In Vivo Efficacy of SQ109 against Leishmania donovani, Trypanosoma spp. and Toxoplasma gondii and In Vitro Activity of SQ109 Metabolites
Abstract
1. Introduction
2. Materials and Methods
2.1. Compounds
2.2. In Vitro Activity Assay for T. brucei
2.3. In Vitro Activity Assay for T. cruzi
2.3.1. Tissue Culture Trypomastigotes Assay
2.3.2. Epimastigotes Assay
2.3.3. Intracellular Amastigotes Assay
2.4. In Vitro Activity Assays for L. donovani
2.4.1. Promastigotes Assay
2.4.2. Intracellular Amastigotes Assay
2.5. In Vitro Activity Assay for T. gondii
2.6. T. brucei in Vivo Model and Drug Efficacy Test
2.7. T. cruzi In Vivo Model and Drug Efficacy Test
2.8. L. donovani In Vivo Model and Drug Efficacy Test
2.9. T. gondii In Vivo Model and Drug Efficacy Test
2.10. Statistical Analysis
2.11. Ethics
3. Results
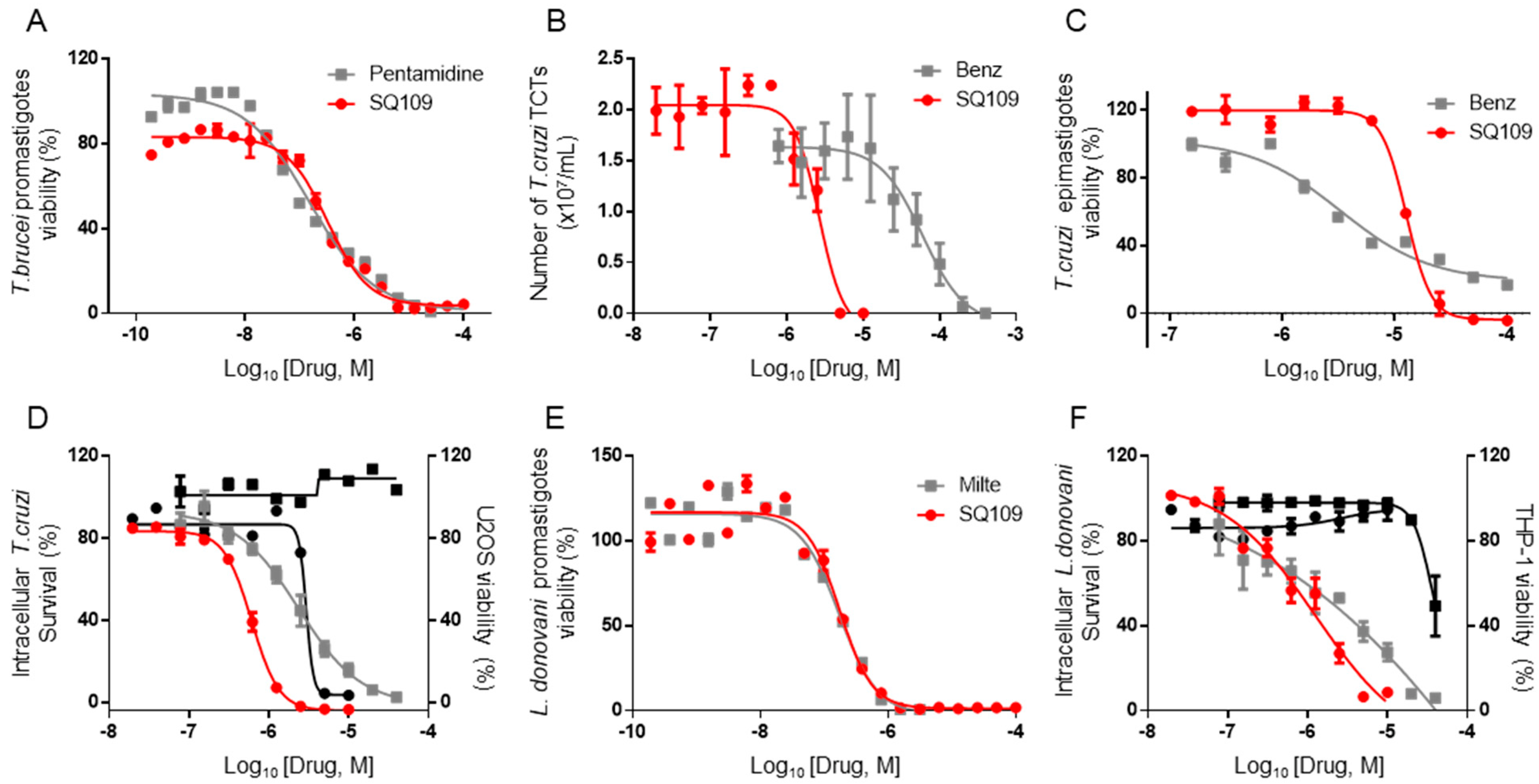
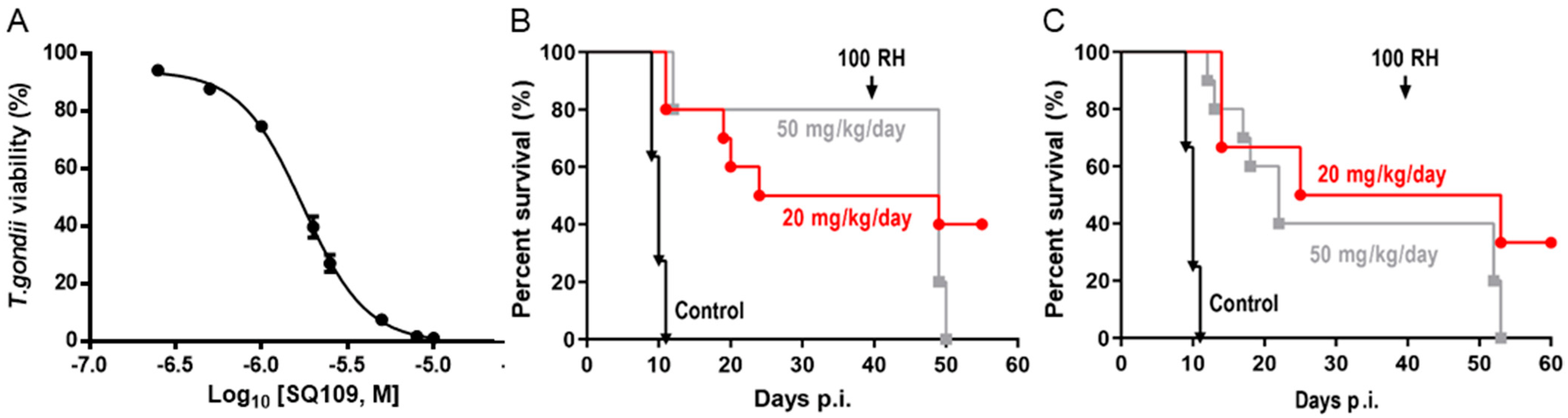
3.1. In Vitro Activity of SQ109 against T. brucei, T. cruzi, and L. donovani
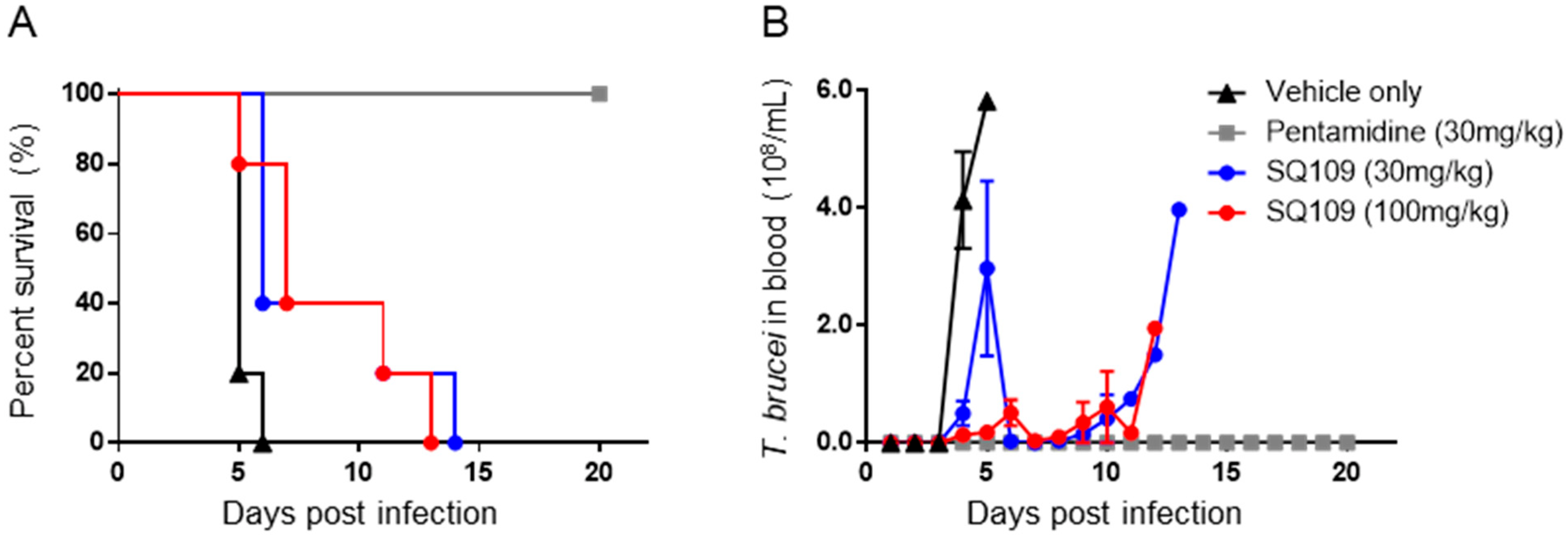
3.2. In Vivo Activity of SQ109 against T. brucei
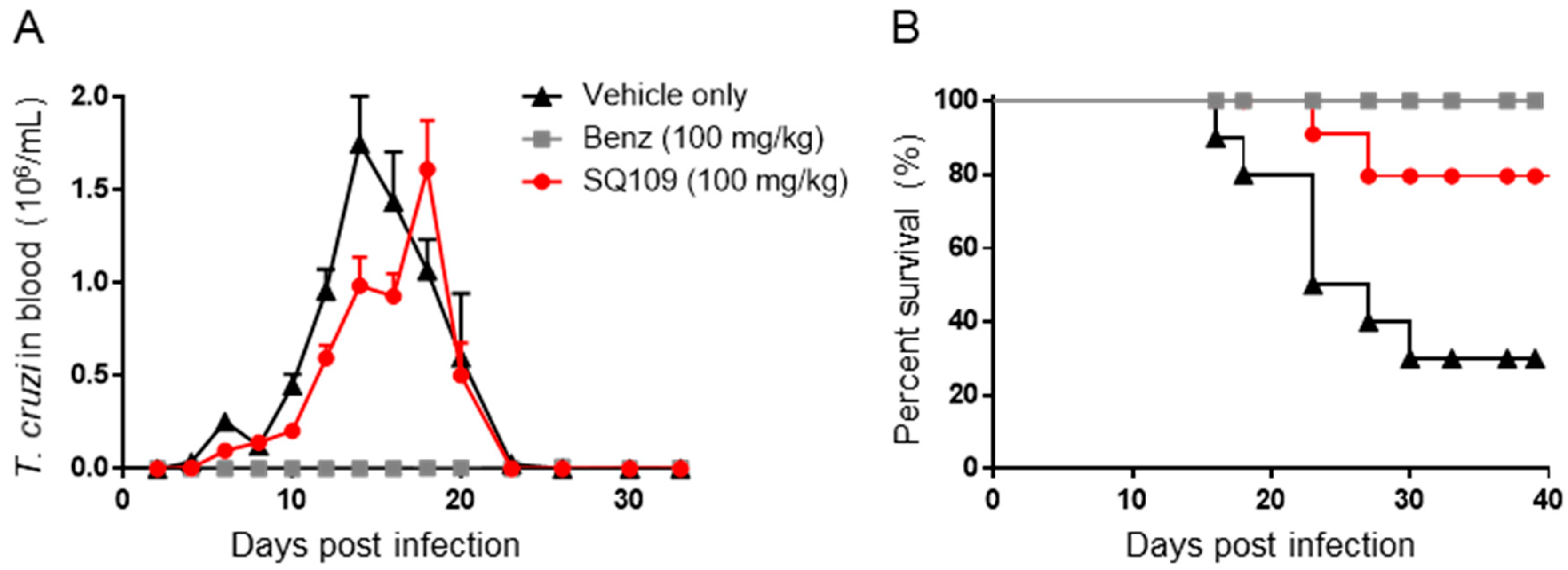
3.3. In Vivo Activity of SQ109 against T. cruzi
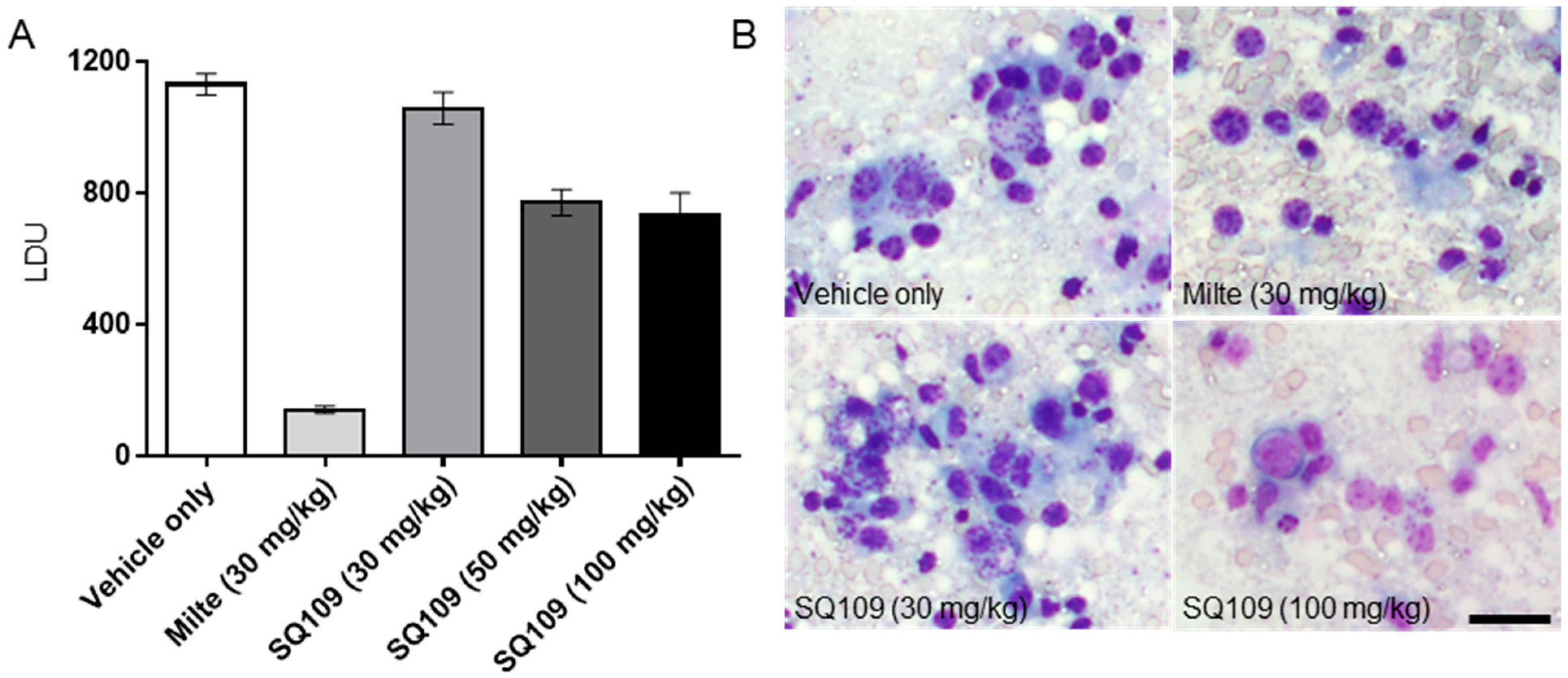
3.4. In Vivo Activity of SQ109 against L. donovani
3.5. In Vitro and In Vivo Activity of SQ109 against T. gondii
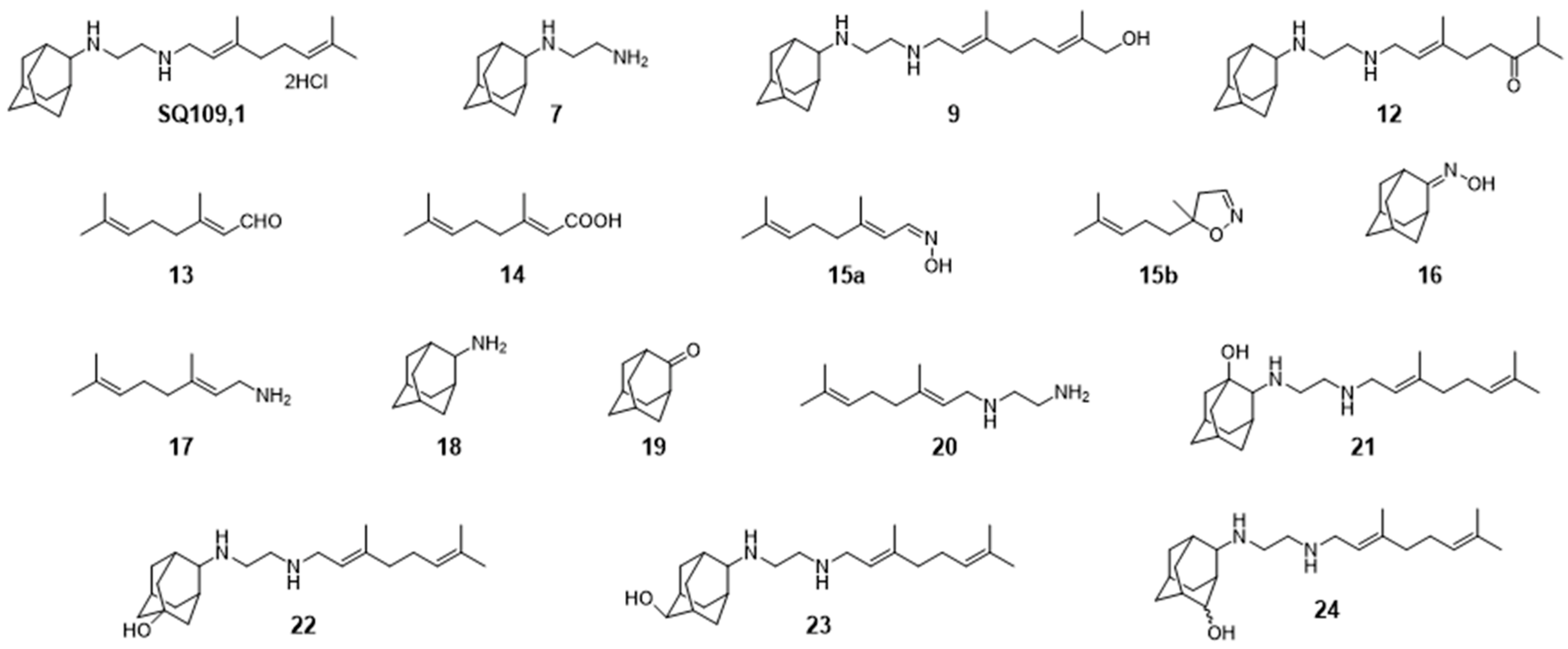
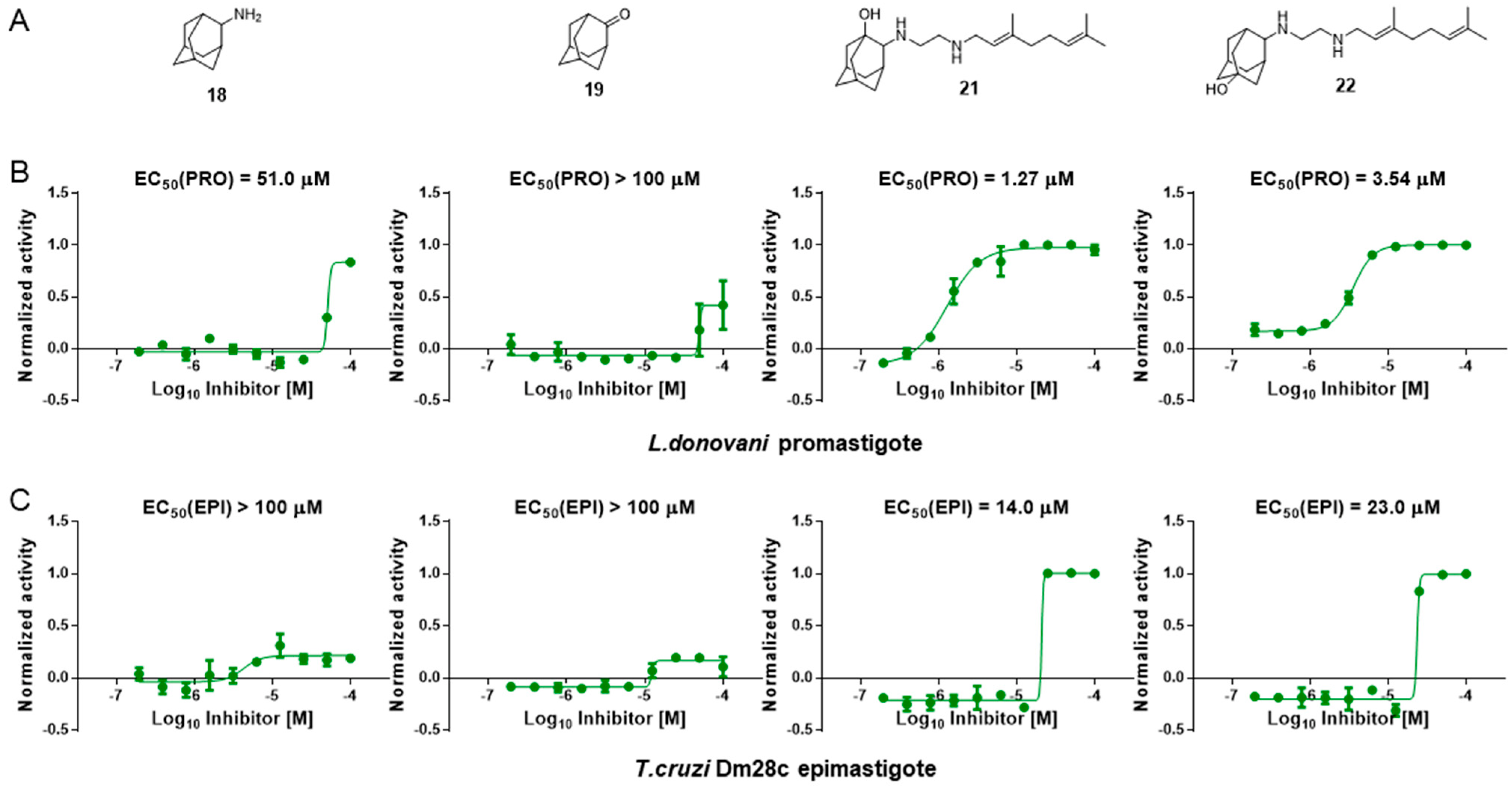
3.6. Activity of SQ109 Metabolites in Protozoa
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Andrews, K.T.; Fisher, G.; Skinner-Adams, T.S. Drug repurposing and human parasitic protozoan diseases. Int. J. Parasitol. Drugs Drug Resist. 2014, 4, 95–111. [Google Scholar] [CrossRef] [PubMed]
