Dysregulation of β-Cell Proliferation in Diabetes: Possibilities of Combination Therapy in the Development of a Comprehensive Treatment
Abstract
1. Introduction
2. Diminished β-Cell Function and Mass in DM
2.1. Pancreatic β-Cell Function in T2DM
2.2. Pancreatic β-Cell Damage and Death in DM
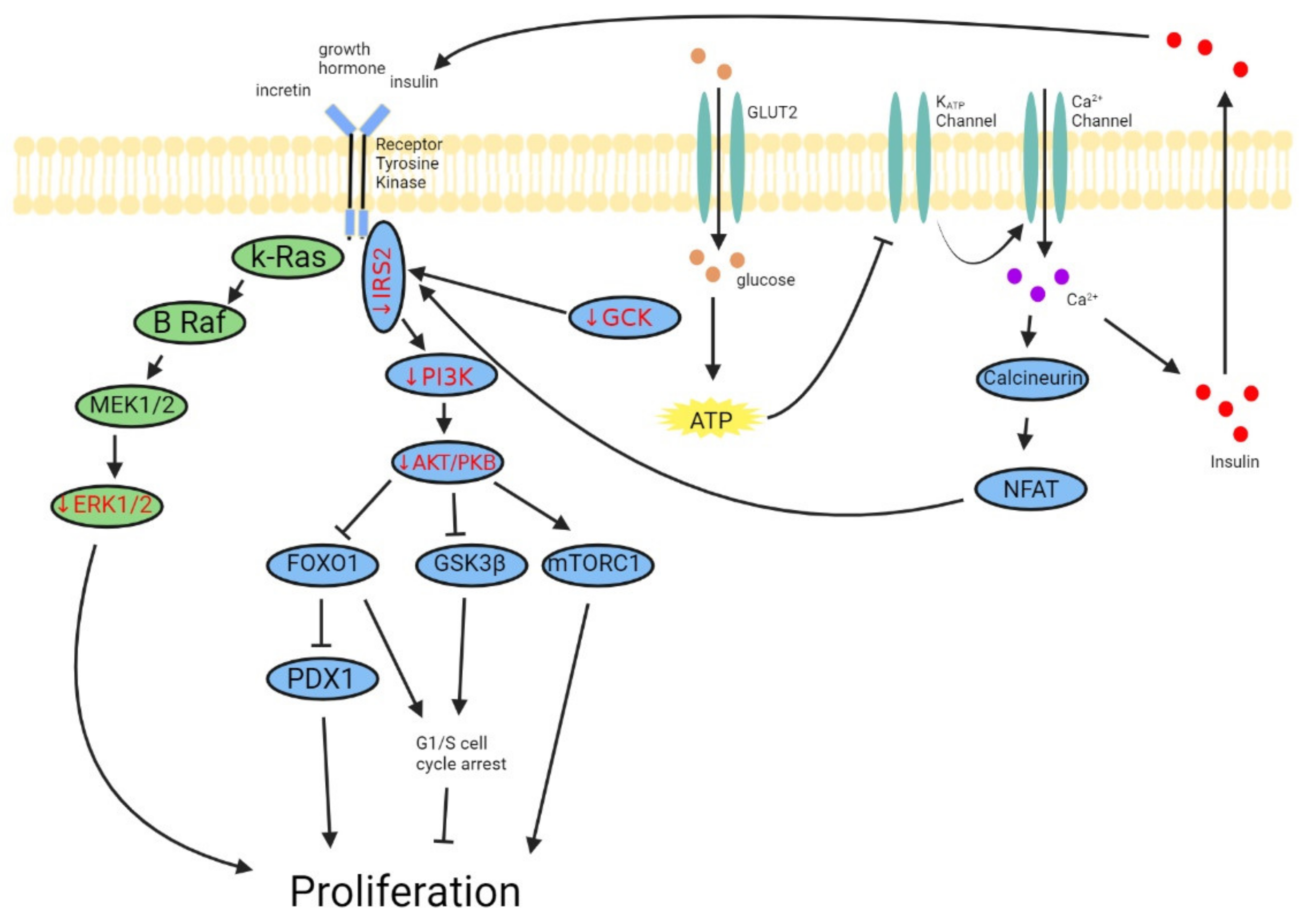
3. Dysregulation of Pathways Regulating β-Cell Mass in DM
3.1. PI3K-AKT/PKB Pathway
3.2. ERK1/2 Pathway
3.3. Altered Cell Cycle Dynamics
4. Current Therapeutic Agents
4.1. Therapeutic Agents for Juvenile Human Pancreatic β-Cells
4.1.1. GLP-1 Analogs
4.1.2. Prolactin
4.1.3. PDGF
4.1.4. WISP1
4.2. Therapeutic Agents for Adult Human Pancreatic β-Cells
4.2.1. Gastrin
4.2.2. DYRK1A Inhibitors
4.2.3. GABA
5. New Therapeutic Approach
5.1. T2DM
5.2. T1DM
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| T1DM | Type 1 Diabetes |
| T2DM | Type 2 Diabetes |
| OGTT | Oral Glucose Tolerance Test |
| HOMA2 | Homeostasis Model Assessment |
| iNOS | Nitric Oxide Synthase |
| PI3K—AKT/PKB | Phosphoinositide 3 Kinases-AKT/Protein Kinase B |
| FOXO1 | Forkhead Box Protein O1 |
| GSK3 | Glycogen Synthase Kinase-3 |
| mTOR | Mammalian Target Rapamycin |
| IRS2 | Insulin Receptor Substrate 2 |
| GCK | Glucokinase |
| ERK | Extracellular Signal-Regulated Kinase |
| MAPK | Mitogen-Activated Protein Kinase |
| MafA | MAF BZIP Transcription Factor A |
