The “Third Violin” in the Cytoskeleton Orchestra—The Role of Intermediate Filaments in the Endothelial Cell’s Life
Abstract
1. Introduction
2. Features of Intermediate Filaments—The Third Component of the Cytoskeleton
3. Classification of Intermediate Filaments and Its Types Found in Endothelial Cells
4. Molecular Structure of Vimentin Filaments as the Basis of Their Remarkable Mechanical Properties
5. Vimentin Filaments: The Role of Post-Translational Modifications in the Regulation of Depolymerization and Stabilization of Vimentin Filaments
6. Features of Intracellular Functions in the Endothelium and Dynamics of Vimentin Filaments
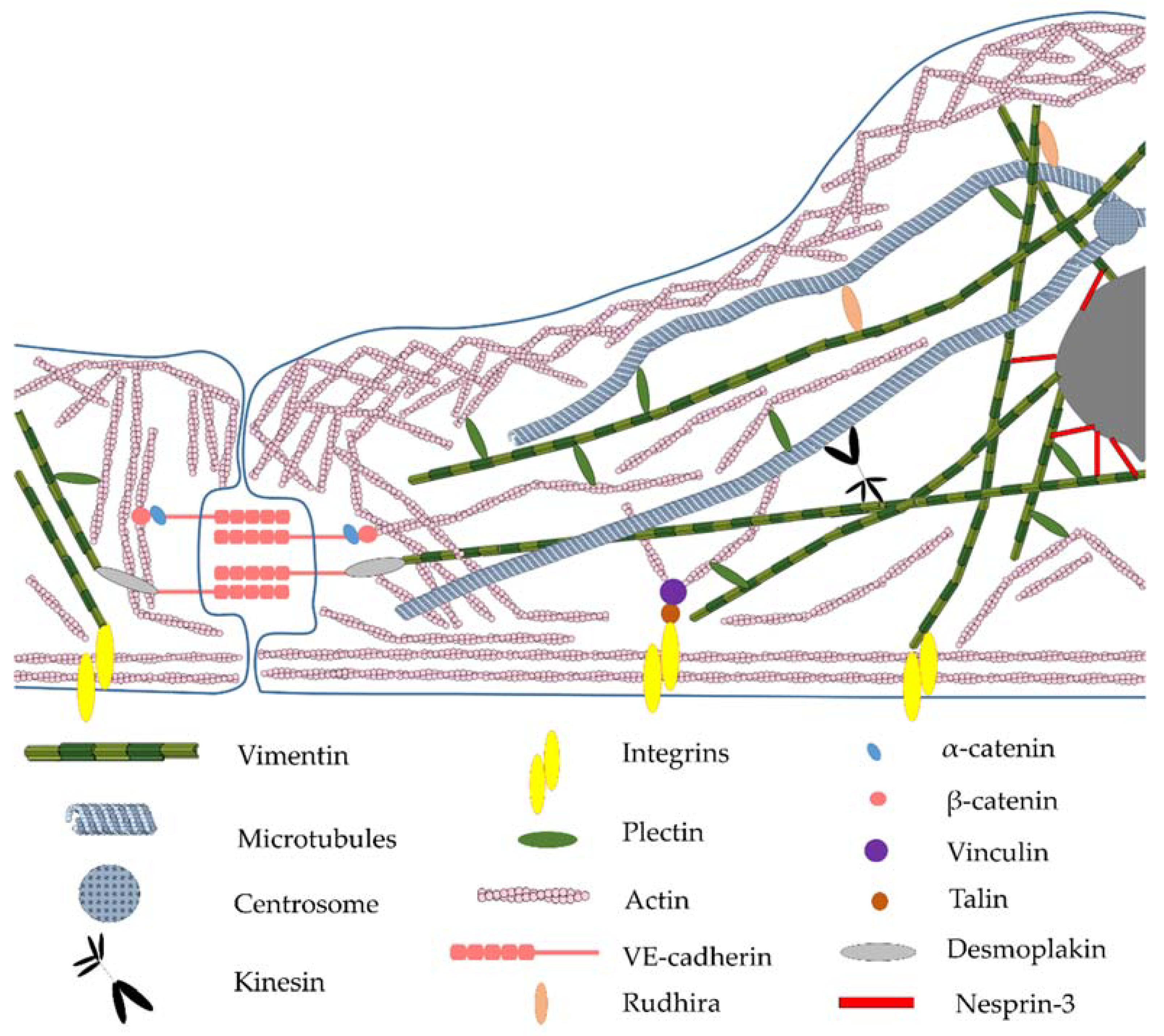
7. Linker Proteins—The Connection of Vimentin Filaments with Other Cellular Structures
8. The Role of Vimentin in Angiogenesis, the Development of Inflammatory Processes and Vascular Pathologies
9. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guo, M.; Ehrlicher, A.J.; Mahammad, S.; Fabich, H.; Jensen, M.H.; Moore, J.R.; Fredberg, J.J.; Goldman, R.D.; Weitz, D.A. The role of vimentin intermediate filaments in cortical and cytoplasmic mechanics. Biophys. J. 2013, 105, 1562–1568. [Google Scholar] [CrossRef] [PubMed]
- Strouhalova, K.; Přechová, M.; Gandalovičová, A.; Brábek, J.; Gregor, M.; Rosel, D. Vimentin intermediate filaments as potential target for cancer treatment. Cancers 2020, 12, 184. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Syder, A.J.; Yu, Q.C.; Letal, A.; Paller, A.S.; Fuchs, E. The genetic basis of epidermolytic hyperkeratosis: A disorder of differentiation-specific epidermal keratin genes. Cell 1992, 70, 811–819. [Google Scholar] [CrossRef]
- Chipev, C.C.; Korge, B.P.; Markova, N.; Bale, S.J.; DiGiovanna, J.J.; Compton, J.G.; Steinert, P.M. A leucine→proline mutation in the H1 subdomain of keratin 1 causes epidermolytic hyperkeratosis. Cell 1992, 70, 821–828. [Google Scholar] [CrossRef]
- Côté, F.; Collard, J.F.; Julien, J.P. Progressive neuronopathy in transgenic mice expressing the human neurofilament heavy gene: A mouse model of amyotrophic lateral sclerosis. Cell 1993, 73, 35–46. [Google Scholar] [CrossRef]
- Di Somma, S.; De Divitiis, O.; Marotta, M.; Salvatore, G.; Cudemo, G.; Cuda, G.; De Vivo, F.; Di Benedetto, M.P.; Ciaramella, F.; Caputo, G. Changes in myocardial cytoskeletal intermediate filaments and myocyte contractile dysfunction in dilated cardiomyopathy: An in vivo study in humans. Heart 2000, 84, 659–667. [Google Scholar] [CrossRef][Green Version]
- Dave, J.M.; Bayless, K.J. Vimentin as an integral regulator of cell adhesion and endothelial sprouting. Microcirculation 2014, 21, 333–344. [Google Scholar] [CrossRef]
- Bruneel, A.; Labas, V.; Mailloux, A.; Sharma, S.; Vinh, J.; Vaubourdolle, M.; Baudin, B. Proteomic study of human umbilical vein endothelial cells in culture. Proteomics 2003, 3, 714–723. [Google Scholar] [CrossRef]
- Liu, T.; Guevara, O.E.; Warburton, R.R.; Hill, N.S.; Gaestel, M.; Kayyali, U.S. Regulation of vimentin intermediate filaments in endothelial cells by hypoxia. Am. J. Physiol. Cell Physiol. 2010, 299, C363–C373. [Google Scholar] [CrossRef]
