Cholinergic Regulation of Hippocampal Theta Rhythm
Abstract
1. Introduction
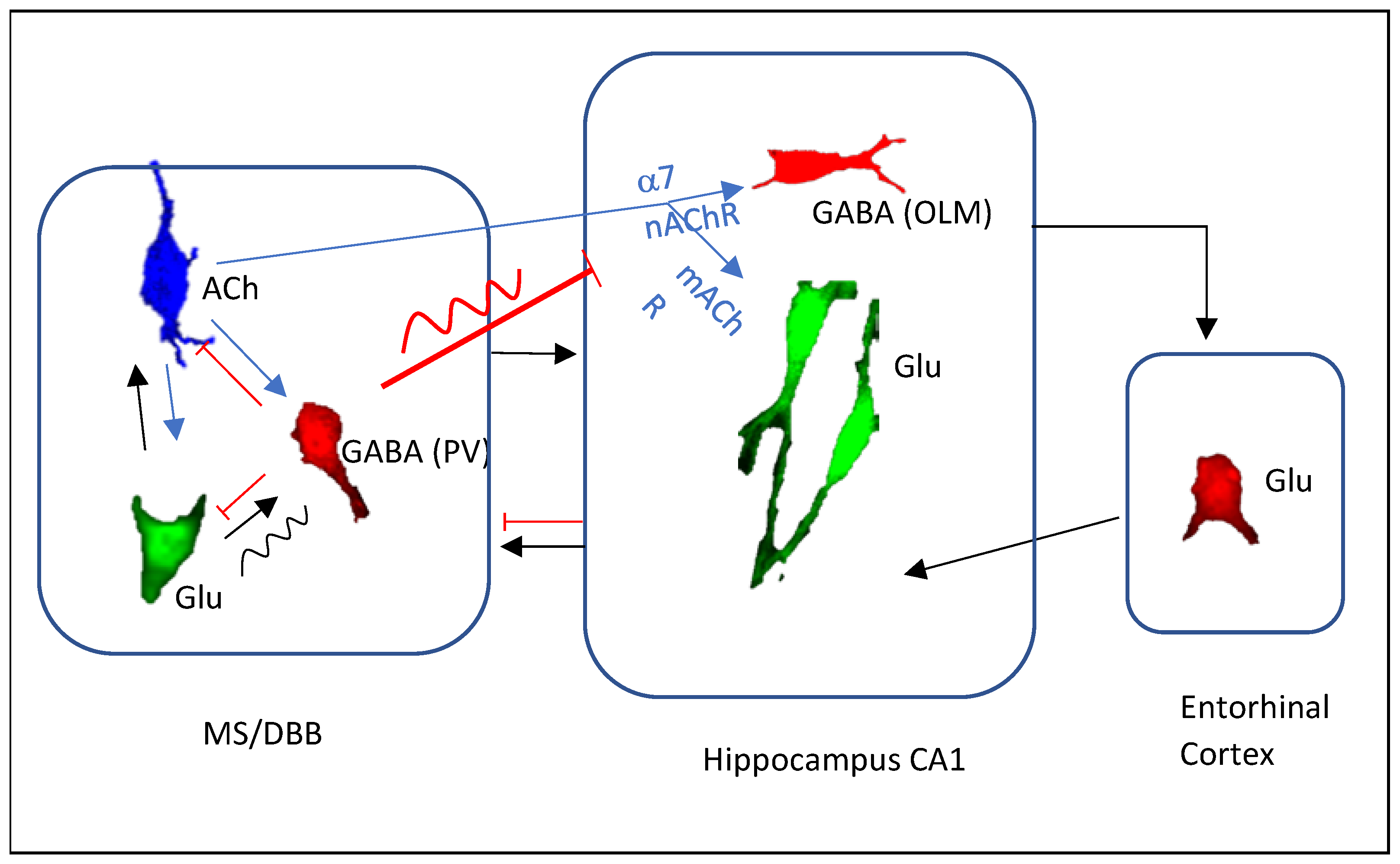
2. MSDB Cholinergic Neuronal Activities Correlate with Theta States
3. Cholinergic Regulation of Theta through Direct Septohippocampal Cholinergic Pathway
4. Cholinergic Regulation of Theta through Septal GABAergic and Glutamatergic Neurons
5. Cholinergic Regulation of Theta Frequency
6. Cholinergic Regulation of Theta-Gamma Coupling
7. Concluding Remarks and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Witter, M.P.; Naber, P.A.; van Haeften, T.; Machielsen, W.C.; Rombouts, S.A.; Barkhof, F.; Scheltens, P.; Lopes da Silva, F.H. Cortico-hippocampal communication by way of parallel parahippocampal-subicular pathways. Hippocampus 2000, 10, 398–410. [Google Scholar] [CrossRef]
- Amaral, D.G.; Scharfman, H.E.; Lavenex, P. The dentate gyrus: Fundamental neuroanatomical organization (dentate gyrus for dummies). Prog. Brain Res. 2007, 163, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cao, L.; Varga, V.; Jing, M.; Karadas, M.; Li, Y.; Buzsaki, G. Cholinergic suppression of hippocampal sharp-wave ripples impairs working memory. Proc. Natl. Acad. Sci. USA 2021, 118, e2016432118. [Google Scholar] [CrossRef]
- Hasselmo, M.E. The role of acetylcholine in learning and memory. Curr. Opin. Neurobiol. 2006, 16, 710–715. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Hwaun, E.; Loza, C.A.; Colgin, L.L. Hippocampal place cell sequences differ during correct and error trials in a spatial memory task. Nat. Commun. 2021, 12, 3373. [Google Scholar] [CrossRef] [PubMed]
- Qasim, S.E.; Fried, I.; Jacobs, J. Phase precession in the human hippocampus and entorhinal cortex. Cell 2021, 184, 3242–3255.e10. [Google Scholar] [CrossRef] [PubMed]
- Nunez, A.; Buno, W. The Theta Rhythm of the Hippocampus: From Neuronal and Circuit Mechanisms to Behavior. Front. Cell Neurosci. 2021, 15, 31. [Google Scholar] [CrossRef] [PubMed]
- Buzsaki, G.; Moser, E.I. Memory, navigation and theta rhythm in the hippocampal-entorhinal system. Nat. Neurosci. 2013, 16, 130–138. [Google Scholar] [CrossRef]
- Lopez-Madrona, V.J.; Perez-Montoyo, E.; Alvarez-Salvado, E.; Moratal, D.; Herreras, O.; Pereda, E.; Mirasso, C.R.; Canals, S. Different theta frameworks coexist in the rat hippocampus and are coordinated during memory-guided and novelty tasks. eLife 2020, 9, e57313. [Google Scholar] [CrossRef]
- Hasselmo, M.E. What is the function of hippocampal theta rhythm?—Linking behavioral data to phasic properties of field potential and unit recording data. Hippocampus 2005, 15, 936–949. [Google Scholar] [CrossRef]
- Kramis, R.; Vanderwolf, C.H.; Bland, B.H. Two types of hippocampal rhythmical slow activity in both the rabbit and the rat: Relations to behavior and effects of atropine, diethyl ether, urethane, and pentobarbital. Exp. Neurol. 1975, 49, 58–85. [Google Scholar] [CrossRef]
