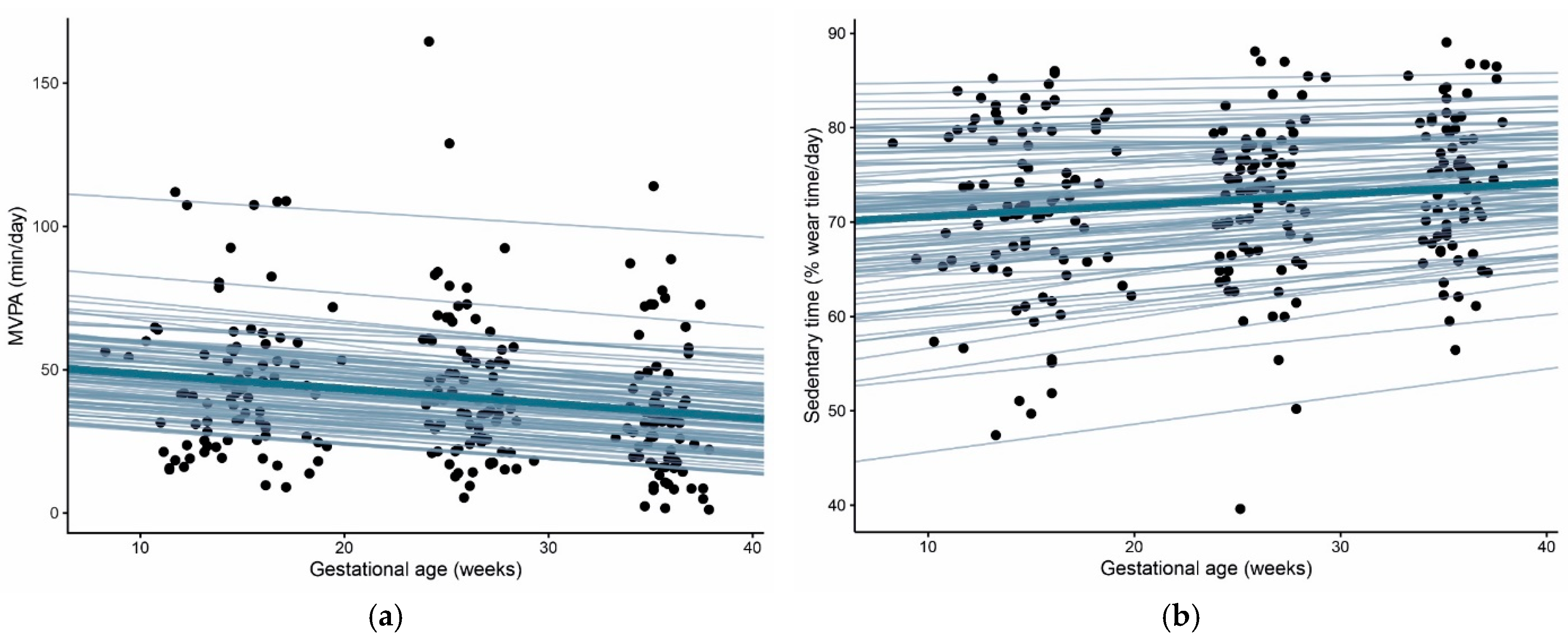
3.1. Physical Activity and Sedentary Time throughout Pregnancy
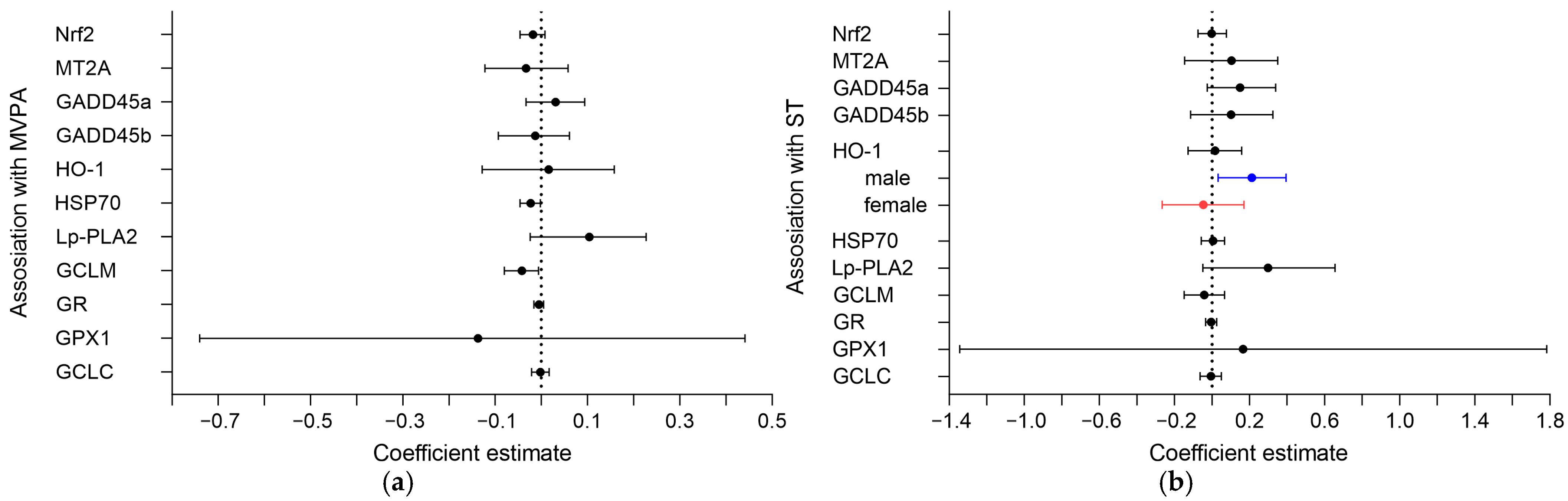
3.2. Association of MVPA and ST with Oxidative Markers in the Placenta
3.3. Sex-Specific Associations of MVPA and ST with Oxidative Markers in the Placenta
| Mean MVPA (10 min) Estimate (95% CI) | Change (Slope) MVPA (10 min/4 Weeks) Estimate (95% CI) | Mean % ST (10% Wear Time) Estimate (95% CI) | Change (Slope) % ST (10% Wear Time/4 Weeks) Estimate (95% CI) | |
|---|---|---|---|---|
| Nrf2, AU | −0.02 (−0.05, 0.01) | 0.54 (−0.87, 1.98) | −0.002 (−0.07, 0.08) | 0.97 (−0.63, 2.57) |
| MT2A, AU | −0.03 (−0.12, 0.06) | 2.33 (−2.15, 6.55) | 0.10 (−0.15, 0.35) | 2.91 (−2.16, 8.00) |
| GADD45a, AU | 0.03 (−0.03, 0.09) | −0.35 (−3.48, 2.76) | 0.15 (−0.03, 0.34) | 1.27 (−2.19, 4.83) |
| GADD45b, AU | −0.01 (−0.09, 0.06) | 0.63 (−3.14, 4.48) | 0.10 (−0.11, 0.32) | −0.67 (−4.74, 3.67) |
| HO-1, AU | 0.01 (−0.04, 0.06) | 0.78 (−1.73, 3.33) | 0.02 (−0.13, 0.16) | 2.37 (−0.53, 5.27) |
| Male (n = 50) | 0.01 (−0.05, 0.07) | 0.58 (−2.90, 3.90) | 0.21 (0.03, 0.39) | 5.84 (1.85, 9.55) |
| Female (n = 43) | 0.034 (−0.05, 0.12) | 1.48 (−2.00, 5.14) | −0.05 (−0.27, 0.17) | 3.58 (−0.78, 7.88) |
| HSP70, AU | −0.02 (−0.05, −0.002) | −0.78 (−1.85, 0.30) | 0.01 (−0.06, 0.07) | 0.88 (−0.29, 2.06) |
| Lp-PLA2, AU | 0.10 (−0.02, 0.23) | 0.58 (−6.00, 7.04) | 0.30 (−0.05, 0.66) | −2.46 (−9.91, 4.87) |
| GCLM, AU | −0.04 (−0.08, −0.01) | 0.21 (−1.62, 1.84) | −0.04 (−0.15, 0.07) | 0.64 (−1.15, 2.19) |
| GR, AU | −0.001 (−0.02, 0.01) | −0.35 (−0.93, 0.25) | −0.01 (−0.03, 0.03) | 0.24 (−0.40, 0.85) |
| GPX1, AU | −0.14 (−0.74, 0.44) | −6.37 (−31.73, 21.13) | 0.17 (−1.34, 1.78) | 3.07 (−23.83, 30.50) |
| GCLC, AU | −0.002 (−0.02, 0.02) | 0.60 (−0.26, 1.47) | −0.01 (−0.06, 0.05) | −0.56 (−1.49, 0.32) |
4. Discussion