- Charlton, R.L.; Rossi-Bergmann, B.; Denny, P.; Steel, P.G. Repurposing as a strategy for the discovery of new anti-leishmanials: The-state-of-the-art. Parasitology 2017, 145, 219–236. [Google Scholar] [CrossRef] [PubMed]
- Deeks, E.D. Fexinidazole: First Global Approval. Drugs 2019, 79, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Vermelho, A.B.; Rodrigues, G.C.; Supuran, C.T. Why hasn’t there been more progress in new Chagas disease drug discovery? Expert Opin. Drug Discov. 2019, 15, 145–158. [Google Scholar] [CrossRef]
- Benaim, G.; Sanders, J.M.; Garcia-Marchán, Y.; Colina, C.; Lira, R.; Caldera, A.R.; Payares, G.; Sanoja, C.; Burgos, J.M.; Leon-Rossell, A.; et al. Amiodarone Has Intrinsic Anti-Trypanosoma cruzi Activity and Acts Synergistically with Posaconazole. J. Med. Chem. 2006, 49, 892–899. [Google Scholar] [CrossRef]
- Madigan, R.; Majoy, S.; Ritter, K.; Concepción, J.L.; Márquez, M.E.; Silva, S.C.; Zao, C.-L.; Alvarez, A.P.; Rodriguez-Morales, A.J.; Mogollón-Mendoza, A.C.; et al. Investigation of a combination of amiodarone and itraconazole for treatment of American trypanosomiasis (Chagas disease) in dogs. J. Am. Veter- Med. Assoc. 2019, 255, 317–329. [Google Scholar] [CrossRef]
- Benaim, G.; Paniz-Mondolfi, A.E.; Sordillo, E.M.; Martinez-Sotillo, N. Disruption of Intracellular Calcium Homeostasis as a Therapeutic Target against Trypanosoma cruzi. Front. Cell. Infect. Microbiol. 2020, 10, 46. [Google Scholar] [CrossRef]
- Li, K.; Wang, Y.; Yang, G.; Byun, S.; Rao, G.; Shoen, C.; Yang, H.; Gulati, A.; Crick, D.C.; Cynamon, M.; et al. Oxa, Thia, Heterocycle, and Carborane Analogues of SQ109: Bacterial and Protozoal Cell Growth Inhibitors. ACS Infect. Dis. 2015, 1, 215–221. [Google Scholar] [CrossRef]