- Buzsaki, G. Theta oscillations in the hippocampus. Neuron 2002, 33, 325–340. [Google Scholar] [CrossRef]
- Gu, Z.; Alexander, G.M.; Dudek, S.M.; Yakel, J.L. Hippocampus and Entorhinal Cortex Recruit Cholinergic and NMDA Receptors Separately to Generate Hippocampal Theta Oscillations. Cell Rep. 2017, 21, 3585–3595. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.; Smith, K.G.; Alexander, G.M.; Guerreiro, I.; Dudek, S.M.; Gutkin, B.; Jensen, P.; Yakel, J.L. Hippocampal Interneuronal alpha7 nAChRs Modulate Theta Oscillations in Freely Moving Mice. Cell Rep. 2020, 31, 107740. [Google Scholar] [CrossRef] [PubMed]
- Dannenberg, H.; Pabst, M.; Braganza, O.; Schoch, S.; Niediek, J.; Bayraktar, M.; Mormann, F.; Beck, H. Synergy of direct and indirect cholinergic septo-hippocampal pathways coordinates firing in hippocampal networks. J. Neurosci. 2015, 35, 8394–8410. [Google Scholar] [CrossRef] [PubMed]
- Vandecasteele, M.; Varga, V.; Berenyi, A.; Papp, E.; Bartho, P.; Venance, L.; Freund, T.F.; Buzsaki, G. Optogenetic activation of septal cholinergic neurons suppresses sharp wave ripples and enhances theta oscillations in the hippocampus. Proc. Natl. Acad. Sci. USA 2014, 111, 13535–13540. [Google Scholar] [CrossRef] [PubMed]
- Quirk, C.R.; Zutshi, I.; Srikanth, S.; Fu, M.L.; Devico Marciano, N.; Wright, M.K.; Parsey, D.F.; Liu, S.; Siretskiy, R.E.; Huynh, T.L.; et al. Precisely timed theta oscillations are selectively required during the encoding phase of memory. Nat. Neurosci. 2021, 24, 1614–1627. [Google Scholar] [CrossRef] [PubMed]
- King, C.; Recce, M.; O’Keefe, J. The rhythmicity of cells of the medial septum/diagonal band of Broca in the awake freely moving rat: Relationships with behaviour and hippocampal theta. Eur. J. Neurosci. 1998, 10, 464–477. [Google Scholar] [CrossRef]
- Zhang, H.; Lin, S.C.; Nicolelis, M.A. Spatiotemporal coupling between hippocampal acetylcholine release and theta oscillations in vivo. J. Neurosci. 2010, 30, 13431–13440. [Google Scholar] [CrossRef]
- Marrosu, F.; Portas, C.; Mascia, M.S.; Casu, M.A.; Fa, M.; Giagheddu, M.; Imperato, A.; Gessa, G.L. Microdialysis measurement of cortical and hippocampal acetylcholine release during sleep-wake cycle in freely moving cats. Brain Res. 1995, 671, 329–332. [Google Scholar] [CrossRef]
- Ma, X.; Zhang, Y.; Wang, L.; Li, N.; Barkai, E.; Zhang, X.; Lin, L.; Xu, J. The Firing of Theta State-Related Septal Cholinergic Neurons Disrupt Hippocampal Ripple Oscillations via Muscarinic Receptors. J. Neurosci. 2020, 40, 3591–3603. [Google Scholar] [CrossRef] [PubMed]
- Giovannini, M.G.; Rakovska, A.; Benton, R.S.; Pazzagli, M.; Bianchi, L.; Pepeu, G. Effects of novelty and habituation on acetylcholine, GABA, and glutamate release from the frontal cortex and hippocampus of freely moving rats. Neuroscience 2001, 106, 43–53. [Google Scholar] [CrossRef]