Strengths and Weaknesses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Andreasen, K.R.; Andersen, M.L.; Schantz, A.L. Obesity and pregnancy. Acta Obstet. Gynecol. Scand. 2004, 83, 1022–1029. [Google Scholar] [CrossRef] [PubMed]
- Linne, Y. Effects of obesity on women’s reproduction and complications during pregnancy. Obes. Rev. 2004, 5, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Catalano, P.M. The impact of gestational diabetes and maternal obesity on the mother and her offspring. J. Dev. Orig. Health Dis. 2010, 1, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Kershaw, E.E.; Flier, J.S. Adipose Tissue as an Endocrine Organ. J. Clin. Endocrinol. Metab. 2004, 89, 2548–2556. [Google Scholar] [CrossRef]
- Saben, J.; Lindsey, F.; Zhong, Y.; Thakali, K.; Badger, T.; Andres, A.; Gomez-Acevedo, H.; Shankar, K. Maternal obesity is associated with a lipotoxic placental environment. Placenta 2014, 35, 171–177. [Google Scholar] [CrossRef] [Green Version]
- Myatt, L.; Maloyan, A. Obesity and Placental Function. Semin. Reprod. Med. 2016, 34, 042–049. [Google Scholar] [CrossRef] [Green Version]
- Roberts, V.; Smith, J.; McLea, S.; Heizer, A.; Richardson, J.; Myatt, L. Effect of Increasing Maternal Body Mass Index on Oxidative and Nitrative Stress in The Human Placenta. Placenta 2009, 30, 169–175. [Google Scholar] [CrossRef] [Green Version]
- Ďuračková, Z. Some Current Insights into Oxidative Stress. Physiol. Res. 2010, 59, 459–469. [Google Scholar] [CrossRef]
- Schoots, M.H.; Gordijn, S.J.; Scherjon, S.A.; van Goor, H.; Hillebrands, J.-L. Oxidative stress in placental pathology. Placenta 2018, 69, 153–161. [Google Scholar] [CrossRef]
- Wu, F.; Tian, F.-J.; Lin, Y. Oxidative Stress in Placenta: Health and Diseases. Bio. Med. Res. Int. 2015, 2015, 293271. [Google Scholar] [CrossRef] [Green Version]