- García-García, V.; Oldfield, E.; Benaim, G. Inhibition of Leishmania mexicana Growth by the Tuberculosis Drug SQ109. Antimicrob. Agents Chemother. 2016, 60, 6386–6389. [Google Scholar] [CrossRef]
- Gil, Z.; Martinez-Sotillo, N.; Pinto-Martinez, A.; Mejias, F.; Martinez, J.C.; Galindo, I.; Oldfield, E.; Benaim, G. SQ109 inhibits proliferation of Leishmania donovani by disruption of intracellular Ca2+ homeostasis, collapsing the mitochondrial electrochemical potential (ΔΨm) and affecting acidocalcisomes. Parasitol. Res. 2020, 119, 649–657. [Google Scholar] [CrossRef]
- Veiga-Santos, P.; Li, K.; Lameira, L.; de Carvalho, T.M.U.; Huang, G.; Galizzi, M.; Shang, N.; Li, Q.; Gonzalez-Pacanowska, D.; Hernandez-Rodriguez, V.; et al. SQ109, a New Drug Lead for Chagas Disease. Antimicrob. Agents Chemother. 2015, 59, 1950–1961. [Google Scholar] [CrossRef]
- Li, K.; Schurig-Briccio, L.A.; Feng, X.; Upadhyay, A.; Pujari, V.; Lechartier, B.; Fontes, F.L.; Yang, H.; Rao, G.; Zhu, W.; et al. Multitarget Drug Discovery for Tuberculosis and Other Infectious Diseases. J. Med. Chem. 2014, 57, 3126–3139. [Google Scholar] [CrossRef]
- Reader, J.; van der Watt, M.E.; Taylor, D.; Le Manach, C.; Mittal, N.; Ottilie, S.; Theron, A.; Moyo, P.; Erlank, E.; Nardini, L.; et al. Multistage and transmission-blocking targeted antimalarials discovered from the open-source MMV Pandemic Response Box. Nat. Commun. 2021, 12, 269. [Google Scholar] [CrossRef]
- Yang, G.; Zhu, W.; Wang, Y.; Huang, G.; Byun, S.Y.; Choi, G.; Li, K.; Huang, Z.; Docampo, R.; Oldfield, E.; et al. In Vitro and in Vivo Activity of Multitarget Inhibitors against Trypanosoma brucei. ACS Infect. Dis. 2015, 1, 388–398. [Google Scholar] [CrossRef]
