Cord Blood Advanced Lipoprotein Testing Reveals an Interaction between Gestational Diabetes and Birth-Weight and Suggests a New Early Biomarker of Infant Obesity
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Subjects
2.2. Clinical and Demographic Data
2.3. Infant Growth and Child BMI
2.4. Umbilical Cord Blood Collection
2.5. Laboratory Analysis
2.6. 1H-NMR Spectroscopy-Based Cord Blood Lipoprotein Profiling
2.7. Statistical Analysis
3. Results
3.1. Clinical Characteristics and Cord Blood 1H-NMR-Based Lipoprotein Profile of the Studied Population
3.2. GDM Alters the Cord Blood Lipoprotein Profile across Birth-Weight Categories
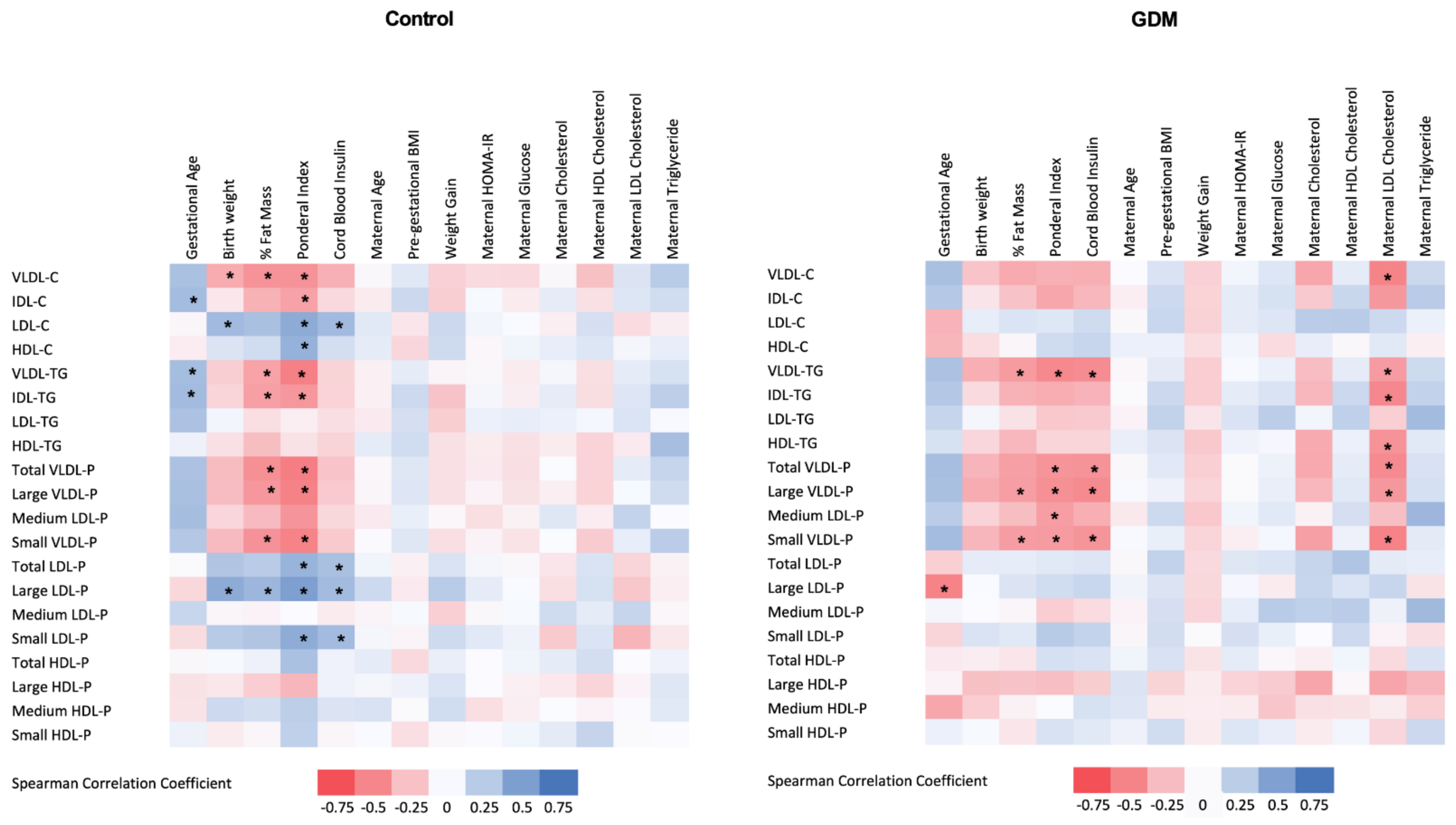
3.3. Relationship of 1H-NMR-Assessed Lipoprotein Profile with Clinical and Laboratory Parameters
3.4. Cord Blood 1H-NMR Lipoprotein Profile Is Associated with Obesity at Two Years
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barker, D.J. The Fetal and Infant Origins of Disease. Eur. J. Clin. Investig. 1995, 25, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Bao, W.; Srinivasan, S.R.; Wattigney, W.A.; Bao, W.; Berenson, G.S. Usefulness of Childhood Low-Density Lipoprotein Cholesterol Level in Predicting Adult Dyslipidemia and Other Cardiovascular Risks. Arch. Intern. Med. 1996, 156, 1315–1320. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.S. Fetal and Childhood Onset of Adult Cardiovascular Diseases. Pediatr. Clin. N. Am. 2004, 51, 1697–1719. [Google Scholar] [CrossRef] [PubMed]
- Loughrey, C.M.; Rimm, E.; Heiss, G.; Rifai, N. Race and Gender Differences in Cord Blood Lipoproteins. Atherosclerosis 2000, 148, 57–65. [Google Scholar] [CrossRef]
- Lane, D.M.; McConathy, W.J. Factors Affecting the Lipid and Apolipoprotein Levels of Cord Sera. Pediatr. Res. 1983, 17, 83–91. [Google Scholar] [CrossRef]
- Aletayeb, S.M.H.; Dehdashtian, M.; Aminzadeh, M.; Moghaddam, A.R.E.; Mortazavi, M.; Malamiri, R.A.; Habibzadeh, M.; Javaherizadeh, H. Correlation between Umbilical Cord Blood Lipid Profile and Neonatal Birth Weight. Pediatr. Pol. 2013, 88, 521–525. [Google Scholar] [CrossRef][Green Version]
- Kumar, A.; Gupta, A.; Malhotra, V.K.; Agarwal, P.S.; Thirupuram, S.; Gaind, B. Cord Blood Lipid Levels in Low Birth Weight Newborns. Indian Pediatr. 1989, 26, 571–574. [Google Scholar]
- Koklu, E.; Akcakus, M.; Kurtoglu, S.; Koklu, S.; Yikilmaz, A.; Coskun, A.; Gunes, T. Aortic Intima-Media Thickness and Lipid Profile in Macrosomic Newborns. Eur. J. Pediatr. 2007, 166, 333–338. [Google Scholar] [CrossRef]
- Miranda, J.; Simões, R.V.; Paules, C.; Cañueto, D.; Pardo-Cea, M.A.; García-Martín, M.L.; Crovetto, F.; Fuertes-Martin, R.; Domenech, M.; Gómez-Roig, M.D.; et al. Metabolic Profiling and Targeted Lipidomics Reveals a Disturbed Lipid Profile in Mothers and Fetuses with Intrauterine Growth Restriction. Sci. Rep. 2018, 8, 13614. [Google Scholar] [CrossRef]
