Cerebrospinal Fluid Neurofilament Light Predicts Risk of Dementia Onset in Cognitively Healthy Individuals and Rate of Cognitive Decline in Mild Cognitive Impairment: A Prospective Longitudinal Study
Abstract
:1. Introduction
2. Methods
2.1. Participants
2.2. Ethics Approval
2.3. Brain Amyloid-β (Aβ) Imaging and Stratification of Cognitively Healthy Participants
2.4. Cognitive Assessments
2.5. CSF Samples
2.6. Biomarker Analyses
2.7. Statistical Analyses
3. Results
3.1. Utility of CSF NfL to Screen Preclinical AD and Predict Dementia Onset
3.1.1. Screening of Preclinical AD
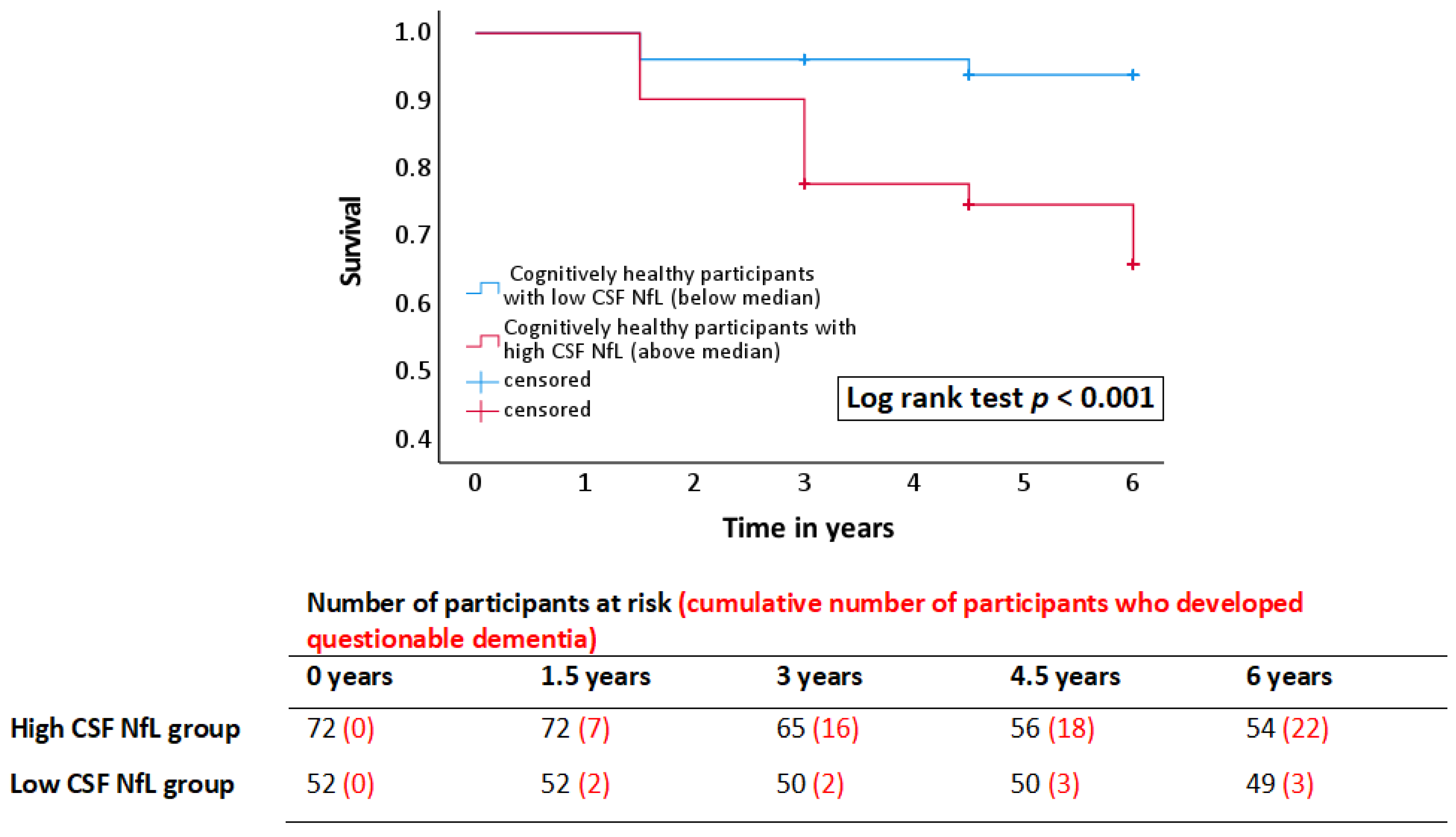
3.1.2. Prediction of Dementia Onset
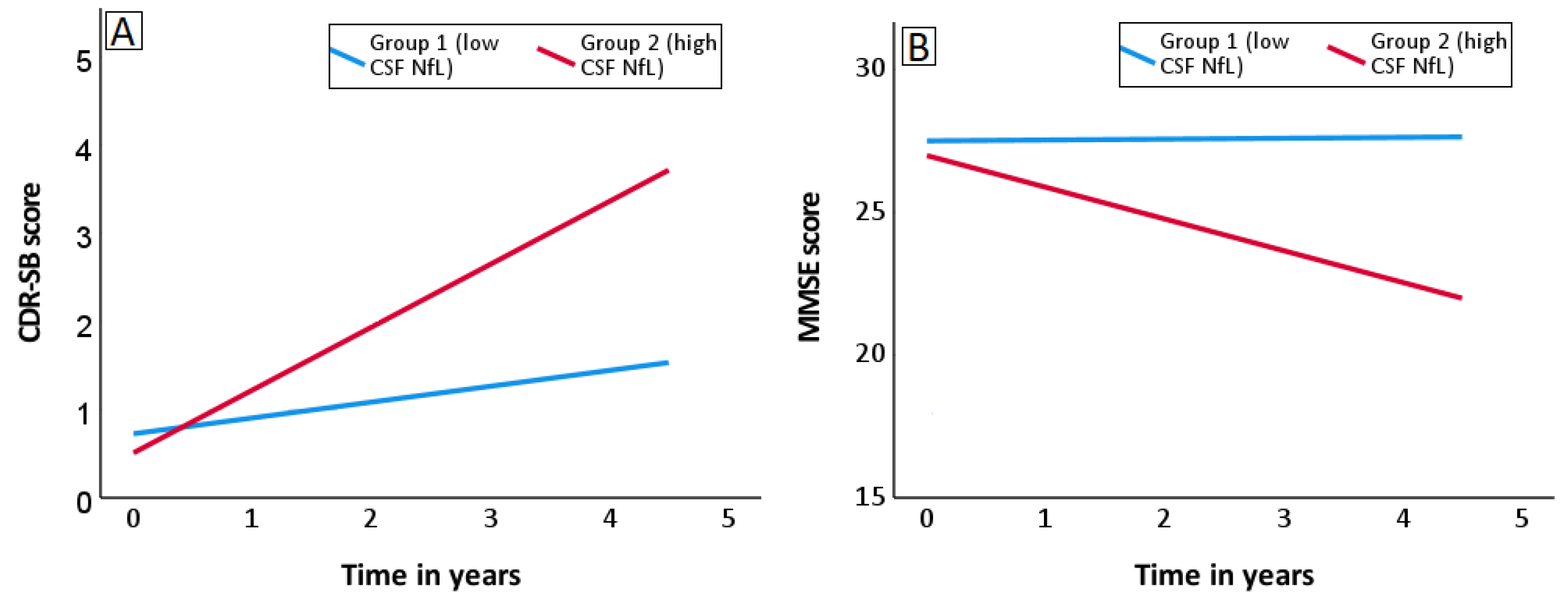
3.2. Utility of CSF NfL for Predicting Rate of Cognitive Decline in MCI
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dhiman, K.; Blennow, K.; Zetterberg, H.; Martins, R.N.; Gupta, V.B. Cerebrospinal fluid biomarkers for understanding multiple aspects of Alzheimer’s disease pathogenesis. Cell. Mol. Life Sci. 2019, 76, 1833–1863. [Google Scholar] [CrossRef] [PubMed]
- Dubois, B.; Hampel, H.; Feldman, H.H.; Scheltens, P.; Aisen, P.; Andrieu, S.; Bakardjian, H.; Benali, H.; Bertram, L.; Blennow, K. Preclinical Alzheimer’s disease: Definition, natural history, and diagnostic criteria. Alzheimers Dement. 2016, 12, 292–323. [Google Scholar] [CrossRef] [PubMed]
- Sperling, R.A.; Aisen, P.S.; Beckett, L.A.; Bennett, D.A.; Craft, S.; Fagan, A.M.; Iwatsubo, T.; Jack, C.R., Jr.; Kaye, J.; Montine, T.J.; et al. Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011, 7, 280–292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jack, C.R.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Dunn, B.; Haeberlein, S.B.; Holtzman, D.M.; Jagust, W.; Jessen, F.; Karlawish, J.; et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018, 14, 535–562. [Google Scholar] [CrossRef] [PubMed]
- Magalingam, K.B.; Radhakrishnan, A.; Ping, N.S.; Haleagrahara, N. Current concepts of neurodegenerative mechanisms in Alzheimer’s disease. BioMed Res. Int. 2018, 2018, 3740461. [Google Scholar] [CrossRef] [Green Version]
- Zetterberg, H.; Skillbäck, T.; Mattsson, N.; Trojanowski, J.Q.; Portelius, E.; Shaw, L.M.; Weiner, M.W.; Blennow, K. Association of cerebrospinal fluid neurofilament light concentration with alzheimer disease progression. JAMA Neurol. 2016, 73, 60–67. [Google Scholar] [CrossRef]