- Gauster, M.; Desoye, G.; Tötsch, M.; Hiden, U. The Placenta and Gestational Diabetes Mellitus. Curr. Diabetes Rep. 2012, 12, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Bidne, K.L.; Rister, A.L.; McCain, A.R.; Hitt, B.D.; Dodds, E.D.; Wood, J.R. Maternal obesity alters placental lysophosphatidylcholines, lipid storage, and the expression of genes associated with lipid metabolism. Biol. Reprod. 2021, 104, 197–210. [Google Scholar] [CrossRef] [PubMed]
- Mele, J.; Muralimanoharan, S.; Maloyan, A.; Myatt, L. Impaired mitochondrial function in human placenta with increased maternal adiposity. Am. J. Physiol. Endocrinol. Metab. 2014, 307, E419–E425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lappas, M.; Hiden, U.; Desoye, G.; Froehlich, J.; Mouzon, S.H.-D.; Jawerbaum, A. The Role of Oxidative Stress in the Pathophysiology of Gestational Diabetes Mellitus. Antioxid. Redox Signal. 2011, 15, 3061–3100. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, P.; Cortijo, D.R.; Reyes-Hernández, C.G.; de Pablo, A.L.L.; González, M.C.; Arribas, S.M. Implication of Oxidative Stress in Fetal Programming of Cardiovascular Disease. Front. Physiol. 2018, 9, 602. [Google Scholar] [CrossRef] [Green Version]
- Van Poppel, M.N.M.; Simmons, D.; Devlieger, R.; van Assche, F.A.; Jans, G.; Galjaard, S.; Corcoy, R.; Adelantado, J.M.; Dunne, F.; Harreiter, J.; et al. A reduction in sedentary behaviour in obese women during pregnancy reduces neonatal adiposity: The DALI randomised controlled trial. Diabetology 2019, 62, 915–925. [Google Scholar] [CrossRef] [Green Version]
- Berghella, V.; Saccone, G. Exercise in pregnancy! Am. J. Obs. Gynecol. 2017, 216, 335–337. [Google Scholar] [CrossRef]
- ACOG Committee on Obstetric Practice Committee opinion #267: Exercise during pregnancy and the postpartum period. Obstet. Gynecol. 2002, 99, 171–173. [CrossRef]
- American College of Sports Medicine. ACSM Information on Pregnancy Physical Activity. Available online: https://www.acsm.org/docs/default-source/files-for-resource-library/pregnancy-physical-activity.pdf?sfvrsn=12a73853_4 (accessed on 21 February 2022).