- Kim, S.M.; Lee, S.M.; Kim, S.J.; Kim, B.J.; Shin, S.; Kim, J.R.; Cho, K.H. Cord and Maternal Sera from Small Neonates Share Dysfunctional Lipoproteins with Proatherogenic Properties: Evidence for Barker’s Hypothesis. J. Clin. Lipidol. 2017, 11, 1318–1328.e3. [Google Scholar] [CrossRef]
- Herrera, E.; Desoye, G. Maternal and Fetal Lipid Metabolism under Normal and Gestational Diabetic Conditions. Horm. Mol. Biol. Clin. Investig. 2016, 26, 109–127. [Google Scholar] [CrossRef]
- Fordyce, M.K.; Duncan, R.; Chao, R.; Christakis, M.; Hsia, S.L.; Robertson, E.; Kafatos, A.; Christakis, G. Cord Blood Serum in Newborns of Diabetic Mothers. J. Chronic Dis. 1983, 36, 263–268. [Google Scholar] [CrossRef]
- Kilby, M.D.; Neary, R.H.; Mackness, M.I.; Durrington, P.N. Fetal and Maternal Lipoprotein Metabolism in Human Pregnancy Complicated by Type I Diabetes Mellitus 1. J. Clin. Endocrinol. Metab. 1998, 83, 1736–1741. [Google Scholar] [CrossRef]
- Chan, T.C.; Schwartz, J.J.; Garcia, R.E.; Chin, H.P.; Barndt, R. Total Serum Cholesterol and Plasma Lipoprotein Cholesterol Concentrations in Cord Sera of Newborns from Hispanic Mothers with Gestational Diabetes. Artery 1988, 15, 203–216. [Google Scholar]
- Sreckovic, I.; Birner-Gruenberger, R.; Besenboeck, C.; Miljkovic, M.; Stojakovic, T.; Scharnagl, H.; Marsche, G.; Lang, U.; Kotur-Stevuljevic, J.; Jelic-Ivanovic, Z.; et al. Gestational Diabetes Mellitus Modulates Neonatal High-Density Lipoprotein Composition and Its Functional Heterogeneity. Biochim. Biophys. Acta BBA Mol. Cell Biol. Lipids 2014, 1841, 1619–1627. [Google Scholar] [CrossRef]
- Miettinen, H.E.; Rönö, K.; Koivusalo, S.B.; Eriksson, J.G.; Gylling, H. Effect of Gestational Diabetes Mellitus on Newborn Cholesterol Metabolism. Atherosclerosis 2018, 275, 346–351. [Google Scholar] [CrossRef]
- Mallol, R.; Rodriguez, M.A.; Brezmes, J.; Masana, L.; Correig, X. Human Serum/Plasma Lipoprotein Analysis by NMR: Application to the Study of Diabetic Dyslipidemia. Prog. Nucl. Magn. Reson. Spectrosc. 2013, 70, 1–24. [Google Scholar] [CrossRef]
- Mallol, R.; Amigó, N.; Rodríguez, M.A.; Heras, M.; Vinaixa, M.; Plana, N.; Rock, E.; Ribalta, J.; Yanes, O.; Masana, L.; et al. Liposcale: A Novel Advanced Lipoprotein Test Based on 2D Diffusion-Ordered 1H NMR Spectroscopy. J. Lipid Res. 2015, 56, 737–746. [Google Scholar] [CrossRef]
- Grupo Español de Diabetes y Embarazo (GEDE); Grupo Español de Diabetes y Embarazo Asistencia a la Gestante con Diabetes. Guía de práctica clínica actualizada en 2014 Avances en Diabetología. Av. Diabetol. 2015, 31, 45–59. [Google Scholar] [CrossRef]
- Alberti, K.G.; Zimmet, P.Z. Definition, Diagnosis and Classification of Diabetes Mellitus and Its Complications. Part 1: Diagnosis and Classification of Diabetes Mellitus Provisional Report of a WHO Consultation. Diabet. Med. 1998, 15, 539–553. [Google Scholar] [CrossRef]