- Mokrý, J.; Cízková, D.; Filip, S.; Ehrmann, J.; Österreicher, J.; Kolár, Z.; English, D. Nestin Expression by Newly Formed Human Blood Vessels. Stem Cells Dev. 2004, 13, 658–664. [Google Scholar] [CrossRef]
- Mokrý, J.; Ehrmann, J.; Karbanová, J.; Cízková, D.; Soukup, T.; Suchánek, J.; Filip, S.; Kolár, Z. Expression of intermediate filament nestin in blood vessels of neural and non-neural tissues. Acta Med. 2008, 51, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Cizkova, D.; Soukup, T.; Mokry, J. Nestin expression reflects formation, revascularization and reinnervation of new myofibers in regenerating rat hind limb skeletal muscles. Cells Tissues Organs 2009, 189, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Rusu, M.C.; Jianu, A.M.; Pop, F.; Hostiuc, S.; Leonardi, R.; Curcă, G.C. Immunolocalization of 200 kDa neurofilaments in human cardiac endothelial cells. Acta Histochem. 2012, 114, 842–845. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, K.I.; Kurihara, H.; Negishi, M.; Saito, N.; Nakazato, Y.; Sasaki, T.; Takeuchi, T. Nestin as a marker for proliferative endothelium in gliomas. Lab. Investig. 2002, 82, 345–351. [Google Scholar] [CrossRef]
- Nowak, A.; Grzegrzolka, J.; Paprocka, M.; Piotrowska, A.; Rys, J.; Matkowski, R.; Dziegiel, P. Nestin-positive microvessel density is an independent prognostic factor in breast cancer. Int. J. Oncol. 2017, 51, 668–676. [Google Scholar] [CrossRef][Green Version]
- Dusart, P.; Fagerberg, L.; Perisic, L.; Civelek, M.; Struck, E.; Hedin, U.; Uhlén, M.; Trégouët, D.A.; Renné, T.; Odeberg, J.; et al. A systems-approach reveals human nestin is an endothelial-enriched, angiogenesis-independent intermediate filament protein. Sci. Rep. 2018, 8, 14668. [Google Scholar] [CrossRef]
- Herrmann, H.; Bär, H.; Kreplak, L.; Strelkov, S.V.; Aebi, U. Intermediate filaments: From cell architecture to nanomechanics. Mol. Cell Biol. 2007, 8, 562–573. [Google Scholar] [CrossRef]
- Qin, Z.; Buehler, M.J. Structure and dynamics of human vimentin intermediate filament dimer and tetramer in explicit and implicit solvent models. J. Mol. Model. 2011, 17, 37–48. [Google Scholar] [CrossRef]
- Strelkov, S.V.; Herrmann, H.; Aebi, U. Molecular architecture of intermediate filaments. BioEssays 2003, 25, 243–251. [Google Scholar] [CrossRef]
- Patteson, A.E.; Carroll, R.J.; Iwamoto, D.V.; Janmey, P.A. The vimentin cytoskeleton: When polymer physics meets cell biology. Phys. Biol. 2020, 18, 011001. [Google Scholar] [CrossRef]
- Snider, N.T.; Omary, M.B. Post-translational modifications of intermediate filament proteins: Mechanisms and functions. Nat. Rev. Mol. Cell Biol. 2014, 15, 163–177. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, J.E.; He, T.; Trejo-Skalli, A.V.; Harmala-Brasken, A.S.; Hellman, J.; Chou, Y.-H.H.; Goldman, R.D.; Härmälä-Braskén, A.-S.; Hellman, J.; Chou, Y.-H.H.; et al. Specific in vivo phosphorylation sites determine the assembly dynamics of vimentin intermediate filaments. J. Cell Sci. 2004, 117, 919–932. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, M.; Nishi, Y.; Nishizawa, K.; Matsuyama, M.; Sato, C. Site-specific phosphorylation induces disassembly of vimentin filaments in vitro. Nature 1987, 328, 649–652. [Google Scholar] [CrossRef] [PubMed]