- De Sousa, C.V.; Sales, M.M.; Rosa, T.S.; Lewis, J.E.; de Andrade, R.V.; Simões, H.G. The antioxidant effect of exercise: A systematic review and meta-analysis. Sports Med. 2017, 47, 277–293. [Google Scholar] [CrossRef]
- Jackson, M.; Gott, P.; Lye, S.; Ritchie, J.K.; Clapp, J. The effects of maternal aerobic exercise on human placental development: Placental volumetric composition and surface areas. Placenta 1995, 16, 179–191. [Google Scholar] [CrossRef]
- Bergmann, A.; Zygmunt, M.; Clapp, J. Running throughout pregnancy: Effect on placental villous vascular volume and cell proliferation. Placenta 2004, 25, 694–698. [Google Scholar] [CrossRef] [PubMed]
- Clapp, J.F. The effects of maternal exercise on fetal oxygenation and feto-placental growth. Eur. J. Obstet. Gynecol. Reprod. Biol. 2003, 110, S80–S85. [Google Scholar] [CrossRef]
- Tinius, R.A.; Cahill, A.G.; Strand, E.A.; Todd Cade, W. Maternal inflammation during late pregnancy is lower in physically active compared to inactive obese women. Appl. Physiol. Nutr. Metab. 2016, 41, 191–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramírez-Vélez, R.; Bustamante, J.; Czerniczyniec, A.; de Plata, A.C.A.; Lores-Arnaiz, S. Effect of Exercise Training on Enos Expression, NO Production and Oxygen Metabolism in Human Placenta. PLoS ONE 2013, 8, e80225. [Google Scholar] [CrossRef] [Green Version]
- Loiselle, J.; Fatica, T.; Tzaneva, V.; Vuong, N.; Holcik, M.; Adamo, K.B. Maternal physical activity significantly alters the placental transcriptome. Placenta 2020, 100, 111–121. [Google Scholar] [CrossRef]
- Acosta-Manzano, P.; Leopold-Posch, B.; Simmons, D.; Devlieger, R.; Galjaard, S.; Corcoy, R.; Adelantado, J.M.; Dunne, F.; Harreiter, J.; Kautzky-Willer, A.; et al. The unexplored role of sedentary time and physical activity in glucose and lipid metabolism-related placental m, RNAs in pregnant women who are obese: The DALI lifestyle randomised controlled trial. BJOG Int. J. Obstet. Gynaecol. 2021, 129, 708–721. [Google Scholar] [CrossRef]
- Welk, G. Physical Activity Assessments for Health-Related Research; Welk, G.J., Ed.; Human Kinetics: Champaign, IL, USA, 2002; ISBN 0736037489. [Google Scholar]
- International Association of Diabetes and Pregnancy Study Groups Consensus Panel. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 2010, 33, 676–682. [Google Scholar] [CrossRef] [Green Version]
- Freedson, P.S.; Melanson, E.; Sirard, J. Calibration of the Computer Science and Applications, Inc. accelerometer. Med. Sci. Sports Exerc. 1998, 30, 777–781. [Google Scholar] [CrossRef]
- Esliger, D.W.; Copeland, J.L.; Barnes, J.D.; Tremblay, M.S. Standardizing and Optimizing the Use of Accelerometer Data for Free-Living Physical Activity Monitoring. J. Phys. Act. Health 2005, 2, 366–383. [Google Scholar] [CrossRef]
- Ma, Q. Role of Nrf2 in Oxidative Stress and Toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef] [Green Version]
- Welten, M.; de Kroon, M.L.A.; Renders, C.M.; Steyerberg, E.W.; Raat, H.; Twisk, J.W.R.; Heymans, M.W. Repeatedly measured predictors: A comparison of methods for prediction modeling. Diagn. Progn. Res. 2018, 2, 5. [Google Scholar] [CrossRef] [PubMed]
- IBM SPSS Statistics 27 Documentation. Available online: https://www.ibm.com/support/pages/node/3006603 (accessed on 20 December 2021).