- Mattsson, N.; Insel, P.S.; Palmqvist, S.; Portelius, E.; Zetterberg, H.; Weiner, M.; Blennow, K.; Hansson, O. Cerebrospinal fluid tau, neurogranin, and neurofilament light in Alzheimer’s disease. EMBO Mol. Med. 2016, 8, 1184–1196. [Google Scholar] [CrossRef]
- Dhiman, K.; Gupta, V.B.; Villemagne, V.L.; Eratne, D.; Graham, P.L.; Fowler, C.; Bourgeat, P.; Li, Q.-X.; Collins, S.; Bush, A.I.; et al. Cerebrospinal fluid neurofilament light concentration predicts brain atrophy and cognition in Alzheimer’s disease. Alzheimer’s Dement. 2020, 12, e12005. [Google Scholar] [CrossRef]
- Olsson, B.; Portelius, E.; Cullen, N.C.; Sandelius, A.; Zetterberg, H.; Andreasson, U.; Hoglund, K.; Irwin, D.J.; Grossman, M.; Weintraub, D.; et al. Association of Cerebrospinal Fluid Neurofilament Light Protein Levels with Cognition in Patients with Dementia, Motor Neuron Disease, and Movement Disorders. JAMA Neurol. 2019, 76, 318–325. [Google Scholar] [CrossRef]
- Andersson, E.; Janelidze, S.; Lampinen, B.; Nilsson, M.; Leuzy, A.; Stomrud, E.; Blennow, K.; Zetterberg, H.; Hansson, O. Blood and cerebrospinal fluid neurofilament light differentially detect neurodegeneration in early Alzheimer’s disease. Neurobiol. Aging 2020, 95, 143–153. [Google Scholar] [CrossRef]
- Yuan, A.; Rao, M.V.; Nixon, R.A. Neurofilaments and Neurofilament Proteins in Health and Disease. Cold Spring Harb. Perspect Biol. 2017, 9, a018309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaiottino, J.; Norgren, N.; Dobson, R.; Topping, J.; Nissim, A.; Malaspina, A.; Bestwick, J.P.; Monsch, A.U.; Regeniter, A.; Lindberg, R.L.; et al. Increased neurofilament light chain blood levels in neurodegenerative neurological diseases. PLoS ONE 2013, 8, e75091. [Google Scholar] [CrossRef] [PubMed]
- Lewczuk, P.; Ermann, N.; Andreasson, U.; Schultheis, C.; Podhorna, J.; Spitzer, P.; Maler, J.M.; Kornhuber, J.; Blennow, K.; Zetterberg, H. Plasma neurofilament light as a potential biomarker of neurodegeneration in Alzheimer’s disease. Alzheimers Res. Ther. 2018, 10, 71. [Google Scholar] [CrossRef] [PubMed]
- Mattsson, N.; Cullen, N.C.; Andreasson, U.; Zetterberg, H.; Blennow, K. Association between Longitudinal Plasma Neurofilament Light and Neurodegeneration in Patients with Alzheimer Disease. JAMA Neurol. 2019, 76, 791–799. [Google Scholar] [CrossRef]