- Shoeman, R.L.; Hartig, R.; Berthel, M.; Traub, P. Deletion mutagenesis of the amino-terminal head domain of vimentin reveals dispensability of large internal regions for intermediate filament assembly and stability. Exp. Cell Res. 2002, 279, 344–353. [Google Scholar] [CrossRef]
- Asaga, H.; Yamada, M.; Senshu, T. Selective deimination of vimentin in calcium ionophore-induced apoptosis of mouse peritoneal macrophages. Biochem. Biophys. Res. Commun. 1998, 243, 641–646. [Google Scholar] [CrossRef]
- Farach, A.M.; Galileo, D.S. O-GlcNAc modification of radial glial vimentin filaments in the developing chick brain. Brain Cell Biol. 2008, 36, 191–202. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, J.; Banerjee, S.; Barnes, L.; Sajja, V.; Liu, Y.; Guo, B.; Du, Y.; Agarwal, M.K.; Wald, D.N.; et al. Sumoylation of vimentin354 is associated with PIAS3 inhibition of glioma cell migration. Oncotarget 2010, 1, 620–627. [Google Scholar] [CrossRef]
- Colucci-Guyon, E.; Portier, M.-M.; Dunia, I.; Paulin, D.; Pournin, S.; Babinet, C. Mice lacking vimentin develop and reproduce without an obvious phenotype. Cell 1994, 79, 679–694. [Google Scholar] [CrossRef]
- Schaffeld, M.; Herrmann, H.; Schultess, J.; Markl, J. Vimentin and desmin of a cartilaginous fish, the shark Scyliorhinus stellaris: Sequence, expression patterns and in vitro assembly. Eur. J. Cell Biol. 2001, 80, 692–702. [Google Scholar] [CrossRef]
- Peuhu, E.; Virtakoivu, R.; Mai, A.; Wärri, A.; Ivaska, J. Epithelial vimentin plays a functional role in mammary gland development. Development 2017, 144, 4103–4113. [Google Scholar] [CrossRef]
- Antfolk, D.; Sjöqvist, M.; Cheng, F.; Isoniemi, K.; Duran, C.L.; Rivero-Muller, A.; Antila, C.; Niemi, R.; Landor, S.; Bouten, C.V.C.; et al. Selective regulation of Notch ligands during angiogenesis is mediated by vimentin. Proc. Natl. Acad. Sci. USA 2017, 114, E4574–E4581. [Google Scholar] [CrossRef] [PubMed]
- Langlois, B.; Belozertseva, E.; Parlakian, A.; Bourhim, M.; Gao-Li, J.; Blanc, J.; Tian, L.; Coletti, D.; Labat, C.; Ramdame-Cherif, Z.; et al. Vimentin knockout results in increased expression of sub-endothelial basement membrane components and carotid stiffness in mice. Sci. Rep. 2017, 7, 11628. [Google Scholar] [CrossRef] [PubMed]
- Schnittler, H.J.; Schmandra, T.; Drenckhahn, D. Correlation of endothelial vimentin content with hemodynamic parameters. Histochem. Cell Biol. 1998, 110, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, E.; Weber, K. Intermediate filaments: Structure, Dynamics, Function and Disease. Annu. Rev. Biochem. 1994, 63, 345–382. [Google Scholar] [CrossRef] [PubMed]
- Minin, A.A.; Moldaver, M.V. Intermediate vimentin filaments and their role in intracellular organelle distribution. Biochem. 2008, 73, 1453–1466. [Google Scholar] [CrossRef]
- Lazarides, E. Intermediate Filaments: A Chemically Heterogeneous, Developmentally Regulated Class of Proteins. Annu. Rev. Biochem. 1982, 51, 219–250. [Google Scholar] [CrossRef]