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria; Available online: https://www.R-project.org/ (accessed on 9 November 2021).
- Bürkner, P.-C. brms: An R Package for Bayesian Multilevel Models Using Stan. J. Stat. Softw. 2017, 80, 1–28. [Google Scholar] [CrossRef] [Green Version]
- Stan Development Team. Stan User’s Guide. Available online: https://mc-stan.org/docs/2_28/stan-users-guide-2_28.pdf (accessed on 9 November 2021).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; ISBN 978-3-319-24277. Available online: https://ggplot2.tidyverse.org (accessed on 21 February 2022).
- Fundu, T.M.; Kapepula, P.M.; Esimo, J.M.; Remacle, J.; Ngombe, N.K. Subcellular Localization of Glutathione Peroxidase, Change in Glutathione System during Ageing and Effects on Cardiometabolic Risks and Associated Diseases. In Glutathione System and Oxidative Stress in Health and Disease; InTechOpen: London, UK, 2019. [Google Scholar]
- Malti, N.; Merzouk, H.; Merzouk, S.; Loukidi, B.; Karaouzene, N.; Malti, A.; Narce, M. Oxidative stress and maternal obesity: Feto-placental unit interaction. Placenta 2014, 35, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.C.; Fragoso, M.B.T.; Tenório, M.C.D.S.; Martins, A.S.D.P.; Borbely, A.U.; Moura, F.A.; Goulart, M.O.F.; de Oliveira, A.C.M. Biomarkers of placental redox imbalance in pregnancies with preeclampsia and consequent perinatal outcomes. Arch. Biochem. Biophys. 2020, 691, 108464. [Google Scholar] [CrossRef] [PubMed]
- Uhlen, M.; Oksvold, P.; Fagerberg, L.; Lundberg, E.; Jonasson, K.; Forsberg, M.; Zwahlen, M.; Kampf, C.; Wester, K.; Hober, S.; et al. Towards a knowledge-based Human Protein Atlas. Nat. Biotechnol. 2010, 28, 1248–1250. [Google Scholar] [CrossRef]
- Ponomarenko, M.; Stepanenko, I.; Kolchanov, N. Heat Shock Proteins. In Brenner’s Encyclopedia of Genetics; Maloy, S., Hughes, K., Eds.; Academic Press: San Diego, CA, USA, 2013; Volume 3, pp. 402–405. ISBN 978-0-08-096156-9. [Google Scholar]
- Lau, S.S.; Griffin, T.M.; Mestril, R. Protection against endotoxemia by HSP70 in rodent cardiomyocytes. Am. J. Physiol. Circ. Physiol. 2000, 278, H1439–H1445. [Google Scholar] [CrossRef]
- Chen, H.; Wu, Y.; Zhang, Y.; Jin, L.; Luo, L.; Xue, B.; Lu, C.; Zhang, X.; Yin, Z. Hsp70 inhibits lipopolysaccharide-induced NF-κB activation by interacting with TRAF6 and inhibiting its ubiquitination. FEBS Lett. 2006, 580, 3145–3152. [Google Scholar] [CrossRef] [Green Version]