- Catalano, P.M.; Thomas, A.J.; Avallone, D.A. Amini SB Anthropometric Estimation of Neonatal Body Composition. Am. J. Obs. Gynecol. 1995, 173, 1176–1181. [Google Scholar] [CrossRef]
- WHO Multicentre Growth Reference Study Group. WHO Child Growth Standards: Length/Height-for-Age, Weight-for-Age, Weightfor- Length, Weight-for-Height and Body Mass Index-for-Age: Methods and Development; World Health Organization: Geneva, Switzerland, 2006. [Google Scholar]
- Martinez-Perez, B.; Ejarque, M.; Gutierrez, C.; Nuñez-Roa, C.; Roche, K.; Vila-Bedmar, R.; Ballesteros, M.; Redondo-Angulo, I.; Planavila, A.; Villarroya, F.; et al. Angiopoietin-like Protein 8 (ANGPTL8) in Pregnancy: A Brown Adipose Tissue–Derived Endocrine Factor with a Potential Role in Fetal Growth. Transl. Res. 2016, 178, 12. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis Model Assessment: Insulin Resistance and Beta Cell Function from Fasting Plasma Glucose and Insulin Concentrations in Man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef]
- Pichler, G.; Amigo, N.; Tellez-Plaza, M.; Pardo-Cea, M.; Dominguez-Lucas, A.; VG, M.; Monleon, D.; Martin-Escudero, J.; Ascaso, J.; Chaves, F.; et al. LDL Particle Size and Composition and Incident Cardiovascular Disease in a South-European Population: The Hortega-Liposcale Follow-up Study. Int. J. Cardiol. 2018, 264, 172–178. [Google Scholar] [CrossRef]
- Haynes, W. Benjamini–Hochberg Method. In Encyclopedia of Systems Biology; Springer: New York, NY, USA, 2013; p. 78. [Google Scholar]
- Koklu, E.; Kurtoglu, S.; Akcakus, M.; Koklu, S.; Buyukkayhan, D.; Gumus, H.; Yikilmaz, A. Increased Aortic Intima-Media Thickness Is Related to Lipid Profile in Newborns with Intrauterine Growth Restriction. Horm. Res. Paediatr. 2006, 65, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Schaefer-Graf, U.M.; Graf, K.; Kulbacka, I.; Kjos, S.L.; Dudenhausen, J.; Vetter, K.; Herrera, E. Maternal Lipids as Strong Determinants of Fetal Environment and Growth in Pregnancies with Gestational Diabetes Mellitus. Diabetes Care 2008, 31, 1858–1863. [Google Scholar] [CrossRef] [PubMed]
- Schaefer-Graf, U.M.; Meitzner, K.; Ortega-Senovilla, H.; Graf, K.; Vetter, K.; Abou-Dakn, M.; Herrera, E. Differences in the Implications of Maternal Lipids on Fetal Metabolism and Growth between Gestational Diabetes Mellitus and Control Pregnancies. Diabet. Med. 2011, 28, 1053–1059. [Google Scholar] [CrossRef] [PubMed]
- Hassing, H.C.; Surendran, R.P.; Mooij, H.L.; Stroes, E.S.; Nieuwdorp, M.; Dallinga-Thie, G.M. Pathophysiology of Hypertriglyceridemia. Biochim. Biophys. Acta BBA Mol. Cell Biol. Lipids 2012, 1821, 826–832. [Google Scholar] [CrossRef]
- Chatterjee, C.; Sparks, D.L. Hepatic Lipase, High Density Lipoproteins, and Hypertriglyceridemia. Am. J. Pathol. 2011, 178, 1429–1433. [Google Scholar] [CrossRef]