- Bianchi, L.; Ballini, C.; Colivicchi, M.A.; Della Corte, L.; Giovannini, M.G.; Pepeu, G. Investigation on acetylcholine, aspartate, glutamate and GABA extracellular levels from ventral hippocampus during repeated exploratory activity in the rat. Neurochem. Res. 2003, 28, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.; Yakel, J.L. Inducing theta oscillations in the entorhinal hippocampal network in vitro. Brain Struct. Funct. 2017, 222, 943–955. [Google Scholar] [CrossRef] [PubMed]
- Stoiljkovic, M.; Kelley, C.; Nagy, D.; Leventhal, L.; Hajos, M. Selective activation of alpha7 nicotinic acetylcholine receptors augments hippocampal oscillations. Neuropharmacology 2016, 110, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Siok, C.J.; Rogers, J.A.; Kocsis, B.; Hajos, M. Activation of alpha7 acetylcholine receptors augments stimulation-induced hippocampal theta oscillation. Eur. J. Neurosci. 2006, 23, 570–574. [Google Scholar] [CrossRef] [PubMed]
- Cobb, S.R.; Bulters, D.O.; Suchak, S.; Riedel, G.; Morris, R.G.; Davies, C.H. Activation of nicotinic acetylcholine receptors patterns network activity in the rodent hippocampus. J. Physiol. 1999, 518, 131–140. [Google Scholar] [CrossRef]
- Letsinger, A.C.; Gu, Z.; Yakel, J.L. alpha7 nicotinic acetylcholine receptors in the hippocampal circuit: Taming complexity. Trends Neurosci. 2022, 45, 145–157. [Google Scholar] [CrossRef]
- Haam, J.; Zhou, J.; Cui, G.; Yakel, J.L. Septal cholinergic neurons gate hippocampal output to entorhinal cortex via oriens lacunosum moleculare interneurons. Proc. Natl. Acad. Sci. USA 2018, 115, E1886–E1895. [Google Scholar] [CrossRef]
- Siwani, S.; Franca, A.S.C.; Mikulovic, S.; Reis, A.; Hilscher, M.M.; Edwards, S.J.; Leao, R.N.; Tort, A.B.L.; Kullander, K. OLMalpha2 Cells Bidirectionally Modulate Learning. Neuron 2018, 99, 404–412. [Google Scholar] [CrossRef]
- Sekulic, V.; Yi, F.; Garrett, T.; Guet-McCreight, A.; Lawrence, J.J.; Skinner, F.K. Integration of Within-Cell Experimental Data With Multi-Compartmental Modeling Predicts H-Channel Densities and Distributions in Hippocampal OLM Cells. Front. Cell Neurosci. 2020, 14, 277. [Google Scholar] [CrossRef] [PubMed]
- Salimi-Nezhad, N.; Hasanlou, M.; Amiri, M.; Keliris, G.A. A neuromimetic realization of hippocampal CA1 for theta wave generation. Neural Netw. 2021, 142, 548–563. [Google Scholar] [CrossRef] [PubMed]
- Leao, R.N.; Mikulovic, S.; Leao, K.E.; Munguba, H.; Gezelius, H.; Enjin, A.; Patra, K.; Eriksson, A.; Loew, L.M.; Tort, A.B.; et al. OLM interneurons differentially modulate CA3 and entorhinal inputs to hippocampal CA1 neurons. Nat. Neurosci. 2012, 15, 1524–1530. [Google Scholar] [CrossRef]