- Zhou, W.; Zhang, J.; Ye, F.; Xu, G.; Su, H.; Su, Y.; Zhang, X. Plasma neurofilament light chain levels in Alzheimer’s disease. Neurosci. Lett. 2017, 650, 60–64. [Google Scholar] [CrossRef] [Green Version]
- Sjögren, M.; Rosengren, L.; Minthon, L.; Davidsson, P.; Blennow, K.; Wallin, A. Cytoskeleton proteins in CSF distinguish frontotemporal dementia from AD. Neurology 2000, 54, 1960–1964. [Google Scholar] [CrossRef]
- Bridel, C.; Van Wieringen, W.N.; Zetterberg, H.; Tijms, B.M.; Teunissen, C.E.; Alvarez-Cermeño, J.C.; Andreasson, U.; Axelsson, M.; Bäckström, D.C.; Bartos, A. Diagnostic value of cerebrospinal fluid neurofilament light protein in neurology: A systematic review and meta-analysis. JAMA Neurol. 2019, 76, 1035–1048. [Google Scholar] [CrossRef]
- Forgrave, L.M.; Ma, M.; Best, J.R.; DeMarco, M.L. The diagnostic performance of neurofilament light chain in CSF and blood for Alzheimer’s disease, frontotemporal dementia, and amyotrophic lateral sclerosis: A systematic review and meta-analysis. Alzheimers Dement. 2019, 11, 730–743. [Google Scholar] [CrossRef]
- Kern, S.; Syrjanen, J.A.; Blennow, K.; Zetterberg, H.; Skoog, I.; Waern, M.; Hagen, C.E.; Van Harten, A.C.; Knopman, D.S.; Jack, C.R. Association of cerebrospinal fluid neurofilament light protein with risk of mild cognitive impairment among individuals without cognitive impairment. JAMA Neurol. 2019, 76, 187–193. [Google Scholar] [CrossRef]
- Ellis, K.A.; Bush, A.I.; Darby, D.; De Fazio, D.; Foster, J.; Hudson, P.; Lautenschlager, N.T.; Lenzo, N.; Martins, R.N.; Maruff, P.; et al. The Australian Imaging, Biomarkers and Lifestyle (AIBL) study of aging: Methodology and baseline characteristics of 1112 individuals recruited for a longitudinal study of Alzheimer’s disease. Int. Psychogeriatr. 2009, 21, 672–687. [Google Scholar] [CrossRef] [Green Version]
- Petersen, R.C.; Smith, G.E.; Waring, S.C.; Ivnik, R.J.; Tangalos, E.G.; Kokmen, E. Mild cognitive impairment: Clinical characterization and outcome. Arch. Neurol. 