- Gillman, M.W.; Oakey, H.; Baghurst, P.A.; Volkmer, R.E.; Robinson, J.S.; Crowther, C.A.; Gillman, M.W.; Oakey, H.; Baghurst, P.A.; Volkmer, R.E.; et al. Effect of Treatment of Gestational Diabetes Mellitus on Obesity in the next Generation. Diabetes Care 2010, 33, 964–968. [Google Scholar] [CrossRef]
- Landon, M.B.; Rice, M.M.; Varner, M.W.; Casey, B.M.; Reddy, U.M.; Wapner, R.J.; Rouse, D.J.; Biggio, J.R.; Thorp, J.M.; Chien, E.K.; et al. Mild Gestational Diabetes Mellitus and Long-Term Child Health. Diabetes Care 2015, 38, 445–452. [Google Scholar] [CrossRef]
- Ruiz-Palacios, M.L.; Ruiz-Alcaraz, A.J.; Sanchez-Campillo, M.; Larqué, E. Role of Insulin in Placental Transport of Nutrients in Gestational Diabetes Mellitus. Annu. Nutr. Metab. 2017, 70, 16–25. [Google Scholar] [CrossRef]
- Fujita, H.; Okada, T.; Inami, I.; Makimoto, M.; Hosono, S.; Minato, M.; Takahashi, S.; Mugishima, H.; Yamamoto, T. Heterogeneity of High-Density Lipoprotein in Cord Blood and Its Postnatal Change. Clin. Chim. Acta 2008, 389, 93–97. [Google Scholar] [CrossRef]
- Sreckovic, I.; Birner-Gruenberger, R.; Obrist, B.; Stojakovic, T.; Scharnagl, H.; Holzer, M.; Scholler, M.; Philipose, S.; Marsche, G.; Lang, U.; et al. Distinct Composition of Human Fetal HDL Attenuates Its Anti-Oxidative Capacity. Biochim. Biophys. Acta BBA Mol. Cell Biol. Lipids 2013, 1831, 737–746. [Google Scholar] [CrossRef]
- Gugliucci, A.; Numaguchi, M.; Caccavello, R.; Kimura, S. Small-Dense Low-Density Lipoproteins Are the Predominant ApoB-100-Containing Lipoproteins in Cord Blood. Clin. Biochem. 2014, 47, 475–477. [Google Scholar] [CrossRef]
- Shokry, E.; Marchioro, L.; Uhl, O.; Bermúdez, M.G.; García-Santos, J.A.; Segura, M.T.; Campoy, C.; Koletzko, B. Transgenerational Cycle of Obesity and Diabetes: Investigating Possible Metabolic Precursors in Cord Blood from the PREOBE Study. Acta Diabetol. 2019, 56, 1073–1082. [Google Scholar] [CrossRef]
- Simpson, J.; Smith, A.D.; Fraser, A.; Sattar, N.; Callaway, M.; Lindsay, R.S.; Lawlor, D.A.; Nelson, S.M. Cord Blood Adipokines and Lipids and Adolescent Nonalcoholic Fatty Liver Disease. J. Clin. Endocrinol. Metab. 2016, 101, 4661–4668. [Google Scholar] [CrossRef]
- Hellmuth, C.; Uhl, O.; Standl, M.; Demmelmair, H.; Heinrich, J.; Koletzko, B.; Thiering, E. Cord Blood Metabolome Is Highly Associated with Birth Weight, but Less Predictive for Later Weight Development. Obes. Facts 2017, 10, 85–100. [Google Scholar] [CrossRef]
- Stratakis, N.; Gielen, M.; Margetaki, K.; de Groot, R.H.M.; Apostolaki, M.; Chalkiadaki, G.; Vafeiadi, M.; Leventakou, V.; Karachaliou, M.; Godschalk, R.W.; et al. Polyunsaturated Fatty Acid Status at Birth, Childhood Growth, and Cardiometabolic Risk: A Pooled Analysis of the MEFAB and RHEA Cohorts. Eur. J. Clin. Nutr. 2018, 18, 175. [Google Scholar] [CrossRef]