- Perez-Molina, J.A.; Perez-Ayala, A.; Moreno, S.; Fernández-González, M.C.; Zamora, J.; López-Velez, R. Use of benznidazole to treat chronic Chagas’ disease: A systematic review with a meta-analysis. J. Antimicrob. Chemother. 2009, 64, 1139–1147. [Google Scholar] [CrossRef]
- Vermeersch, M.; da Luz, R.I.; Toté, K.; Timmermans, J.-P.; Cos, P.; Maes, L. In Vitro Susceptibilities of Leishmania donovani Promastigote and Amastigote Stages to Antileishmanial Reference Drugs: Practical Relevance of Stage-Specific Differences. Antimicrob. Agents Chemother. 2009, 53, 3855–3859. [Google Scholar] [CrossRef]
- Bowling, T.; Mercer, L.; Don, R.; Jacobs, R.; Nare, B. Application of a resazurin-based high-throughput screening assay for the identification and progression of new treatments for human African trypanosomiasis. Int. J. Parasitol. Drugs Drug Resist. 2012, 2, 262–270. [Google Scholar] [CrossRef]
- Yang, G.; Lee, N.; Ioset, J.-R.; No, J.H. Evaluation of Parameters Impacting Drug Susceptibility in Intracellular Trypanosoma cruzi Assay Protocols. SLAS Discov. Adv. Sci. Drug Discov. 2016, 22, 125–134. [Google Scholar] [CrossRef]
- Monteiro, A.C.; Schmitz, V.; Morrot, A.; De Arruda, L.B.; Nagajyothi, F.; Granato, A.; Pesquero, J.B.; Müller-Esterl, W.; Tanowitz, H.B.; Scharfstein, J. Bradykinin B2 Receptors of Dendritic Cells, Acting as Sensors of Kinins Proteolytically Released by Trypanosoma cruzi, Are Critical for the Development of Protective Type-1 Responses. PLOS Pathog. 2007, 3, e185. [Google Scholar] [CrossRef]