| PDX-1 | Pancreatic and Duodenal Homeobox 1 |
| GLUT2 | Glucose Transporter 2 |
| PCNA | Proliferating Cell Nuclear Antigen |
| CDK2 | Cyclin-Dependent Kinase 2 |
| NOD mouse | Non-Obese Diabetic Mouse |
| IPGTT | Intraperitoneal Glucose Tolerance Test |
| GLP1 | Glucagon-Like Peptide 1 |
| HbA1C | Hemoglobin A1C |
| NFAT | Nuclear Factor of Activated T-Cells |
| PRLR | Prolactin Receptor |
| PDGF | Platelet-Derived Growth Factor |
| WISP1 | Wnt-Induced Signaling Protein 1 |
| PPI | Proton Pump Inhibitors |
| EGF | Epidermal Growth Factor |
| INS | Insulin |
| NKX1.6 | NKX Homeobox 1 |
| NKX2.2 | NK2 Homeobox 2 |
| MNX1 | Motor Neuron and Pancreas Homeobox 1 |
| CCKBR | Dholecystokinin B Receptor |
| DYRK1A | Dual Specificity Tyrosine Phosphorylation Regulated Kinase 1A |
| IEQ | Islet Equivalent |
| GABA | γ-Aminobutyric Acid |
| CREB | cAMP Response Element Binding Protein |
| TGF β | Transforming Growth Factor β |
| STZ | Streptozotocin |
| NSG mouse | NOD Scid Gamma Mouse |
| Nrf2 | Nuclear Factor Erythroid Factor 2-Related Factor 2 |
| HO-1 | Heme Oxygenase 1 |
| G6PD | Glucose 6 Phosphate Dehydrogenase |
| TXNRD1 | Thioredoxin Reductase 1 |
| DMF | Dimethyl Fumarate |
| MS | Multiple Sclerosis |
| Th1 | T-Helper 1 |
References
- Disease, National Institute of Diabetes and Digestive and Kidney; National Institute of Health: Bethesda, MD, USA, 2020.