- Yoon, M.; Moir, R.D.; Prahlad, V.; Goldman, R.D. Motile Properties of Vimentin Intermediate Filament Networks in Living Cells. J. Cell Biol. 1998, 143, 147–157. [Google Scholar] [CrossRef]
- Saxton, W.M.; Stemple, D.L.; Leslie, R.J.; Salmon, E.D.; Zavortink, M.; McIntosh, J.R. Tubulin dynamics in cultured mammalian cells. J. Cell Biol. 1984, 99, 2175–2186. [Google Scholar] [CrossRef]
- Kreis, T.E.; Geiger, B.; Schlessinger, J. Mobility of microinjected rhodamine actin within living chicken gizzard cells determined by fluorescence photobleaching recovery. Cell 1982, 29, 835–845. [Google Scholar] [CrossRef]
- Helmke, B.P.; Goldman, R.D.; Davies, P.F. Rapid displacement of vimentin intermediate filaments in living endothelial cells exposed to flow. Circ. Res. 2000, 86, 745–752. [Google Scholar] [CrossRef]
- Helmke, B.P.; Thakker, D.B.; Goldman, R.D.; Davies, P.F. Spatiotemporal analysis of flow-induced intermediate filament displacement in living endothelial cells. Biophys. J. 2001, 80, 184–194. [Google Scholar] [CrossRef]
- Helfand, B.T.; Chang, L.; Goldman, R.D. Intermediate filaments are dynamic and motile elements of cellular architecture. J. Cell Sci. 2004, 117, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Liem, R.K.H. Intermediate Filaments: Not Just for Structure Anymore. Curr. Biol. 2013, 23, R322–R324. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wiche, G.; Winter, L. Plectin isoforms as organizers of intermediate filament cytoarchitecture. Bioarchitecture 2011, 1, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Morgan, J.T.; Pfeiffer, E.R.; Thirkill, T.L.; Kumar, P.; Peng, G.; Fridolfsson, H.N.; Douglas, G.C.; Starr, D.A.; Barakat, A.I. Nesprin-3 regulates endothelial cell morphology, perinuclear cytoskeletal architecture, and flow-induced polarization. Mol. Biol. Cell 2011, 22, 4324–4334. [Google Scholar] [CrossRef]
- Maro, B.; Paintrand, M.; Sauron, M.-E.; Paulin, D.; Bornens, M. Vimentin Filaments and Centrosomes. Are They Associated? Exp. Cell Res. 1984, 150, 452–458. [Google Scholar] [CrossRef]
- Alieva, I.B.; Nadezhdina, E.S.; Vaisberg, E.A.; Vorobjev, A. Microtubule and Intermediate Filament Patterns around the Centrosome in Interphase Cells. Centrosome 1992, 15, 103–129. [Google Scholar]
- Trevor, K.T.; McGuire, J.G.; Leonova, E. V Association of vimentin intermediate filaments with the centrosome. J. Cell Sci. 1995, 108 Pt 1, 343–356. [Google Scholar] [CrossRef]
- Niwa, T.; Saito, H.; Imajoh-ohmi, S.; Kaminishi, M.; Seto, Y.; Miki, Y.; Nakanishi, A. BRCA2 interacts with the cytoskeletal linker protein plectin to form a complex controlling centrosome localization. Cancer Sci. 2009, 100, 2115–2125. [Google Scholar] [CrossRef]
- Robert, A.; Herrmann, H.; Davidson, M.W.; Gelfand, V.I. Microtubule-dependent transport of vimentin filament precursors is regulated by actin and by the concerted action of Rho- and p21-activated kinases. FASEB J. 2014, 28, 2879–2890. [Google Scholar] [CrossRef]
- Hookway, C.; Ding, L.; Davidson, M.W.; Rappoport, J.Z.; Danuser, G.; Gelfand, V.I. Microtubule-dependent transport and dynamics of vimentin intermediate filaments. Mol. Biol. Cell 2015, 26, 1675–1686. [Google Scholar] [CrossRef] [PubMed]