- Baek, K.-H.; Piel, L.; Rosazza, T.; Prina, E.; Späth, G.F.; No, J.H. Infectivity and Drug Susceptibility Profiling of Different Leishmania-Host Cell Combinations. Pathogens 2020, 9, 393. [Google Scholar] [CrossRef]
- Pescher, P.; Blisnick, T.; Bastin, P.; Späth, G.F. Quantitative proteome profiling informs on phenotypic traits that adapt Leishmania donovani for axenic and intracellular proliferation. Cell. Microbiol. 2011, 13, 978–991. [Google Scholar] [CrossRef] [PubMed]
- Barja, P.P.; Pescher, P.; Bussotti, G.; Dumetz, F.; Imamura, H.; Kedra, D.; Domagalska, M.; Chaumeau, V.; Himmelbauer, H.; Pages, M.; et al. Haplotype selection as an adaptive mechanism in the protozoan pathogen Leishmania donovani. Nat. Ecol. Evol. 2017, 1, 1961–1969. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.-H.; Li, C.; Szajnman, S.H.; Rodriguez, J.B.; Moreno, S.N.J. Synergistic Activity between Statins and Bisphosphonates against Acute Experimental Toxoplasmosis. Antimicrob. Agents Chemother. 2017, 61. [Google Scholar] [CrossRef] [PubMed]
- Szajnman, S.H.; Galaka, T.; Li, Z.-H.; Li, C.; Howell, N.M.; Chao, M.N.; Striepen, B.; Muralidharan, V.; Moreno, S.N.J.; Rodriguez, J.B. In Vitro and In Vivo Activities of Sulfur-Containing Linear Bisphosphonates against Apicomplexan Parasites. Antimicrob. Agents Chemother. 2017, 61. [Google Scholar] [CrossRef]
- Phan, T.-N.; Baek, K.-H.; Lee, N.; Byun, S.Y.; Shum, D.; No, J.H. In Vitro and in Vivo Activity of mTOR Kinase and PI3K Inhibitors Against Leishmania donovani and Trypanosoma brucei. Molecules 2020, 25, 1980. [Google Scholar] [CrossRef]