- Kulkarni, R.N.; Mizrachi, E.B.; Ocana, A.G.; Stewart, A.F. Human Beta-Cell Proliferation and Intracellular Signaling: Driving in the Dark without a Road Map. Diabetes 2012, 61, 2205–2213. [Google Scholar] [CrossRef]
- Utzschneider, K.M.; Younes, N.; Rasouli, N.; Barzilay, J.I.; Banerji, M.A.; Cohen, R.M.; Gonzalez, E.V.; Ismail-Beigi, F.; Mather, K.J.; Raskin, P.; et al. Shape of the Ogtt Glucose Response Curve: Relationship with Beta-Cell Function and Differences by Sex, Race, and Bmi in Adults with Early Type 2 Diabetes Treated with Metformin. BMJ Open Diabet. Res. Care 2021, 9, e002264. [Google Scholar] [CrossRef]
- Kanauchi, M.; Kimura, K.; Saito, Y. Beta-cell function and insulin sensitivity contribute to the shape of plasma glucose curve during an oral glucose tolerance test in non-diabetic individuals. Int. J. Clin. Pract. 2005, 59, 427–432. [Google Scholar] [CrossRef]
- Wallace, T.M.; Levy, J.C.; Matthews, D.R. Use and Abuse of HOMA Modeling. Diabet. Care 2004, 27, 1487–1495. [Google Scholar] [CrossRef]
- Schofield, C.J.; Sutherland, C. Disordered insulin secretion in the development of insulin resistance and Type 2 diabetes. Diabet. Med. 2012, 29, 972–979. [Google Scholar] [CrossRef]
- Marchetti, P.; Bugliani, M.; De Tata, V.; Suleiman, M.; Marselli, L. Pancreatic Beta Cell Identity in Humans and the Role of Type 2 Diabetes. Front. Cell Dev. Biol. 2017, 5, 55. [Google Scholar] [CrossRef]
- Supale, S.; Li, N.; Brun, T.; Maechler, P. Mitochondrial Dysfunction in Pancreatic Beta Cells. Trends Endocrinol. Metab. 2012, 23, 477–487. [Google Scholar] [CrossRef] [PubMed]
- Gerber, P.A.; Rutter, G.A. The Role of Oxidative Stress and Hypoxia in Pancreatic Beta-Cell Dysfunction in Diabetes Mellitus. Antioxid. Redox Signal. 2017, 26, 501–518. [Google Scholar] [CrossRef] [PubMed]
- Ferrannini, E.; Gastaldelli, A.; Miyazaki, Y.; Matsuda, M.; Mari, A.; DeFronzo, R.A. Beta-Cell Function in Subjects Spanning the Range from Normal Glucose Tolerance to Overt Diabetes: A New Analysis. J. Clin. Endocrinol. Metab. 2005, 90, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Ferrannini, E. The Stunned Beta Cell: A Brief History. Cell Metab. 2010, 11, 349–352. [Google Scholar] [CrossRef] [PubMed]
- Kanauchi, M.; Nakajima, M.; Saito, Y.; Kanauchi, K. Pancreatic beta-cell function and insulin sensitivity in japanese subjects with impaired glucose tolerance and newly diagnosed type 2 diabetes mellitus. Metabolism 2003, 52, 476–481. [Google Scholar] [CrossRef] [PubMed]
- Laurenti, M.; Matveyenko, A.; Vella, A. Measurement of Pulsatile Insulin Secretion: Rationale and Methodology. Metabolites 2021, 11, 409. [Google Scholar] [CrossRef] [PubMed]
- Kreutzberger, A.J.B.; Kiessling, V.; Doyle, C.A.; Schenk, N.; Upchurch, C.M.; Elmer-Dixon, M.; Ward, A.E.; Preobraschenski, J.; Hussein, S.S.; Tomaka, W.; et al. Distinct insulin granule subpopulations implicated in the secretory pathology of diabetes types 1 and 2. eLife 2020, 9, e62506. [Google Scholar] [CrossRef] [PubMed]