- Castañón, M.J.; Walko, G.; Winter, L.; Wiche, G. Plectin-intermediate filament partnership in skin, skeletal muscle, and peripheral nerve. Histochem. Cell Biol. 2013, 140, 33–53. [Google Scholar] [CrossRef] [PubMed]
- Jiu, Y.; Lehtimäki, J.; Tojkander, S.; Cheng, F.; Jäälinoja, H.; Liu, X.; Varjosalo, M.; Eriksson, J.E.; Lappalainen, P. Bidirectional Interplay between Vimentin Intermediate Filaments and Contractile Actin Stress Fibers. Cell Rep. 2015, 11, 1511–1518. [Google Scholar] [CrossRef] [PubMed]
- Osmanagic-Myers, S.; Rus, S.; Wolfram, M.; Brunner, D.; Goldmann, W.H.; Bonakdar, N.; Fischer, I.; Reipert, S.; Zuzuarregui, A.; Walko, G.; et al. Plectin reinforces vascular integrity by mediating vimentin-actin network crosstalk. J. Cell Sci. 2015, 44, 4138–4150. [Google Scholar] [CrossRef]
- Burgstaller, G.; Gregor, M.; Winter, L.; Wiche, G. Keeping the Vimentin Network under Control: Cell–Matrix Adhesion–associated Plectin 1f Affects Cell Shape and Polarity of Fibroblasts. Mol. Biol. Cell 2010, 21, 3362–3375. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Yang, C.; Kim, E.J.; Jang, J.; Kim, S.J.; Kang, S.M.; Kim, M.G.; Jung, H.; Park, D.; Kim, C. Vimentin filaments regulate integrin-ligand interactions by binding to the cytoplasmic tail of integrin β3. J. Cell Sci. 2016, 129, 2030–2042. [Google Scholar] [CrossRef]
- Kreis, S.; Schönfeld, H.J.; Melchior, C.; Steiner, B.; Kieffer, N. The intermediate filament protein vimentin binds specifically to a recombinant integrin α2/β1 cytoplasmic tail complex and co-localizes with native α2/β1 in endothelial cell focal adhesions. Exp. Cell Res. 2005, 305, 110–121. [Google Scholar] [CrossRef]
- De Pascalis, C.; Pérez-González, C.; Seetharaman, S.; Boëda, B.; Vianay, B.; Burute, M.; Leduc, C.; Borghi, N.; Trepat, X.; Etienne-Manneville, S. Intermediate filaments control collective migration by restricting traction forces and sustaining cell-cell contacts. J. Cell Biol. 2018, 217, 3031–3044. [Google Scholar] [CrossRef]
- Gonzales, M.; Weksler, B.; Tsuruta, D.; Goldman, R.D.; Yoon, K.J.; Hopkinson, S.B.; Flitney, F.W.; Jones, J.C.R. Structure and function of a vimentin-associated matrix adhesion in endothelial cells. Mol. Biol. Cell 2001, 12, 85–100. [Google Scholar] [CrossRef][Green Version]
- Battaglia, R.A.; Delic, S.; Herrmann, H.; Snider, N.T. Vimentin on the move: New developments in cell migration. F1000Research 2018, 7, 1796. [Google Scholar] [CrossRef]
- Gan, Z.; Ding, L.; Burckhardt, C.J.; Lowery, J.; Zaritsky, A.; Sitterley, K.; Mota, A.; Costigliola, N.; Starker, C.G.; Voytas, D.F.; et al. Vimentin Intermediate Filaments Template Microtubule Networks to Enhance Persistence in Cell Polarity and Directed Migration. Cell Syst. 2016, 3, 252–263.e8. [Google Scholar] [CrossRef] [PubMed]
- Shakhov, A.S.; Alieva, I.B. The centrosome as the main integrator of endothelial cell functional activity. Biochemistry 2017, 82, 663–677. [Google Scholar] [CrossRef] [PubMed]