- Mikulovic, S.; Restrepo, C.E.; Siwani, S.; Bauer, P.; Pupe, S.; Tort, A.B.L.; Kullander, K.; Leao, R.N. Ventral hippocampal OLM cells control type 2 theta oscillations and response to predator odor. Nat. Commun. 2018, 9, 3638. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Neville, K.R.; Goldstein, N.; Kabu, S.; Kausar, N.; Ye, R.; Nguyen, T.T.; Gelwan, N.; Hyman, B.T.; Gomperts, S.N. Cholinergic modulation of hippocampal calcium activity across the sleep-wake cycle. eLife 2019, 8, e39777. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.D.; Bolstad, M.; Lee, A.K. Experience-dependent shaping of hippocampal CA1 intracellular activity in novel and familiar environments. eLife 2017, 6, e23040. [Google Scholar] [CrossRef]
- Dragoi, G.; Buzsaki, G. Temporal encoding of place sequences by hippocampal cell assemblies. Neuron 2006, 50, 145–157. [Google Scholar] [CrossRef]
- Pastalkova, E.; Itskov, V.; Amarasingham, A.; Buzsaki, G. Internally generated cell assembly sequences in the rat hippocampus. Science 2008, 321, 1322–1327. [Google Scholar] [CrossRef]
- Venditto, S.J.C.; Le, B.; Newman, E.L. Place cell assemblies remain intact, despite reduced phase precession, after cholinergic disruption. Hippocampus 2019, 29, 1075–1090. [Google Scholar] [CrossRef]
- Newman, E.L.; Venditto, S.J.C.; Climer, J.R.; Petter, E.A.; Gillet, S.N.; Levy, S. Precise spike timing dynamics of hippocampal place cell activity sensitive to cholinergic disruption. Hippocampus 2017, 27, 1069–1082. [Google Scholar] [CrossRef]
- Schlesiger, M.I.; Cannova, C.C.; Boublil, B.L.; Hales, J.B.; Mankin, E.A.; Brandon, M.P.; Leutgeb, J.K.; Leibold, C.; Leutgeb, S. The medial entorhinal cortex is necessary for temporal organization of hippocampal neuronal activity. Nat. Neurosci. 2015, 18, 1123–1132. [Google Scholar] [CrossRef] [PubMed]
- Feng, T.; Silva, D.; Foster, D.J. Dissociation between the experience-dependent development of hippocampal theta sequences and single-trial phase precession. J. Neurosci. 2015, 35, 4890–4902. [Google Scholar] [CrossRef] [PubMed]
- Dragoi, G.; Tonegawa, S. Distinct preplay of multiple novel spatial experiences in the rat. Proc. Natl. Acad. Sci. USA 2013, 110, 9100–9105. [Google Scholar] [CrossRef] [PubMed]
- Dragoi, G.; Tonegawa, S. Preplay of future place cell sequences by hippocampal cellular assemblies. Nature 2011, 469, 397–401. [Google Scholar] [CrossRef]
- Rogers, J.L.; Kesner, R.P. Cholinergic modulation of the hippocampus during encoding and retrieval. Neurobiol. Learn. Mem. 2003, 80, 332–342. [Google Scholar] [CrossRef]
- Yoder, R.M.; Pang, K.C. Involvement of GABAergic and cholinergic medial septal neurons in hippocampal theta rhythm. Hippocampus 2005, 15, 381–392. [Google Scholar] [CrossRef]
- Bland, B.H.; Oddie, S.D.; Colom, L.V. Mechanisms of neural synchrony in the septohippocampal pathways underlying hippocampal theta generation. J. Neurosci. 1999, 19, 3223–3237. [Google Scholar] [CrossRef]
- Lee, M.G.; Chrobak, J.J.; Sik, A.; Wiley, R.G.; Buzsaki, G. Hippocampal theta activity following selective lesion of the septal cholinergic system. Neuroscience 1994, 62, 1033–1047. [Google Scholar] [CrossRef]