- Malwal, S.R.; Zimmerman, M.D.; Alvarez, N.; Sarathy, J.P.; Dartois, V.; Nacy, C.A.; Oldfield, E. Structure, In Vivo Detection, and Antibacterial Activity of Metabolites of SQ109, an Anti-Infective Drug Candidate. ACS Infect. Dis. 2021, 7, 2492–2507. [Google Scholar] [CrossRef]
- Egbelowo, O.; Sarathy, J.P.; Gausi, K.; Zimmerman, M.D.; Wang, H.; Wijnant, G.-J.; Kaya, F.; Gengenbacher, M.; Van, N.; Degefu, Y.; et al. Pharmacokinetics and Target Attainment of SQ109 in Plasma and Human-Like Tuberculosis Lesions in Rabbits. Antimicrob. Agents Chemother. 2021, 65, AAC0002421. [Google Scholar] [CrossRef]
- Seifert, K.; Escobar, P.; Croft, S.L. In vitro activity of anti-leishmanial drugs against Leishmania donovani is host cell dependent. J. Antimicrob. Chemother. 2010, 65, 508–511. [Google Scholar] [CrossRef]
- Protopopova, M.; Hanrahan, C.; Nikonenko, B.; Samala, R.; Chen, P.; Gearhart, J.; Einck, L.; Nacy, C.A. Identification of a new antitubercular drug candidate, SQ109, from a combinatorial library of 1,2-ethylenediamines. J. Antimicrob. Chemother. 2005, 56, 968–974. [Google Scholar] [CrossRef]
- Jia, L.; Tomaszewski, J.E.; Hanrahan, C.; Coward, L.; Noker, P.; Gorman, G.; Nikonenko, B.; Protopopova, M. Pharmacodynamics and pharmacokinetics of SQ109, a new diamine-based antitubercular drug. J. Cereb. Blood Flow Metab. 2005, 144, 80–87. [Google Scholar] [CrossRef]
- Jia, L.; Noker, P.E.; Coward, L.; Gorman, G.S.; Protopopova, M.; Tomaszewski, J.E. Interspecies pharmacokinetics and in vitro metabolism of SQ109. J. Cereb. Blood Flow Metab. 2006, 147, 476–485. [Google Scholar] [CrossRef]
- Bukhdruker, S.; Varaksa, T.; Grabovec, I.; Marin, E.; Shabunya, P.; Kadukova, M.; Grudinin, S.; Kavaleuski, A.; Gusach, A.; Gilep, A.; et al. Hydroxylation of Antitubercular Drug Candidate, SQ109, by Mycobacterial Cytochrome P450. Int. J. Mol. Sci. 2020, 21, 7683. [Google Scholar] [CrossRef]