- Standl, M.; Thiering, E.; Demmelmair, H.; Koletzko, B.; Heinrich, J. Age-Dependent Effects of Cord Blood Long-Chain PUFA Composition on BMI during the First 10 Years of Life. Br. J. Nutr. 2014, 111, 2024–2031. [Google Scholar] [CrossRef]
- Donahue, S.M.A.; Rifas-Shiman, S.L.; Gold, D.R.; Jouni, Z.E.; Gillman, M.W.; Oken, E. Prenatal Fatty Acid Status and Child Adiposity at Age 3 y: Results from a US Pregnancy Cohort. Am. J. Clin. Nutr. 2011, 93, 780–788. [Google Scholar] [CrossRef]
- Hillier, T.A.; Pedula, K.L.; Schmidt, M.M.; Mullen, J.A.; Charles, M.-A.; Pettitt, D.J. Childhood Obesity and Metabolic Imprinting. Diabetes Care 2007, 30, 2287–2292. [Google Scholar] [CrossRef]
- Voerman, E.; Santos, S.; Patro Golab, B.; Amiano, P.; Ballester, F.; Barros, H.; Bergström, A.; Charles, M.-A.; Chatzi, L.; Chevrier, C.; et al. Maternal Body Mass Index, Gestational Weight Gain, and the Risk of Overweight and Obesity across Childhood: An Individual Participant Data Meta-Analysis. PLoS Med. 2019, 16, e1002744. [Google Scholar] [CrossRef]
- Pathirana, M.M.; Lassi, Z.S.; Roberts, C.T.; Andraweera, P.H. Cardiovascular Risk Factors in Offspring Exposed to Gestational Diabetes Mellitus in Utero: Systematic Review and Meta-Analysis. J. Dev. Orig. Health Dis. 2020, 18, 850. [Google Scholar] [CrossRef]

| Maternal and Neonatal Clinical Characteristics | |||
| Control (n = 74) | GDM (n = 62) | p-Value | |
| Maternal age (years) | 32.5 ± 5.4 | 33.5 ± 4.3 | 0.257 |
| Pre-gestational BMI (kg/m2) | 25.5 ± 5.2 | 26.6 ± 5.1 | 0.207 |
| Gestational weight gain (kg) | 12.3 ± 6.2 | 8.6 ± 4.9 | <0.001 |
| Final BMI (kg/m2) | 30.1 ± 4.8 | 30.0 ± 4.7 | 0.872 |
| Smoking, n (%) | 14 (18.9) | 8 (12.9) | 0.363 |
| M cholesterol (mg/dL) * | 246 ± 40 | 236 ± 44 | 0.165 |
| M HDL cholesterol (mg/dL) * | 73 ± 13 | 72 ± 15 | 0.530 |
| M LDL cholesterol (mg/dL) * | 121 ± 54 | 124 ± 36 | 0.775 |
| M triglycerides (mg/dL) * | 205 ± 79 | 203 ± 81 | 0.898 |
| HOMA-IR * | 2.0 (1.2–3.3) | 2.9 (1.66–4.22) | 0.052 |
| Gestational age (weeks) | 39 (38–40) | 39 (38–40) | 0.749 |
| Vaginal delivery n (%) | 51 (68.9) | 50 (80.6) | 0.119 |
| Birth weight (g) | 3259 ± 603 | 3310 ± 697 | 0.645 |
| Male sex n (%) | 38 (51.4) | 32 (51.6) | 0.976 |
| SGA/AGA/LGA (n) | 25/25/24 | 14/25/23 | 0.353 |
| Fat mass (%) | 11.7 ± 4.3 | 11.9 ± 3.8 | 0.789 |
| Ponderal Index (g/cm3) | 2.7 ± 0.3 | 2.7 ± 0.3 | 0.633 |
| Cord blood insulin (mcUI/mL) | 4.5 (2.1–8.0) | 6.3 (2.9–12.1) | 0.058 |
| Cord blood 1H-NMR-assessed lipoprotein profile | |||
| Control (n = 74) | GDM (n = 62) | p-value | |
| VLDL-cholesterol (mg/dL) | 7.2 ± 3.3 | 7.6 ± 4.0 | 0.495 |
| IDL-cholesterol (mg/dL) | 4.6 ± 1.6 | 5.0 ± 2.0 | 0.291 |
| LDL-cholesterol (mg/dL) | 70.1 ± 10.1 | 70.6 ± 8.7 | 0.767 |
| HDL-cholesterol (mg/dL) | 40.9 ± 8.8 | 40.6 ± 8.1 | 0.827 |
| VLDL-triglycerides (mg/dL) | 29.4 ± 8.6 | 30.7 ± 9.9 | 0.415 |