1999, 56, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Clark, C.M.; Schneider, J.A.; Bedell, B.J.; Beach, T.G.; Bilker, W.B.; Mintun, M.A.; Pontecorvo, M.J.; Hefti, F.; Carpenter, A.P.; Flitter, M.L. Use of florbetapir-PET for imaging β-amyloid pathology. JAMA 2011, 305, 275–283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vandenberghe, R.; Van Laere, K.; Ivanoiu, A.; Salmon, E.; Bastin, C.; Triau, E.; Hasselbalch, S.; Law, I.; Andersen, A.; Korner, A. 18F-flutemetamol amyloid imaging in Alzheimer disease and mild cognitive impairment: A phase 2 trial. Ann. Neurol. 2010, 68, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Rowe, C.C.; Ellis, K.A.; Rimajova, M.; Bourgeat, P.; Pike, K.E.; Jones, G.; Fripp, J.; Tochon-Danguy, H.; Morandeau, L.; O’Keefe, G.; et al. Amyloid imaging results from the Australian Imaging, Biomarkers and Lifestyle (AIBL) study of aging. Neurobiol. Aging 2010, 31, 1275–1283. [Google Scholar] [CrossRef] [PubMed]
- Villemagne, V.L.; Doré, V.; Yates, P.; Brown, B.; Mulligan, R.; Bourgeat, P.; Veljanoski, R.; Rainey-Smith, S.R.; Ong, K.; Rembach, A. En attendant centiloid. Adv. Res. 2014, 2, 723–729. [Google Scholar] [CrossRef] [Green Version]
- Harrington, K.D.; Dang, C.; Lim, Y.Y.; Ames, D.; Laws, S.M.; Pietrzak, R.H.; Rainey-Smith, S.; Robertson, J.; Rowe, C.C.; Salvado, O.; et al. The effect of preclinical Alzheimer’s disease on age-related changes in intelligence in cognitively normal older adults. Intelligence 2018, 70, 22–29. [Google Scholar] [CrossRef]
- Li, Q.X.; Villemagne, V.L.; Doecke, J.D.; Rembach, A.; Sarros, S.; Varghese, S.; McGlade, A.; Laughton, K.M.; Pertile, K.K.; Fowler, C.J.; et al. Alzheimer’s Disease Normative Cerebrospinal Fluid Biomarkers Validated in PET Amyloid-β Characterized Subjects from the Australian Imaging, Biomarkers and Lifestyle (AIBL) study. J. Alzheimers Dis. 2015, 48, 175–187. [Google Scholar] [CrossRef]
- Vanderstichele, H.; Bibl, M.; Engelborghs, S.; Le Bastard, N.; Lewczuk, P.; Molinuevo, J.L.; Parnetti, L.; Perret-Liaudet, A.; Shaw, L.M.; Teunissen, C. Standardization of preanalytical aspects of cerebrospinal fluid biomarker testing for Alzheimer’s disease diagnosis: A consensus paper from the Alzheimer’s Biomarkers Standardization Initiative. Alzheimer Dement. 2012, 8, 65–73. [Google Scholar] [CrossRef]
- Fluss, R.; Faraggi, D.; Reiser, B. Estimation of the Youden Index and its Associated Cutoff Point. Biom. J. 2005, 47, 458–472. [Google Scholar] [CrossRef] [Green Version]