- Chen, P.; Gearhart, J.; Protopopova, M.; Einck, L.; Nacy, C.A. Synergistic interactions of SQ109, a new ethylene diamine, with front-line antitubercular drugs in vitro. J. Antimicrob. Chemother. 2006, 58, 332–337. [Google Scholar] [CrossRef]
- Malwal, S.R.; Oldfield, E. Mycobacterial membrane protein Large 3-like-family proteins in bacteria, protozoa, fungi, plants, and animals: A bioinformatics and structural investigation. Proteins 2021, 90, 776–790. [Google Scholar] [CrossRef]

| Compd. No. | Structure | T. cruzi Dm28c | L. donovani EC50 (PRO) μM | T. brucei BSF EC50 (μM) | ||
|---|---|---|---|---|---|---|
| EC50 (EPI) μM | EC50 (AMA) μM | CC50 (U2OS) μM | ||||
| 1 | 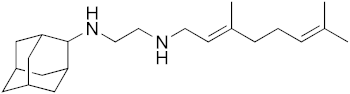 | 4.5 | 1.1 | 3.6 | 0.28 | 0.81 |
| 7 | 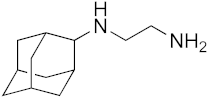 | 56 | 25 | >100 | 22 | 4.6 |
| 9 |  | 28 | 4.9 | 27 | 2.9 | 5.4 |
| 12 | 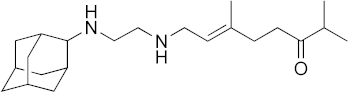 | 45 | 6.9 | 32 | 0.55 | 2.0 |
| 13 | 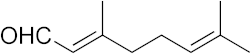 | >100 | >100 | >100 | >100 | 2.3 |
| 14 | 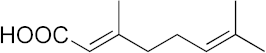 | >100 | >100 | >100 | >100 | 13 |
| 15a |  | >100 | >100 | >100 | >100 | >200 |
| 15b | 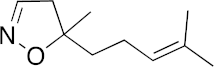 | >100 | >100 | >100 | >100 | >200 |
| 16 | 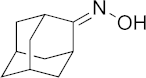 | >100 | >100 | >100 | >100 | >200 |
| 17 | 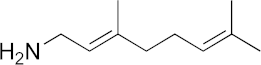 | >100 | 40 | >100 | 21 | 32 |
| 18 | 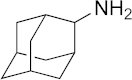 | >100 | 50 | >100 | 51 | 71 |
| 19 | 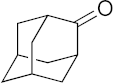 | >100 | >100 | >100 | >100 | >200 |