- Tosi, M.E.R.; Bocanegra, V.; Manucha, W.; Gil Lorenzo, A.; Vallés, P.G. The Nrf2–Keap1 cellular defense pathway and heat shock protein 70 (Hsp70) response. Role in protection against oxidative stress in early neonatal unilateral ureteral obstruction (UUO). Cell Stress Chaperones 2010, 16, 57–68. [Google Scholar] [CrossRef] [Green Version]
- Jee, B.; Dhar, R.; Singh, S.; Karmakar, S. Heat shock proteins and their role in pregnancy: Redefining the function of “Old Rum in a New Bottle”. Front. Cell Dev. Biol. 2021, 9, 648463. [Google Scholar] [CrossRef]
- Molvarec, A.; Rigó, J.; Nagy, B.; Walentin, S.; Szalay, J.; Füst, G.; Karádi, I.; Prohászka, Z. Serum heat shock protein 70 levels are decreased in normal human pregnancy. J. Reprod. Immunol. 2007, 74, 163–169. [Google Scholar] [CrossRef]
- Fukushima, A.; Kawahara, H.; Isurugi, C.; Syoji, T.; Oyama, R.; Sugiyama, T.; Horiuchi, S. Changes in serum levels of heat shock protein 70 in preterm delivery and pre-eclampsia. J. Obstet. Gynaecol. Res. 2005, 31, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Molvarec, A.; Rigó, J.; Lázár, L.; Balogh, K.; Makó, V.; Cervenak, L.; Mézes, M.; Prohászka, Z. Increased serum heat-shock protein 70 levels reflect systemic inflammation, oxidative stress and hepatocellular injury in preeclampsia. Cell Stress Chaperones 2008, 14, 151–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garamvölgyi, Z.; Prohászka, Z.; Rigo, J.; Kecskeméti, A.; Molvarec, A. Increased circulating heat shock protein 70 (HSPA1A) levels in gestational diabetes mellitus: A pilot study. Cell Stress Chaperones 2015, 20, 575–581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodrigues-Krause, J.; Krause, M.; O’Hagan, C.; de Vito, G.; Boreham, C.; Murphy, C.; Newsholme, P.; Colleran, G. Divergence of intracellular and extracellular HSP72 in type 2 diabetes: Does fat matter? Cell Stress Chaperones 2012, 17, 293–302. [Google Scholar] [CrossRef] [Green Version]
- Gauster, M.; Majali-Martinez, A.; Maninger, S.; Gutschi, E.; Greimel, P.H.; Ivanisevic, M.; Djelmis, J.; Desoye, G.; Hiden, U. Maternal Type 1 diabetes activates stress response in early placenta. Placenta 2017, 50, 110–116. [Google Scholar] [CrossRef]
- Araújo, J.; Ramalho, C.; Correia-Branco, A.; Faria, A.; Ferraz, T.; Keating, E.; Martel, F. A parallel increase in placental oxidative stress and antioxidant defenses occurs in pre-gestational type 1 but not gestational diabetes. Placenta 2013, 34, 1095–1098. [Google Scholar] [CrossRef]
- Barut, F.; Barut, A.; Gun, B.D.; Kandemir, N.O.; Aktunc, E.; Harma, M.; Harma, M.I.; Ozdamar, S.O. Expression of heat shock protein 70 and endothelial nitric oxide synthase in placental tissue of preeclamptic and intrauterine growth-restricted pregnancies. Pathol. Res. Pr. 2010, 206, 651–656. [Google Scholar] [CrossRef]