- Przedborski, S.; Vila, M.; Jackson-Lewis, V. Neurodegeneration: What is it and where are we? J. Clin. Investig. 2003, 111, 3–10. [Google Scholar] [CrossRef] [Green Version]
- Lim, B.; Grøntvedt, G.R.; Bathala, P.; Kale, S.S.; Campbell, C.T.; Stengelin, M.; Sando, S.B.; Prassas, I.; Diamandis, E.P.; Bråthen, G. CSF neurofilament light may predict progression from amnestic mild cognitive impairment to Alzheimer’s disease dementia. Neurobiol. Aging 2021, 107, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Reiman, E.M.; Langbaum, J.B.; Tariot, P.N.; Lopera, F.; Bateman, R.J.; Morris, J.C.; Sperling, R.A.; Aisen, P.S.; Roses, A.D.; Welsh-Bohmer, K.A.; et al. CAP—advancing the evaluation of preclinical Alzheimer disease treatments. Nat. Rev. Neurol. 2015, 12, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olsson, B.; Alberg, L.; Cullen, N.C.; Michael, E.; Wahlgren, L.; Kroksmark, A.-K.; Rostasy, K.; Blennow, K.; Zetterberg, H.; Tulinius, M. NFL is a marker of treatment response in children with SMA treated with nusinersen. J. Neurol. 2019, 266, 2129–2136. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Participant Characteristics | Cognitively Healthy (n = 141) | |||
|---|---|---|---|---|
| Normal AD Biomarkers (A-T-N-) | Preclinical AD Pathological Change (A+T-N-) | Preclinical AD (A+T+/N±) | Non-AD Pathological Change (A-T+/N+) | |
| Demographics | ||||
| Number of participants | 87 | 42 | 7 | 5 |
| Sex M/F (% females) | 35/52 (60%) | 24/18 (43%) | 2/5 (71%) | 3/2 (40%) |
| Age at LP in years | 72 (5.48) | 74 (4.81) | 70 (4.63) | 76 (9.07) |
| APOE ε4 allele not present/present (% of ε4 carriers) | 74/13 (15%) | 23/19 (45%) $ | 5/2 (29%) | 4/1 (20%) |
| Neurofilament light | ||||
| CSF NfL pg/mL | 709 (241.27) | 775 (223.58) | 1070 (394.11) %,§ | 885 (355.46) |
| Participant Characteristics | Participant Groups | p-Value | |
|---|---|---|---|
| Converters | Non-Converters | ||
| Demographics | |||
| Number of participants (n = 124) | 25 | 99 | |
| Sex M/F (% females) | 14/11 (44%) | 42/57 (58%) | 0.223 |
| Age at LP in years | 75 (4.82) | 72 (5.68) | 0.011 |
| APOE ε4 allele not present/present (% of ε4 carriers) | 18/7 (28%) | 77/22 (22%) | 0.542 |
| Neurofilament light | |||
| CSF NfL pg/mL | 877 (256.24) | 714 (252.29) | 0.045 |
| CDR-SB | ||||
|---|---|---|---|---|
| Biomarker | Group 1 (low CSF Biomarker) | p-Value | Group 2 (High CSF Biomarker) | p-Value |