- Goginashvili, A.; Zhang, Z.; Erbs, E.; Spiegelhalter, C.; Kessler, P.; Mihlan, M.; Pasquier, A.; Krupina, K.; Schieber, N.; Cinque, L.; et al. Insulin Granules. Insulin Secretory Granules Control Autophagy in Pancreatic Beta Cells. Science 2015, 347, 878–882. [Google Scholar] [CrossRef]
- Laedtke, T.; Kjems, L.; Pørksen, N.; Schmitz, O.; Veldhuis, J.; Kao, P.C.; Butler, P. Overnight inhibition of insulin secretion restores pulsatility and proinsulin/insulin ratio in type 2 diabetes. Am. J. Physiol. Metab. 2000, 279, E520–E528. [Google Scholar] [CrossRef]
- Stadler, M.; Pacini, G.; Petrie, J.; Luger, A.; Anderwald, C.; on behalf of the RISC Investigators. Beta cell (dys)function in non-diabetic offspring of diabetic patients. Diabetologia 2009, 52, 2435–2444. [Google Scholar] [CrossRef]
- O’Rahilly, S.; Turner, R.C.; Matthews, D.R. Impaired Pulsatile Secretion of Insulin in Relatives of Patients with Non-Insulin-Dependent Diabetes. N. Engl. J. Med. 1988, 318, 1225–1230. [Google Scholar] [CrossRef]
- Petrie, J.R.; Pearson, E.; Sutherland, C. Implications of genome wide association studies for the understanding of type 2 diabetes pathophysiology. Biochem. Pharmacol. 2011, 81, 471–477. [Google Scholar] [CrossRef]
- Thomsen, S.K.; Gloyn, A.L. The Pancreatic Beta Cell: Recent Insights from Human Genetics. Trends Endocrinol. Metab. 2014, 25, 425–434. [Google Scholar] [CrossRef]
- Kettunen, J.; Tuomi, T.; Jarno, L.K. Human Physiology of Genetic Defects Causing Beta-cell Dysfunction. J. Mol. Biol. 2020, 432, 1579–1598. [Google Scholar] [CrossRef]
- Cerf, M.E. Beta Cell Dysfunction and Insulin Resistance. Front. Endocrinol. 2013, 4, 37. [Google Scholar] [CrossRef]
- Karaca, M.; Magnan, C.; Kargar, C. Functional pancreatic beta-cell mass: Involvement in type 2 diabetes and therapeutic intervention. Diabetes Metab. 2009, 35, 77–84. [Google Scholar] [CrossRef]
- Ebato, C.; Uchida, T.; Arakawa, M.; Komatsu, M.; Ueno, T.; Komiya, K.; Azuma, K.; Hirose, T.; Tanaka, K.; Kominami, E.; et al. Autophagy Is Important in Islet Homeostasis and Compensatory Increase of Beta Cell Mass in Response to High-Fat Diet. Cell Metab. 2008, 8, 325–332. [Google Scholar] [CrossRef]
- Marchetti, P.; Masini, M. Autophagy and the pancreatic beta-cell in human type 2 diabetes. Autophagy 2009, 5, 1055–1056. [Google Scholar] [CrossRef]
- Stewart, A.F.; Hussain, M.A.; Garcia-Ocana, A.; Vasavada, R.C.; Bhushan, A.; Bernal-Mizrachi, E.; Kulkarni, R.N. Human Beta-Cell Proliferation and Intracellular Signaling: Part 3. Diabetes 2015, 64, 1872–1885. [Google Scholar] [CrossRef]
- Kaneko, K.; Ueki, K.; Takahashi, N.; Hashimoto, S.; Okamoto, M.; Awazawa, M.; Okazaki, Y.; Ohsugi, M.; Inabe, K.; Umehara, T.; et al. Class Ia Phosphatidylinositol 3-Kinase in Pancreatic Beta Cells Controls Insulin Secretion by Multiple Mechanisms. Cell Metab. 2010, 12, 619–632. [Google Scholar] [CrossRef]
- Jiang, W.J.; Peng, Y.C.; Yang, K.M. Cellular Signaling Pathways Regulating Beta-Cell Proliferation as a Promising Therapeutic Target in the Treatment of Diabetes. Exp. Ther. Med. 2018, 16, 3275–3285. [Google Scholar]