- Wataba, K.; Saito, T.; Takeuchi, M.; Nakayama, M.; Suehara, N.; Kudo, R. Changed expression of heat shock proteins in various pathological findings in placentas with intrauterine fetal growth restriction. Med. Mol. Morphol. 2004, 37, 170–176. [Google Scholar] [CrossRef]
- Ryter, S.W.; Alam, J.; Choi, A.M.K. Heme Oxygenase-1/Carbon Monoxide: From Basic Science to Therapeutic Applications. Physiol. Rev. 2006, 86, 583–650. [Google Scholar] [CrossRef]
- Campbell, N.K.; Fitzgerald, H.K.; Dunne, A. Regulation of inflammation by the antioxidant haem oxygenase. Nat. Rev. Immunol. 2021, 21, 411–425. [Google Scholar] [CrossRef]
- Ozen, M.; Zhao, H.; Lewis, D.B.; Wong, R.J.; Stevenson, D.K. Heme oxygenase and the immune system in normal and pathological pregnancies. Front. Pharmacol. 2015, 6, 84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilbert, J.S.; Banek, C.T.; Bauer, A.J.; Gingery, A.; Dreyer, H.C. Placental and vascular adaptations to exercise training before and during pregnancy in the rat. Am. J. Physiol. Integr. Comp. Physiol. 2012, 303, R520–R526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evans, L.; Myatt, L. Sexual dimorphism in the effect of maternal obesity on antioxidant defense mechanisms in the human placenta. Placenta 2017, 51, 64–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dieberger, A.M.; Desoye, G.; Stolz, E.; Hill, D.J.; Corcoy, R.; Simmons, D.; Harreiter, J.; Kautzky-Willer, A.; Dunne, F.; Devlieger, R.; et al. Less sedentary time is associated with a more favourable glucose-insulin axis in obese pregnant women—a secondary analysis of the DALI study. Int. J. Obes. 2021, 45, 296–307. [Google Scholar] [CrossRef]
- Sylvia, L.G.; Bernstein, E.E.; Hubbard, J.L.; Keating, L.; Anderson, E.J. Practical Guide to Measuring Physical Activity. J. Acad. Nutr. Diet. 2014, 114, 199–208. [Google Scholar] [CrossRef] [Green Version]
- Pereira, M.A.; Rifas-Shiman, S.L.; Kleinman, K.; Rich-Edwards, J.W.; Peterson, K.E.; Gillman, M.W. Predictors of Change in Physical Activity During and After Pregnancy: Project Viva. Am. J. Prev. Med. 2007, 32, 312–319. [Google Scholar] [CrossRef] [Green Version]
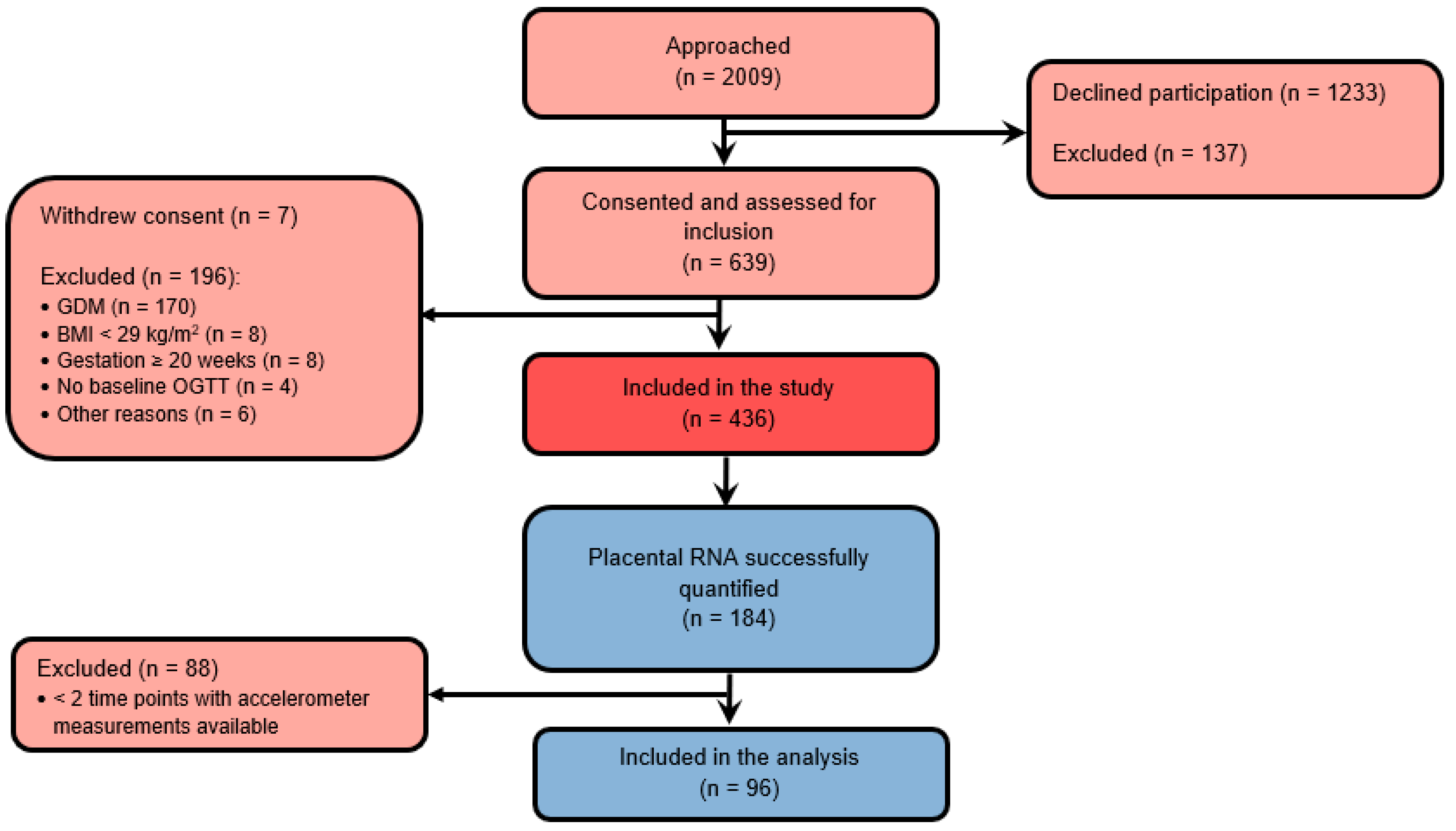
| Maternal Characteristics | n | Included | n | Excluded |
|---|---|---|---|---|
| Age, years, mean ± SD | 96 | 33.3 ± 5.3 | 339 | 31.6 ± 5.3 * |
| Pre-pregnancy BMI, kg/m2, median (IQR) | 96 | 33.7 ± 4.0 | 339 | 33.7 ± 4.0 |
| GWG at 35–37 weeks, kg, mean ± SD | 92 | 8.3 ± 5.2 | 266 | 7.6 ± 4.4 |
| Nulliparity, count (%) | 96 | 50 (52.1%) | 339 | 164 (48.4%) |