| IDL-triglycerides (mg/dL) | 4.4 ± 1.3 | 4.7 ± 1.8 | 0.335 |
| LDL-triglycerides (mg/dL) | 4.5 ± 1.8 | 4.4 ± 1.9 | 0.925 |
| HDL-triglycerides (mg/dL) | 7.9 ± 3.7 | 8.6 ± 3.9 | 0.251 |
| VLDL-P (nmol/L) | 23.1 ± 6.3 | 24.0 ± 7.5 | 0.416 |
| Large VLDL-P (nmol/L) | 0.6 (0.5–0.8) | 0.6 (0.5–0.8) | 0.848 |
| Medium VLDL-P (nmol/L) | 1.1 (0.6–2.0) | 1.1 (0.7–2.4) | 0.471 |
| Small VLDL-P (nmol/L) | 21.0 ± 5.1 | 21.8 ± 6.3 | 0.408 |
| LDL-P (nmol/L) | 484.9 ± 71.7 | 488.0 ± 62.0 | 0.79 |
| Large LDL-P (nmol/L) | 92.5 ± 13.0 | 93.1 ± 11.0 | 0.782 |
| Medium LDL-P (nmol/L) | 61.9 ± 39.3 | 62.1 ± 36.9 | 0.973 |
| Small LDL-P (nmol/L) | 330.4 ± 50.1 | 332.7 ± 42.1 | 0.775 |
| HDL-P (nmol/L) | 17.8 ± 4.2 | 17.4 ± 4.1 | 0.591 |
| Large HDL-P (nmol/L) | 0.3 ± 0.1 | 0.3 ± 0.1 | 0.107 |
| Medium HDL-P (nmol/L) | 9.7 ± 1.5 | 9.9 ± 1.4 | 0.257 |
| Small HDL-P (nmol/L) | 7.8 ± 3.8 | 7.1 ± 3.9 | 0.306 |
| Maternal and Neonatal Clinical Characteristics | ||||
| SGA (n = 39) | AGA (n = 50) | LGA (n = 49) | p-Value | |
| Maternal age (years) | 31.6 ± 4.6 | 33.91 ± 5.0 | 32.8 ± 5.0 | 0.102 |
| Pre-gestational BMI (kg/m2) | 25.3 ± 4.2 | 25.9 ± 5.3 | 26.7 ± 5.7 | 0.445 |
| Gestational weight gain (kg) | 8.6 ± 5.0 | 10.0 ± 5.3 | 12.9 ± 6.3 b | 0.002 |
| Final BMI (kg/m2) | 28.6 ± 4.0 b | 29.7 ± 4.6 | 30.0 ± 4.8 | 0.014 |
| Smoking, n (%) | 11 (28.2) | 5 (10.6) | 6 (12) | 0.053 |
| M cholesterol (mg/dL) * | 247 ± 36 | 235 ± 46 | 244 ± 42 | 0.395 |
| M HDL cholesterol (mg/dL) * | 73 ± 10 | 76 ± 17 | 69 ± 12 | 0.053 |
| M LDL cholesterol (mg/dL) * | 130 ± 47 | 116 ± 35 | 124 ± 57 | 0.439 |
| M triglycerides (mg/dL) * | 191 ± 70 | 204 ± 79 | 209 ± 74 | 0.552 |
| HOMA-IR * | 1.7 (1.2–3.2) | 2.2 (1.4–4.5) | 2.4 (1.6–3.4) | 0.272 |
| Gestational age (weeks) | 39 (39–40) | 39 (38–40) | 39 (38–40) | 0.599 |
| Vaginal delivery n (%) | 27 (69.2) | 40 (80) | 84 (72.3) | 0.480 |
| Birth weight (g) | 2598 ± 279 a | 3268 ± 270 c | 3929 ± 251 b | <0.001 |
| Male sex n (%) | 22 (56.4) | 21 (42) | 27 (57.4) | 0.241 |
| Fat mass (%) | 6.8 ± 3.6 a | 11.6 ± 2.4 c | 12.0 ± 2.0 | <0.001 |
| Ponderal Index (g/cm3) | 2.5 ± 0.2 a | 2.8 ± 0.3 c | 2.9 ± 0.2 b | <0.001 |
| Cb insulin (mcUI(mL) | 2.5 (1.1–4.3) | 4.3 (2.2–4.7) | 8.2 (6.2–14.1) b | <0.001 |
| Cord Blood 1H-NMR-Assessed Lipoprotein Profile | ||||
| SGA (n = 39) | AGA (n = 50) | LGA (n = 49) | p-value | |
| VLDL-cholesterol (mg/dL) | 8.5 ± 3.6 b | 8.1 ± 3.5 c | 5.8 ± 3.3 | 0.001 |
| IDL-cholesterol (mg/dL) | 5.1 ± 1.4 | 4.8 ± 2.0 | 4.5 ± 1.7 | 0.353 |
| LDL-cholesterol (mg/dL) | 66.3 ± 11.3 a,b | 72.1 ± 9.5 | 72.0 ± 7.1 | 0.005 |
| HDL-cholesterol (mg/dL) | 37.3 ± 6.6 a | 44.2 ± 8.7 c | 40.1 ± 8.5 | <0.001 |
| VLDL-triglycerides (mg/dL) | 34.1 ± 10.1 a,b | 29.0 ± 8.2 | 27.5 ± 8.3 | 0.002 |
| IDL-triglycerides (mg/dL) | 4.7 ± 1.3 | 4.8 ± 1.7 | 4.1 ± 1.6 | 0.092 |