- Balcazar Morales, N.; de Plata, C.A. Role of Akt/Mtorc1 Pathway in Pancreatic Beta-Cell Proliferation. Colomb. Med. 2012, 43, 235–243. [Google Scholar] [CrossRef]
- Gunton, J.E.; Kulkarni, R.N.; Yim, S.; Okada, T.; Hawthorne, W.J.; Tseng, Y.H.; Roberson, R.S.; Ricordi, C.; O’Connell, P.J.; Gonzalez, F.J.; et al. Loss of Arnt/Hif1beta Mediates Altered Gene Expression and Pancreatic-Islet Dysfunction in Human Type 2 Diabetes. Cell 2005, 122, 337–349. [Google Scholar] [CrossRef]
- Elghazi, L.; Bernal-Mizrachi, E. Akt and Pten: Beta-Cell Mass and Pancreas Plasticity. Trends Endocrinol. Metab. 2009, 20, 243–251. [Google Scholar] [CrossRef]
- Assmann, A.; Ueki, K.; Winnay, J.N.; Kadowaki, T.; Kulkarni, R.N. Glucose Effects on Beta-Cell Growth and Survival Require Activation of Insulin Receptors and Insulin Receptor Substrate 2. Mol. Cell. Biol. 2009, 29, 3219–3228. [Google Scholar] [CrossRef]
- Mohanty, S.; Spinas, G.A.; Maedler, K.; Zuellig, R.A.; Lehmann, R.; Donath, M.Y.; Trub, T.; Niessen, M. Overexpression of Irs2 in Isolated Pancreatic Islets Causes Proliferation and Protects Human Beta-Cells from Hyperglycemia-Induced Apoptosis. Exp. Cell Res. 2005, 303, 68–78. [Google Scholar] [CrossRef][Green Version]
- Liu, Y.; Zeng, Y.; Miao, Y.; Cheng, X.; Deng, S.; Hao, X.; Jiang, Y.; Wan, Q. Relationships among Pancreatic Beta Cell Function, the Nrf2 Pathway, and Irs2: A Cross-Sectional Study. Postgrad. Med. 2020, 132, 720–726. [Google Scholar] [CrossRef]
- Terauchi, Y.; Takamoto, I.; Kubota, N.; Matsui, J.; Suzuki, R.; Komeda, K.; Hara, A.; Toyoda, Y.; Miwa, I.; Aizawa, S.; et al. Glucokinase and Irs-2 Are Required for Compensatory Beta Cell Hyperplasia in Response to High-Fat Diet-Induced Insulin Resistance. J. Clin. Investig. 2007, 117, 246–257. [Google Scholar] [CrossRef]
- Nakamura, A.; Terauchi, Y.; Ohyama, S.; Kubota, J.; Shimazaki, H.; Nambu, T.; Takamoto, I.; Kubota, N.; Eiki, J.; Yoshioka, N.; et al. Impact of Small-Molecule Glucokinase Activator on Glucose Metabolism and Beta-Cell Mass. Endocrinology 2009, 150, 1147–1154. [Google Scholar] [CrossRef]
- Demozay, D.; Tsunekawa, S.; Briaud, I.; Shah, R.; Rhodes, C.J. Specific Glucose-Induced Control of Insulin Receptor Substrate-2 Expression Is Mediated Via Ca2+-Dependent Calcineurin/Nfat Signaling in Primary Pancreatic Islet Beta-Cells. Diabetes 2011, 60, 2892–2902. [Google Scholar] [CrossRef]
- Del Guerra, S.; Lupi, R.; Marselli, L.; Masini, M.; Bugliani, M.; Sbrana, S.; Torri, S.; Pollera, M.; Boggi, U.; Mosca, F.; et al. Functional and Molecular Defects of Pancreatic Islets in Human Type 2 Diabetes. Diabetes 2005, 54, 727–735. [Google Scholar] [CrossRef]
- Kassem, S.; Bhandari, S.; Rodriguez-Bada, P.; Motaghedi, R.; Heyman, M.; Garcia-Gimeno, M.A.; Cobo-Vuilleumier, N.; Sanz, P.; Maclaren, N.K.; Rahier, J.; et al. Large Islets, Beta-Cell Proliferation, and a Glucokinase Mutation. N. Engl. J. Med. 2010, 362, 1348–1350. [Google Scholar] [CrossRef]
- Kitamura, Y.I.; Kitamura, T.; Kruse, J.P.; Raum, J.C.; Stein, R.; Gu, W.; Accili, D. Foxo1 Protects against Pancreatic Beta Cell Failure through Neurod and Mafa Induction. Cell Metab. 2005, 2, 153–163. [Google Scholar] [CrossRef]