- Simon, A.P.; Poindessous-Jazat, F.; Dutar, P.; Epelbaum, J.; Bassant, M.H. Firing properties of anatomically identified neurons in the medial septum of anesthetized and unanesthetized restrained rats. J. Neurosci. 2006, 26, 9038–9046. [Google Scholar] [CrossRef]
- Sotty, F.; Danik, M.; Manseau, F.; Laplante, F.; Quirion, R.; Williams, S. Distinct electrophysiological properties of glutamatergic, cholinergic and GABAergic rat septohippocampal neurons: Novel implications for hippocampal rhythmicity. J. Physiol. 2003, 551, 927–943. [Google Scholar] [CrossRef]
- Lawson, V.H.; Bland, B.H. The role of the septohippocampal pathway in the regulation of hippocampal field activity and behavior: Analysis by the intraseptal microinfusion of carbachol, atropine, and procaine. Exp. Neurol. 1993, 120, 132–144. [Google Scholar] [CrossRef] [PubMed]
- Broncel, A.; Bocian, R.; Klos-Wojtczak, P.; Konopacki, J. Medial septal cholinergic mediation of hippocampal theta rhythm induced by vagal nerve stimulation. PLoS ONE 2018, 13, e0206532. [Google Scholar] [CrossRef] [PubMed]
- Oddie, S.D.; Bland, B.H. Hippocampal formation theta activity and movement selection. Neurosci. Biobehav. Rev. 1998, 22, 221–231. [Google Scholar] [CrossRef]
- Golebiewski, H.; Eckersdorf, B.; Konopacki, J. Septal cholinergic mediation of hippocampal theta in the cat. Brain Res. Bull. 2002, 58, 323–335. [Google Scholar] [CrossRef]
- Li, S.; Topchiy, I.; Kocsis, B. The effect of atropine administered in the medial septum or hippocampus on high- and low-frequency theta rhythms in the hippocampus of urethane anesthetized rats. Synapse 2007, 61, 412–419. [Google Scholar] [CrossRef]
- Smythe, J.W.; Colom, L.V.; Bland, B.H. The extrinsic modulation of hippocampal theta depends on the coactivation of cholinergic and GABA-ergic medial septal inputs. Neurosci. Biobehav. Rev. 1992, 16, 289–308. [Google Scholar] [CrossRef]
- Manseau, F.; Goutagny, R.; Danik, M.; Williams, S. The hippocamposeptal pathway generates rhythmic firing of GABAergic neurons in the medial septum and diagonal bands: An investigation using a complete septohippocampal preparation in vitro. J. Neurosci. 2008, 28, 4096–4107. [Google Scholar] [CrossRef]
- Robinson, J.; Manseau, F.; Ducharme, G.; Amilhon, B.; Vigneault, E.; El Mestikawy, S.; Williams, S. Optogenetic Activation of Septal Glutamatergic Neurons Drive Hippocampal Theta Rhythms. J. Neurosci. 2016, 36, 3016–3023. [Google Scholar] [CrossRef]
- Ibrahim, K.M.; Ariffin, M.Z.; Khanna, S. Modulation of Septo-Hippocampal Neural Responses in Anesthetized and Behaving Rats by Septal AMPA Receptor Mechanisms. Front. Neural Circuits 2021, 15, 663633. [Google Scholar] [CrossRef]