| LDL-triglycerides (mg/dL) | 4.5 ± 1.7 | 4.6 ± 2.1 | 4.3 ± 1.7 | 0.797 |
| HDL-triglycerides (mg/dL) | 7.4 ± 3.6 a | 10.1 ± 3.2 c | 7.0 ± 3.8 | <0.001 |
| VLDL-P (nmol/L) | 26.1 ± 7.1 b | 23.6 ± 6.1 | 21.2 ± 6.6 | 0.003 |
| Large VLDL-P (nmol/L) | 0.8 ± 0.3 b | 0.7 ± 0.3 | 0.6 ± 0.3 | 0.001 |
| Medium VLDL-P (nmol/L) | 2.0 (0.9–2.7) a,b | 0.7 (0.5–1.5) | 1.1 (0.6–1.5) | 0.001 |
| Small VLDL-P (nmol/L) | 23.20 ± 5.71 b | 21.68 ± 4.82 | 19.35 ± 5.84 | 0.005 |
| LDL-P (nmol/L) | 455.7 ± 79.7 b | 504.0 ± 62.6 | 493.8 ± 50.8 | 0.002 |
| Large LDL-P (nmol/L) | 86.2 ± 12.6 b | 94.1 ± 10.6 | 97.0 ± 10.9 | <0.001 |
| Medium LDL-P (nmol/L) | 67.5 ± 32.1 | 59.0 ± 44.6 | 60.6 ± 35.7 | 0.555 |
| Small LDL-P (nmol/L) | 302.1 ± 48.5 | 350.9 ± 37.4 | 336.9 ± 41.3 | <0.001 |
| HDL-P (nmol/L) | 15.8 ± 3.7 a,b | 19.9 ± 4.1 c | 16.7 ± 3.5 | <0.001 |
| Large HDL-P (nmol/L) | 0.4 ± 0.1 a | 0.3 ± 0.1 | 0.3 ± 0.1 | 0.374 |
| Medium HDL-P (nmol/L) | 9.5 ± 1.2 | 10.0 ± 1.4 | 9.9 ± 1.6 | 0.194 |
| Small HDL-P (nmol/L) | 6.0 ± 3.9 a | 9.6 ± 3.6 c | 6.5 ± 3.0 | <0.001 |
| Control | GDM | p-Values | |||||||
|---|---|---|---|---|---|---|---|---|---|
| SGA (n = 25) | AGA (n = 25) | LGA (n = 24) | SGA (n = 14) | AGA (n = 25) | LGA (n = 23) | GDM | BW | Interaction | |
| VLDL-cholesterol (mg/dL) | 9.0 ± 3.8 | 7.0 ± 2.6 | 5.5 ± 2.4 | 7.6 ± 3.1 | 9.1 ± 3.9 | 6.1 ± 3.9 | 0.474 | 0.001 | 0.06 |
| IDL-cholesterol (mg/dL) | 5.3 ± 1.5 | 4.1 ± 1.5 a | 4.6 ± 1.5 | 4.8 ± 1.6 | 5.7 ± 2.2 a | 4.4 ± 2.0 | 0.338 | 0.403 | 0.006 |
| LDL-cholesterol (mg/dL) | 66.7 ± 12.8 | 70.2 ± 8.1 | 73.6 ± 7.1 | 65.6 ± 8.8 | 74.1 ± 9.7 | 70.2 ± 5.8 | 0.907 | 0.005 | 0.135 |
| HDL-cholesterol (mg/dL) | 37.5 ± 7.2 | 44.5 ± 8.8 | 40.7 ± 9.2 | 36.9 ± 5.5 | 43.8 ± 8.8 | 39.5 ± 7.9 | 0.579 | 0.001 | 0.983 |
| VLDL-triglycerides (mg/dL) | 34.0 ± 11.1 | 26.4 ± 5.8 | 27.8 ± 5.9 | 34.3 ± 8.5 | 31.7 ± 9.6 | 27.3 ± 10.3 | 0.263 | 0.002 | 0.223 |
| IDL-triglycerides (mg/dL) | 4.9 ± 1.4 | 4.1 ± 1.2 a | 4.2 ± 1.2 | 4.3 ± 1.3 | 5.5 ± 1.8 a | 4.1 ± 1.9 | 0.440 | 0.080 | 0.005 |
| LDL-triglycerides (mg/dL) | 4.8 ± 1.8 | 3.9 ± 1.7 a | 4.6 ± 1.9 | 3.9 ± 1.3 | 5.1 ± 2.2 a | 3.9 ± 1.3 | 0.735 | 0.749 | 0.007 |
| HDL-triglycerides (mg/dL) | 7.5 ± 3.4 | 9.6 ± 3.6 | 6.5 ± 3.4 | 7.2 ± 3.9 | 10.7 ± 2.6 | 7.2 ± 3.9 | 0.351 | <0.001 | 0.651 |
| VLDL-P (nmol/L) | 26.3 ± 7.8 | 21.8 ± 4.7 | 21.1 ± 4.5 | 25.8 ± 5.9 | 25.6 ± 6.8 | 21.3 ± 8.4 | 0.307 | 0.004 | 0.244 |
| Large VLDL-P (nmol/L) | 0.8 ± 0.3 | 0.6 ± 0.3 | 0.6 ± 0.2 | 0.8 ± 0.3 | 0.8 ± 0.3 | 0.6 ± 0.3 | 0.217 | 0.001 | 0.361 |
| Medium VLDL-P (nmol/L) | 2.2 ± 1.5 | 0.9 ± 0.6 a | 1.3 ± 0.9 | 1.9 ± 1.2 | 1.7 ± 1.5 a | 1.2 ± 0.8 | 0.452 | 0.002 | 0.038 |
| Small VLDL-P (nmol/L) | 23.3 ± 6.3 | 20.3 ± 5.1 | 19.2 ± 3.8 | 23.1 ± 4.9 | 23.1 ± 5.2 | 19.5 ± 7.5 | 0.312 | 0.006 | 0.365 |
| LDL-P (nmol/L) | 461.4 ± 90.5 | 488.1 ± 57.2 | 505.9 ± 57.3 | 446.1 ± 59.3 | 520.6 ± 64.9 | 481.2 ± 40.3 | 0.823 | 0.001 | 0.070 |