| 20 | 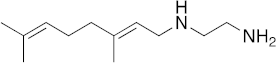 | >100 | >100 | >100 | 6 | 2.2 |
| 21 |  | 14 | 3 | 6.8 | 1.3 | 3.2 |
| 22 | 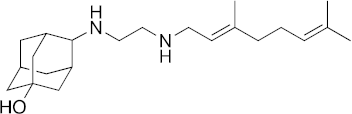 | 23 | 7.5 | 29 | 3.5 | 2.9 |
| 23 |  | 20 | 4.3 | 17 | 2.3 | 1.8 |
| 24 | 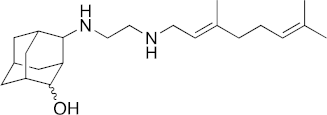 | 14 | 3.2 | 14 | 1.1 | 2.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baek, K.-H.; Phan, T.-N.; Malwal, S.R.; Lee, H.; Li, Z.-H.; Moreno, S.N.J.; Oldfield, E.; No, J.H. In Vivo Efficacy of SQ109 against Leishmania donovani, Trypanosoma spp. and Toxoplasma gondii and In Vitro Activity of SQ109 Metabolites. Biomedicines 2022, 10, 670. https://doi.org/10.3390/biomedicines10030670
Baek K-H, Phan T-N, Malwal SR, Lee H, Li Z-H, Moreno SNJ, Oldfield E, No JH. In Vivo Efficacy of SQ109 against Leishmania donovani, Trypanosoma spp. and Toxoplasma gondii and In Vitro Activity of SQ109 Metabolites. Biomedicines. 2022; 10(3):670. https://doi.org/10.3390/biomedicines10030670
Chicago/Turabian StyleBaek, Kyung-Hwa, Trong-Nhat Phan, Satish R. Malwal, Hyeryon Lee, Zhu-Hong Li, Silvia N. J. Moreno, Eric Oldfield, and Joo Hwan No. 2022. "In Vivo Efficacy of SQ109 against Leishmania donovani, Trypanosoma spp. and Toxoplasma gondii and In Vitro Activity of SQ109 Metabolites" Biomedicines 10, no. 3: 670. https://doi.org/10.3390/biomedicines10030670
APA StyleBaek, K.-H., Phan, T.-N., Malwal, S. R., Lee, H., Li, Z.-H., Moreno, S. N. J., Oldfield, E., & No, J. H. (2022). In Vivo Efficacy of SQ109 against Leishmania donovani, Trypanosoma spp. and Toxoplasma gondii and In Vitro Activity of SQ109 Metabolites. Biomedicines, 10(3), 670. https://doi.org/10.3390/biomedicines10030670