| CSF NfL | 0.19 | 0.003 | 0.68 | <0.001 |
| CSF Aβ42 * | 0.65 | <0.001 | 0.24 | 0.004 |
| CSF T-tau | 0.20 | 0.002 | 0.70 | <0.001 |
| CSF P-tau | 0.18 | 0.003 | 0.78 | <0.001 |
| MMSE | ||||
| Biomarker | Group 1 (low CSF Biomarker) | p-Value | Group 2 (High CSF Biomarker) | p-Value |
| CSF NfL | −0.12 | 0.372 | −0.99 | 0.001 |
| CSF Aβ42 * | −1.17 | <0.001 | −0.02 | 0.875 |
| CSF T-tau | −0.14 | 0.214 | −1.03 | 0.003 |
| CSF P-tau | −0.04 | 0.734 | −1.31 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dhiman, K.; Villemagne, V.L.; Fowler, C.; Bourgeat, P.; Li, Q.-X.; Collins, S.; Bush, A.I.; Rowe, C.C.; Masters, C.L.; Ames, D.; et al. Cerebrospinal Fluid Neurofilament Light Predicts Risk of Dementia Onset in Cognitively Healthy Individuals and Rate of Cognitive Decline in Mild Cognitive Impairment: A Prospective Longitudinal Study. Biomedicines 2022, 10, 1045. https://doi.org/10.3390/biomedicines10051045
Dhiman K, Villemagne VL, Fowler C, Bourgeat P, Li Q-X, Collins S, Bush AI, Rowe CC, Masters CL, Ames D, et al. Cerebrospinal Fluid Neurofilament Light Predicts Risk of Dementia Onset in Cognitively Healthy Individuals and Rate of Cognitive Decline in Mild Cognitive Impairment: A Prospective Longitudinal Study. Biomedicines. 2022; 10(5):1045. https://doi.org/10.3390/biomedicines10051045
Chicago/Turabian StyleDhiman, Kunal, Victor L. Villemagne, Christopher Fowler, Pierrick Bourgeat, Qiao-Xin Li, Steven Collins, Ashley I. Bush, Christopher C. Rowe, Colin L. Masters, David Ames, and et al. 2022. "Cerebrospinal Fluid Neurofilament Light Predicts Risk of Dementia Onset in Cognitively Healthy Individuals and Rate of Cognitive Decline in Mild Cognitive Impairment: A Prospective Longitudinal Study" Biomedicines 10, no. 5: 1045. https://doi.org/10.3390/biomedicines10051045
APA StyleDhiman, K., Villemagne, V. L., Fowler, C., Bourgeat, P., Li, Q.-X., Collins, S., Bush, A. I., Rowe, C. C., Masters, C. L., Ames, D., Blennow, K., Zetterberg, H., Martins, R. N., & Gupta, V. (2022). Cerebrospinal Fluid Neurofilament Light Predicts Risk of Dementia Onset in Cognitively Healthy Individuals and Rate of Cognitive Decline in Mild Cognitive Impairment: A Prospective Longitudinal Study. Biomedicines, 10(5), 1045. https://doi.org/10.3390/biomedicines10051045