- Alieva, I.B.; Zemskov, E.A.; Kireev, I.I.; Gorshkov, B.A.; Wiseman, D.A.; Black, S.M.; Verin, A.D. Microtubules Growth Rate Alteration in Human Endothelial Cells. J. Biomed. Biotechnol. 2010, 2010, 671536. [Google Scholar] [CrossRef] [PubMed]
- Alieva, I.B.; Zemskov, E.A.; Smurova, K.M.; Kaverina, I.N.; Verin, A.D. The leading role of microtubules in endothelial barrier dysfunction: Disassembly of peripheral microtubules leaves behind the cytoskeletal reorganization. J. Cell. Biochem. 2013, 114, 2258–2272. [Google Scholar] [CrossRef] [PubMed]
- Shakhov, A.S.; Verin, A.D.; Alieva, I.B. Reorganization of endothelial cells cytoskeleton during formation of functional monolayer in vitro. Cell Tissue Biol. 2014, 8, 138–151. [Google Scholar] [CrossRef]
- Shakhov, A.S.; Dugina, V.B.; Alieva, I.B. Reorganization of actin and microtubule systems in human vein endothelial cells during intercellular contact formation. Cell Tissue Biol. 2015, 9, 299–309. [Google Scholar] [CrossRef]
- Dugina, V.B.; Shagieva, G.S.; Shakhov, A.S.; Alieva, I.B. The cytoplasmic actins in the regulation of endothelial cell function. Int. J. Mol. Sci. 2021, 22, 7836. [Google Scholar] [CrossRef]
- Eckes, B.; Dogic, D.; Colucci-Guyon, E.; Wang, N.; Maniotis, A.; Ingber, D.; Merckling, A.; Langa, F.; Aumailley, M.; Delouvee, A.; et al. Impaired mechanical stability, migration and contractile capacity in vimentin-deficient fibroblasts. J. Cell Sci. 1998, 111, 1897–1907. [Google Scholar] [CrossRef]
- Lundkvist, A.; Reichenbach, A.; Betsholtz, C.; Carmeliet, P.; Wolburg, H.; Pekny, M. Under stress, the absence of intermediate filaments from Müller cells in the retina has structural and functional consequences. J. Cell Sci. 2004, 117, 3481–3488. [Google Scholar] [CrossRef]
- Dave, J.M.; Kang, H.; Abbey, C.A.; Maxwell, S.A.; Bayless, K.J. Proteomic profiling of endothelial invasion revealed receptor for activated C kinase 1 (RACK1) complexed with vimentin to regulate focal adhesion kinase (FAK). J. Biol. Chem. 2013, 288, 30720–30733. [Google Scholar] [CrossRef]
- Joshi, D.; Inamdar, M.S. Rudhira/BCAS3 couples microtubules and intermediate filaments to promote cell migration for angiogenic remodeling. Mol. Biol. Cell 2019, 30, 1437–1450. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; DeWaal, R.M.; Mor-Vaknin, N.; Hibbard, C.; Markovitz, D.M.; Kahn, M.L. The Endothelial Cell-Specific Antibody PAL-E Identifies a Secreted Form of Vimentin in the Blood Vasculature. Mol. Cell. Biol. 2004, 24, 9198–9206. [Google Scholar] [CrossRef] [PubMed]
- Gong, D.H.; Dai, Y.; Chen, S.; Wang, X.Q.; Yan, X.X.; Shen, Y.; Liu, J.; Yang, Z.K.; Hu, J.; Yu, L.J.; et al. Secretory vimentin is associated with coronary artery disease in patients and induces atherogenesis in ApoE−/− mice. Int. J. Cardiol. 2019, 283, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Cho, W.; Kim, I.; Lee, S.H.; Oh, G.T.; Park, Y.M. Oxidized LDL induces vimentin secretion by macrophages and contributes to atherosclerotic inflammation. J. Mol. Med. 2020, 98, 973–983. [Google Scholar] [CrossRef] [PubMed]