- Manseau, F.; Danik, M.; Williams, S. A functional glutamatergic neurone network in the medial septum and diagonal band area. J. Physiol. 2005, 566, 865–884. [Google Scholar] [CrossRef]
- Puma, C.; Bizot, J.C. Hippocampal theta rhythm in anesthetized rats: Role of AMPA glutamate receptors. Neuroreport 1999, 10, 2297–2300. [Google Scholar] [CrossRef] [PubMed]
- Fuhrmann, F.; Justus, D.; Sosulina, L.; Kaneko, H.; Beutel, T.; Friedrichs, D.; Schoch, S.; Schwarz, M.K.; Fuhrmann, M.; Remy, S. Locomotion, Theta Oscillations, and the Speed-Correlated Firing of Hippocampal Neurons Are Controlled by a Medial Septal Glutamatergic Circuit. Neuron 2015, 86, 1253–1264. [Google Scholar] [CrossRef] [PubMed]
- Jeewajee, A.; Lever, C.; Burton, S.; O’Keefe, J.; Burgess, N. Environmental novelty is signaled by reduction of the hippocampal theta frequency. Hippocampus 2008, 18, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Wells, C.E.; Amos, D.P.; Jeewajee, A.; Douchamps, V.; Rodgers, J.; O’Keefe, J.; Burgess, N.; Lever, C. Novelty and anxiolytic drugs dissociate two components of hippocampal theta in behaving rats. J. Neurosci. 2013, 33, 8650–8667. [Google Scholar] [CrossRef] [PubMed]
- Jeewajee, A.; Barry, C.; O’Keefe, J.; Burgess, N. Grid cells and theta as oscillatory interference: Electrophysiological data from freely moving rats. Hippocampus 2008, 18, 1175–1185. [Google Scholar] [CrossRef] [PubMed]
- Bush, D.; Bisby, J.A.; Bird, C.M.; Gollwitzer, S.; Rodionov, R.; Diehl, B.; McEvoy, A.W.; Walker, M.C.; Burgess, N. Human hippocampal theta power indicates movement onset and distance travelled. Proc. Natl. Acad. Sci. USA 2017, 114, 12297–12302. [Google Scholar] [CrossRef] [PubMed]
- Kropff, E.; Carmichael, J.E.; Moser, E.I.; Moser, M.B. Frequency of theta rhythm is controlled by acceleration, but not speed, in running rats. Neuron 2021, 109, 1029–1039. [Google Scholar] [CrossRef]
- Newman, E.L.; Gillet, S.N.; Climer, J.R.; Hasselmo, M.E. Cholinergic blockade reduces theta-gamma phase amplitude coupling and speed modulation of theta frequency consistent with behavioral effects on encoding. J. Neurosci. 2013, 33, 19635–19646. [Google Scholar] [CrossRef]
- Givens, B.; Olton, D.S. Bidirectional modulation of scopolamine-induced working memory impairments by muscarinic activation of the medial septal area. Neurobiol. Learn. Mem. 1995, 63, 269–276. [Google Scholar] [CrossRef][Green Version]
- Thiel, C.M.; Huston, J.P.; Schwarting, R.K. Hippocampal acetylcholine and habituation learning. Neuroscience 1998, 85, 1253–1262. [Google Scholar] [CrossRef]
- Korotkova, T.; Ponomarenko, A.; Monaghan, C.K.; Poulter, S.L.; Cacucci, F.; Wills, T.; Hasselmo, M.E.; Lever, C. Reconciling the different faces of hippocampal theta: The role of theta oscillations in cognitive, emotional and innate behaviors. Neurosci. Biobehav. Rev. 2018, 85, 65–80. [Google Scholar] [CrossRef] [PubMed]