| Large LDL-P (nmol/L) | 84.4 ± 12.9 | 94.2 ± 10.0 | 99.1 ± 11.6 | 89.0 ± 11.9 | 94.0 ± 11.3 | 94.8 ± 9.7 | 0.998 | <0.001 | 0.200 |
| Medium LDL-P (nmol/L) | 73.8 ± 34.2 | 48.9 ± 39.8 a | 65.2 ± 40.4 | 56.9 ± 25.8 | 71.6 ± 46.7 a | 55.7 ± 30.1 | 0.931 | 0.0740 | 0.021 |
| Small LDL-P (nmol/L) | 303.2 ± 54.4 | 347.0 ± 38.8 | 341.6 ± 45.6 | 300.2 ± 38.2 | 355.0 ± 36.4 | 330.7 ± 36.4 | 0.794 | <0.001 | 0.553 |
| HDL-P (nmol/L) | 16.6 ± 3.8 | 20.0 ± 4.2 | 16.8 ± 3.7 | 14.6 ± 3.4 | 19.8 ± 4.1 | 16.7 ± 3.4 | 0.269 | <0.001 | 0.442 |
| Large HDL-P (nmol/L) | 0.3 ± 0.1 | 0.3 ± 0.1 | 0.3 ± 0.1 | 0.4 ± 0.1 | 0.3 ± 0.1 | 0.3 ± 0.1 | 0.048 | 0.159 | 0.145 |
| Medium HDL-P (nmol/L) | 9.1 ± 1.1 | 9.9 ± 1.3 | 10.0 ± 1.8 | 10.0 ± 1.0 | 10.0 ± 1.5 | 9.8 ± 1.5 | 0.251 | 0.394 | 0.193 |
| Small HDL-P (nmol/L) | 7.1 ± 3.9 | 9.8 ± 3.6 | 6.4 ± 3.3 | 4.2 ± 3.3 | 9.5 ± 3.7 | 6.6 ± 2.8 | 0.089 | <0.001 | 0.101 |
| Model R2 | Exp (B) | 95% CI for Exp (B) | p-Value | |
|---|---|---|---|---|
| Large LDL-P * (nmol/L) | 0.234 | 1.052 | 0.998–1.109 | 0.058 |
| Small LDL-P * (nmol/L) | 0.251 | 1.018 | 1.002–1.034 | 0.023 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Algaba-Chueca, F.; Maymó-Masip, E.; Ballesteros, M.; Guarque, A.; Majali-Martínez, A.; Freixes, O.; Amigó, N.; Fernández-Veledo, S.; Vendrell, J.; Megía, A. Cord Blood Advanced Lipoprotein Testing Reveals an Interaction between Gestational Diabetes and Birth-Weight and Suggests a New Early Biomarker of Infant Obesity. Biomedicines 2022, 10, 1033. https://doi.org/10.3390/biomedicines10051033
Algaba-Chueca F, Maymó-Masip E, Ballesteros M, Guarque A, Majali-Martínez A, Freixes O, Amigó N, Fernández-Veledo S, Vendrell J, Megía A. Cord Blood Advanced Lipoprotein Testing Reveals an Interaction between Gestational Diabetes and Birth-Weight and Suggests a New Early Biomarker of Infant Obesity. Biomedicines. 2022; 10(5):1033. https://doi.org/10.3390/biomedicines10051033
Chicago/Turabian StyleAlgaba-Chueca, Francisco, Elsa Maymó-Masip, Mónica Ballesteros, Albert Guarque, Alejandro Majali-Martínez, Olga Freixes, Núria Amigó, Sonia Fernández-Veledo, Joan Vendrell, and Ana Megía. 2022. "Cord Blood Advanced Lipoprotein Testing Reveals an Interaction between Gestational Diabetes and Birth-Weight and Suggests a New Early Biomarker of Infant Obesity" Biomedicines 10, no. 5: 1033. https://doi.org/10.3390/biomedicines10051033
APA StyleAlgaba-Chueca, F., Maymó-Masip, E., Ballesteros, M., Guarque, A., Majali-Martínez, A., Freixes, O., Amigó, N., Fernández-Veledo, S., Vendrell, J., & Megía, A. (2022). Cord Blood Advanced Lipoprotein Testing Reveals an Interaction between Gestational Diabetes and Birth-Weight and Suggests a New Early Biomarker of Infant Obesity. Biomedicines, 10(5), 1033. https://doi.org/10.3390/biomedicines10051033