| High education, count (%) | 96 | 57 (59.4%) | 339 | 181 (53.4%) |
| European descent, count (%) | 96 | 78 (81.3%) | 339 | 299 (88.2%) |
| Smoking, count (%) | 96 | 11 (11.5%) | 317 | 44 (13.9%) |
| GDM total, count (%) | 93 | 32 (34.4%) | 268 | 101 (37.7%) |
| Neonatal characteristics | ||||
| Birthweight, g, mean ± SD | 96 | 3604.6 ± 499.8 | 297 | 3448.6 ± 554.8 * |
| Placenta weight, g, mean ± SD | 91 | 634.5 ± 147.8 | 207 | 651.0 ± 376.3 |
| Gestational age at birth, weeks, mean ± SD | 96 | 40.0 ± 1.2 | 297 | 39.4 ± 2.4 * |
| Female sex, count (%) | 96 | 44 (45.8%) | 300 | 155 (51.7%) |
| Caesarean section, count (%) | 95 | 26 (27.4%) | 288 | 101 (35.1%) |
| <20 Weeks n = 87 | 24–28 Weeks n = 83 | 35–37 Weeks n = 75 | |
|---|---|---|---|
| MVPA, min/day, median (IQR) | 41.9 (26.0–56.4) | 38.9 (25.9–55.4) * | 31.4 (18.2–43.0) ** |
| Sedentary time, % of wear time, mean ± SD | 71.4 ± 9.1 | 72.5 ± 8.2 * | 74.1 ± 7.2 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zafaranieh, S.; Dieberger, A.M.; Leopold-Posch, B.; Huppertz, B.; Granitzer, S.; Hengstschläger, M.; Gundacker, C.; Desoye, G.; van Poppel, M.N.M.; DALI Core Investigator Group. Physical Activity and Sedentary Time in Pregnancy: An Exploratory Study on Oxidative Stress Markers in the Placenta of Women with Obesity. Biomedicines 2022, 10, 1069. https://doi.org/10.3390/biomedicines10051069
Zafaranieh S, Dieberger AM, Leopold-Posch B, Huppertz B, Granitzer S, Hengstschläger M, Gundacker C, Desoye G, van Poppel MNM, DALI Core Investigator Group. Physical Activity and Sedentary Time in Pregnancy: An Exploratory Study on Oxidative Stress Markers in the Placenta of Women with Obesity. Biomedicines. 2022; 10(5):1069. https://doi.org/10.3390/biomedicines10051069
Chicago/Turabian StyleZafaranieh, Saghi, Anna M. Dieberger, Barbara Leopold-Posch, Berthold Huppertz, Sebastian Granitzer, Markus Hengstschläger, Claudia Gundacker, Gernot Desoye, Mireille N. M. van Poppel, and DALI Core Investigator Group. 2022. "Physical Activity and Sedentary Time in Pregnancy: An Exploratory Study on Oxidative Stress Markers in the Placenta of Women with Obesity" Biomedicines 10, no. 5: 1069. https://doi.org/10.3390/biomedicines10051069
APA StyleZafaranieh, S., Dieberger, A. M., Leopold-Posch, B., Huppertz, B., Granitzer, S., Hengstschläger, M., Gundacker, C., Desoye, G., van Poppel, M. N. M., & DALI Core Investigator Group. (2022). Physical Activity and Sedentary Time in Pregnancy: An Exploratory Study on Oxidative Stress Markers in the Placenta of Women with Obesity. Biomedicines, 10(5), 1069. https://doi.org/10.3390/biomedicines10051069