- Colgin, L.L. Rhythms of the hippocampal network. Nat. Rev. Neurosci. 2016, 17, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Belluscio, M.A.; Mizuseki, K.; Schmidt, R.; Kempter, R.; Buzsaki, G. Cross-frequency phase-phase coupling between theta and gamma oscillations in the hippocampus. J. Neurosci. 2012, 32, 423–435. [Google Scholar] [CrossRef] [PubMed]
- Schomburg, E.W.; Fernandez-Ruiz, A.; Mizuseki, K.; Berenyi, A.; Anastassiou, C.A.; Koch, C.; Buzsaki, G. Theta phase segregation of input-specific gamma patterns in entorhinal-hippocampal networks. Neuron 2014, 84, 470–485. [Google Scholar] [CrossRef]
- Hasselmo, M.E.; Bodelon, C.; Wyble, B.P. A proposed function for hippocampal theta rhythm: Separate phases of encoding and retrieval enhance reversal of prior learning. Neural Comput. 2002, 14, 793–817. [Google Scholar] [CrossRef]
- Colgin, L.L.; Denninger, T.; Fyhn, M.; Hafting, T.; Bonnevie, T.; Jensen, O.; Moser, M.B.; Moser, E.I. Frequency of gamma oscillations routes flow of information in the hippocampus. Nature 2009, 462, 353–357. [Google Scholar] [CrossRef]
- Lisman, J.E.; Jensen, O. The theta-gamma neural code. Neuron 2013, 77, 1002–1016. [Google Scholar] [CrossRef]
- Colgin, L.L. Theta-gamma coupling in the entorhinal-hippocampal system. Curr. Opin. Neurobiol. 2015, 31, 45–50. [Google Scholar] [CrossRef]
- Tort, A.B.; Komorowski, R.W.; Manns, J.R.; Kopell, N.J.; Eichenbaum, H. Theta-gamma coupling increases during the learning of item-context associations. Proc. Natl. Acad. Sci. USA 2009, 106, 20942–20947. [Google Scholar] [CrossRef]
- Tort, A.B.; Kramer, M.A.; Thorn, C.; Gibson, D.J.; Kubota, Y.; Graybiel, A.M.; Kopell, N.J. Dynamic cross-frequency couplings of local field potential oscillations in rat striatum and hippocampus during performance of a T-maze task. Proc. Natl. Acad. Sci. USA 2008, 105, 20517–20522. [Google Scholar] [CrossRef]
- Axmacher, N.; Henseler, M.M.; Jensen, O.; Weinreich, I.; Elger, C.E.; Fell, J. Cross-frequency coupling supports multi-item working memory in the human hippocampus. Proc. Natl. Acad. Sci. USA 2010, 107, 3228–3233. [Google Scholar] [CrossRef] [PubMed]
- Goodman, M.S.; Kumar, S.; Zomorrodi, R.; Ghazala, Z.; Cheam, A.S.M.; Barr, M.S.; Daskalakis, Z.J.; Blumberger, D.M.; Fischer, C.; Flint, A.; et al. Theta-Gamma Coupling and Working Memory in Alzheimer’s Dementia and Mild Cognitive Impairment. Front. Aging Neurosci. 2018, 10, 101. [Google Scholar] [CrossRef] [PubMed]
- Musaeus, C.S.; Nielsen, M.S.; Musaeus, J.S.; Hogh, P. Electroencephalographic Cross-Frequency Coupling as a Sign of Disease Progression in Patients With Mild Cognitive Impairment: A Pilot Study. Front. Neurosci. 2020, 14, 790. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, T.K.; Howe, M.D.; Schmidt, B.; Hinman, J.R.; Escabi, M.A.; Markus, E.J. Hippocampal theta, gamma, and theta-gamma coupling: Effects of aging, environmental change, and cholinergic activation. J. Neurophysiol. 2013, 109, 1852–1865. [Google Scholar] [CrossRef]