- Evrard, S.M.; Lecce, L.; Michelis, K.C.; Nomura-Kitabayashi, A.; Pandey, G.; Purushothaman, K.R.; D’Escamard, V.; Li, J.R.; Hadri, L.; Fujitani, K.; et al. Endothelial to mesenchymal transition is common in atherosclerotic lesions and is associated with plaque instability. Nat. Commun. 2016, 7, 11853. [Google Scholar] [CrossRef]
- Danielsson, F.; Peterson, M.K.; Araújo, H.C.; Lautenschläger, F.; Gad, A.K.B. Vimentin diversity in health and disease. Cells 2018, 7, 147. [Google Scholar] [CrossRef]
- Ranchoux, B.; Antigny, F.; Rucker-Martin, C.; Hautefort, A.; Péchoux, C.; Bogaard, H.J.; Dorfmüller, P.; Remy, S.; Lecerf, F.; Planté, S.; et al. Endothelial-to-mesenchymal transition in pulmonary hypertension. Circulation 2015, 131, 1006–1018. [Google Scholar] [CrossRef]
- Yun, E.; Kook, Y.; Yoo, K.H.; Kim, K., II; Lee, M.S.; Kim, J.; Lee, A. Endothelial to mesenchymal transition in pulmonary vascular diseases. Biomedicines 2020, 8, 639. [Google Scholar] [CrossRef]
- Gorelova, A.; Berman, M.; Al Ghouleh, I. Endothelial-to-mesenchymal transition in pulmonary arterial hypertension. Antioxidants Redox. Signal. 2021, 34, 891–914. [Google Scholar] [CrossRef]
- He, Q.; Wang, F.; Honda, T.; Greis, K.D.; Redington, A.N. Ablation of miR-144 increases vimentin expression and atherosclerotic plaque formation. Sci. Rep. 2020, 10, 6127. [Google Scholar] [CrossRef]
- Nieminen, M.; Henttinen, T.; Merinen, M.; Marttila-Ichihara, F.; Eriksson, J.E.; Jalkanen, S. Vimentin function in lymphocyte adhesion and transcellular migration. Nat. Cell Biol. 2006, 8, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, J.N.; Kim, K.; Zemskova, M.A.; Rafikov, R.; Heeke, B.; Varn, M.N.; Black, S.; Kennedy, T.P.; Verin, A.D.; Zemskov, E.A. Low anticoagulant heparin blocks thrombin-induced endothelial permeability in a PAR-dependent manner. Vascul. Pharmacol. 2014, 62, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Ise, H.; Kobayashi, S.; Goto, M.; Sato, T.; Kawakubo, M.; Takahashi, M.; Ikeda, U.; Akaike, T. Vimentin and desmin possess GlcNAc-binding lectin-like properties on cell surfaces. Glycobiology 2010, 20, 843–864. [Google Scholar] [CrossRef] [PubMed]
- McEver, R.P.; Cummings, R.D. Perspectives series: Cell adhesion in vascular biology. Role of PSGL-1 binding to selectins in leukocyte recruitment. J. Clin. Investig. 1997, 100, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Päll, T.; Pink, A.; Kasak, L.; Turkina, M.; Anderson, W.; Valkna, A.; Kogerman, P. Soluble CD44 interacts with intermediate filament protein vimentin on endothelial cell surface. PLoS ONE 2011, 6, e29305. [Google Scholar] [CrossRef]
- Katayama, Y.; Hidalgo, A.; Chang, J.; Peired, A.; Frenette, P.S. CD44 is a physiological E-selectin ligand on neutrophils. J. Exp. Med. 2005, 201, 1183–1189. [Google Scholar] [CrossRef]
- Lam, F.W.; Da, Q.; Guillory, B.; Cruz, M.A. Recombinant Human Vimentin Binds to P-Selectin and Blocks Neutrophil Capture and Rolling on Platelets and Endothelium. J. Immunol. 2018, 200, ji1700784. [Google Scholar] [CrossRef]
- Da, Q.; Behymer, M.; Correa, J.I.; Vijayan, K.V.; Cruz, M.A. Platelet adhesion involves a novel interaction between vimentin and von Willebrand factor under high shear stress. Blood 2014, 123, 2715–2721. [Google Scholar] [CrossRef]
- Fasipe, T.A.; Hong, S.-H.; Da, Q.; Valladolid, C.; Lahey, M.T.; Richards, L.M.; Dunn, A.K.; Cruz, M.A.; Marrelli, S.P. Extracellular Vimentin/VWF (von Willebrand Factor) Interaction Contributes to VWF String Formation and Stroke Pathology. Stroke 2018, 49, 2536–2540. [Google Scholar] [CrossRef]
- Amraei, R.; Xia, C.; Olejnik, J.; White, M.R.; Napoleon, M.A.; Lotfollahzadeh, S.; Hauser, B.M.; Schmidt, A.G.; Chitalia, V.; Mühlberger, E.; et al. Extracellular vimentin is an attachment factor that facilitates SARS-CoV-2 entry into human endothelial cells. Proc. Natl. Acad. Sci. USA 2022, 119, e2113874119. [Google Scholar] [CrossRef]
| Pathological Processes | Role of Vimentin | References |
|---|---|---|
| Disturbances of angiogenesis and cell motility | Increased vimentin level in the sites of atherosclerotic lesions leads to the endothelial-to-mesenchymal transition Vimentin deletion destabilizes sprouting angiogenesis and disrupts embryonic angiogenesis | Evrard et al., 2016 [75] Antfolk et al., 2017 [31] |
| Importance of protein linker Rudhira for vimentin-microtubules association and angiogenesis | Joshi and Inamdar, 2019 [71] | |
| Secretory vimentin provoke atherogenesis in Apolipoprotein E (ApoE−/−) deficient mice in vivo | Gong et al., 2019 [73] | |
| Inflammation | Prevention of the initial stage of acute inflammatory response using recombinant human vimentin | Lam et al., 2018 [87] |
| Secretory vimentin as an inflammatory trigger (promotion of macrophage-endothelial cell adhesion) | Gong et al., 2019 [73] | |
| Vascular diseases | Reduction of ability to remodeling arteries and enlarged stiffness, contractility and endothelial dysfunction in vimentin-null mice | Langlois et al., 2017 [32] |
| Targeting the vimentin/VWF von Willebrand Factor interaction promote improved reperfusion after ischemic stroke | Fasipe et al., 2018 [89] | |
| Increased serum levels of secretory vimentin are associated with the presence and severity of coronary artery disease | Gong et al., 2019 [73] | |
| Vimentin accumulation in the knockout (miR-144) mice aorta strengthen atherosclerotic plaque formation | He et al., 2020 [80] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shakhov, A.S.; Alieva, I.B. The “Third Violin” in the Cytoskeleton Orchestra—The Role of Intermediate Filaments in the Endothelial Cell’s Life. Biomedicines 2022, 10, 828. https://doi.org/10.3390/biomedicines10040828
Shakhov AS, Alieva IB. The “Third Violin” in the Cytoskeleton Orchestra—The Role of Intermediate Filaments in the Endothelial Cell’s Life. Biomedicines. 2022; 10(4):828. https://doi.org/10.3390/biomedicines10040828
Chicago/Turabian StyleShakhov, Anton S., and Irina B. Alieva. 2022. "The “Third Violin” in the Cytoskeleton Orchestra—The Role of Intermediate Filaments in the Endothelial Cell’s Life" Biomedicines 10, no. 4: 828. https://doi.org/10.3390/biomedicines10040828
APA StyleShakhov, A. S., & Alieva, I. B. (2022). The “Third Violin” in the Cytoskeleton Orchestra—The Role of Intermediate Filaments in the Endothelial Cell’s Life. Biomedicines, 10(4), 828. https://doi.org/10.3390/biomedicines10040828