- Goutagny, R.; Gu, N.; Cavanagh, C.; Jackson, J.; Chabot, J.G.; Quirion, R.; Krantic, S.; Williams, S. Alterations in hippocampal network oscillations and theta-gamma coupling arise before Abeta overproduction in a mouse model of Alzheimer’s disease. Eur. J. Neurosci. 2013, 37, 1896–1902. [Google Scholar] [CrossRef]
- Hentschke, H.; Perkins, M.G.; Pearce, R.A.; Banks, M.I. Muscarinic blockade weakens interaction of gamma with theta rhythms in mouse hippocampus. Eur. J. Neurosci. 2007, 26, 1642–1656. [Google Scholar] [CrossRef]
- Lasztoczi, B.; Klausberger, T. Layer-specific GABAergic control of distinct gamma oscillations in the CA1 hippocampus. Neuron 2014, 81, 1126–1139. [Google Scholar] [CrossRef]
- Howe, W.M.; Gritton, H.J.; Lusk, N.A.; Roberts, E.A.; Hetrick, V.L.; Berke, J.D.; Sarter, M. Acetylcholine Release in Prefrontal Cortex Promotes Gamma Oscillations and Theta-Gamma Coupling during Cue Detection. J. Neurosci. 2017, 37, 3215–3230. [Google Scholar] [CrossRef]
- Yang, Y.; Gritton, H.; Sarter, M.; Aton, S.J.; Booth, V.; Zochowski, M. Theta-gamma coupling emerges from spatially heterogeneous cholinergic neuromodulation. PLoS Comput. Biol. 2021, 17, e1009235. [Google Scholar] [CrossRef]
- Penzo, M.A.; Robert, V.; Tucciarone, J.; De Bundel, D.; Wang, M.; Van Aelst, L.; Darvas, M.; Parada, L.F.; Palmiter, R.D.; He, M.; et al. The paraventricular thalamus controls a central amygdala fear circuit. Nature 2015, 519, 455–459. [Google Scholar] [CrossRef]
- Daigle, T.L.; Madisen, L.; Hage, T.A.; Valley, M.T.; Knoblich, U.; Larsen, R.S.; Takeno, M.M.; Huang, L.; Gu, H.; Larsen, R.; et al. A Suite of Transgenic Driver and Reporter Mouse Lines with Enhanced Brain-Cell-Type Targeting and Functionality. Cell 2018, 174, 465–480. [Google Scholar] [CrossRef] [PubMed]
| Type I Theta | Type II Theta | |
|---|---|---|
| Occurrence | Active exploration | Urethane anesthesia; alert immobility |
| Theta frequency | 6–12 Hz | 4–9 Hz |
| Atropine dependence | Atropine-resistant | Atropine sensitive |
| MS-DBB dependence | Yes | Yes |
| EC dependence | Yes | No |
| NMDAR dependence | Yes | No |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, Z.; Yakel, J.L. Cholinergic Regulation of Hippocampal Theta Rhythm. Biomedicines 2022, 10, 745. https://doi.org/10.3390/biomedicines10040745
Gu Z, Yakel JL. Cholinergic Regulation of Hippocampal Theta Rhythm. Biomedicines. 2022; 10(4):745. https://doi.org/10.3390/biomedicines10040745
Chicago/Turabian StyleGu, Zhenglin, and Jerrel L. Yakel. 2022. "Cholinergic Regulation of Hippocampal Theta Rhythm" Biomedicines 10, no. 4: 745. https://doi.org/10.3390/biomedicines10040745
APA StyleGu, Z., & Yakel, J. L. (2022). Cholinergic Regulation of Hippocampal Theta Rhythm. Biomedicines, 10(4), 745. https://doi.org/10.3390/biomedicines10040745