- Kitamura, T.; Nakae, J.; Kitamura, Y.; Kido, Y.; Biggs, W.H., 3rd; Wright, C.V.; White, M.F.; Arden, K.C.; Accili, D. The Forkhead Transcription Factor Foxo1 Links Insulin Signaling to Pdx1 Regulation of Pancreatic Beta Cell Growth. J. Clin. Investig. 2002, 110, 1839–1847. [Google Scholar] [CrossRef]
- Ueberberg, S.; Tannapfel, A.; Schenker, P.; Viebahn, R.; Uhl, W.; Schneider, S.; Meier, J.J. Differential expression of cell-cycle regulators in human beta-cells derived from insulinoma tissue. Metabolism 2016, 65, 736–746. [Google Scholar] [CrossRef]
- Yuan, T.; Rafizadeh, S.; Gorrepati, K.D.D.; Lupse, B.; Oberholzer, J.; Maedler, K.; Ardestani, A. Reciprocal regulation of mTOR complexes in pancreatic islets from humans with type 2 diabetes. Diabetologia 2016, 60, 668–678. [Google Scholar] [CrossRef]
- Jaafar, R.; Tran, S.; Shah, A.N.; Sun, G.; Valdearcos, M.; Marchetti, P.; Masini, M.; Swisa, A.; Giacometti, S.; Bernal-Mizrachi, E.; et al. Mtorc1 to Ampk Switching Underlies Beta-Cell Metabolic Plasticity During Maturation and Diabetes. J. Clin. Investig. 2019, 129, 4124–4137. [Google Scholar] [CrossRef]
- Jia, Y.F.; Jeeva, S.; Xu, J.; Heppelmann, C.J.; Jang, J.S.; Slama, M.Q.; Tapadar, S.; Oyelere, A.K.; Kang, S.M.; Matveyenko, A.V.; et al. Tbk1 Regulates Regeneration of Pancreatic Beta-Cells. Sci. Rep. 2020, 10, 19374. [Google Scholar] [CrossRef]
- Puri, S.; Roy, N.; Russ, H.A.; Leonhardt, L.; French, E.K.; Roy, R.; Bengtsson, H.; Scott, D.K.; Stewart, A.F.; Hebrok, M. Replication Confers Beta Cell Immaturity. Nat. Commun. 2018, 9, 485. [Google Scholar] [CrossRef]
- Sacco, F.; Seelig, A.; Humphrey, S.; Krahmer, N.; Volta, F.; Reggio, A.; Marchetti, P.; Gerdes, J.; Mann, M. Phosphoproteomics Reveals the GSK3-PDX1 Axis as a Key Pathogenic Signaling Node in Diabetic Islets. Cell Metab. 2019, 29, 1422–1432.e3. [Google Scholar] [CrossRef]
- Sidarala, V.; Kowluru, A. The Regulatory Roles of Mitogen-Activated Protein Kinase (Mapk) Pathways in Health and Diabetes: Lessons Learned from the Pancreatic Beta-Cell. Recent Pat. Endocr. Metab. Immune Drug Discov. 2017, 10, 76–84. [Google Scholar] [CrossRef]
- Ikushima, Y.M.; Awazawa, M.; Kobayashi, N.; Osonoi, S.; Takemiya, S.; Kobayashi, H.; Suwanai, H.; Morimoto, Y.; Soeda, K.; Adachi, J.; et al. Mek/Erk Signaling in Beta-Cells Bifunctionally Regulates Beta-Cell Mass and Glucose-Stimulated Insulin Secretion Response to Maintain Glucose Homeostasis. Diabetes 2021, 70, 1519–1535. [Google Scholar] [CrossRef]
- Orime, K.; Shirakawa, J.; Togashi, Y.; Tajima, K.; Inoue, H.; Ito, Y.; Sato, K.; Nakamura, A.; Aoki, K.; Goshima, Y.; et al. Trefoil Factor 2 Promotes Cell Proliferation in Pancreatic Beta-Cells through Cxcr-4-Mediated Erk1/2 Phosphorylation. Endocrinology 2013, 154, 54–64. [Google Scholar] [CrossRef]
- Fu, Z.; Zhang, W.; Zhen, W.; Lum, H.; Nadler, J.; Bassaganya-Riera, J.; Jia, Z.; Wang, Y.; Misra, H.; Liu, D. Genistein Induces Pancreatic Beta-Cell Proliferation through Activation of Multiple Signaling Pathways and Prevents Insulin-Deficient Diabetes in Mice. Endocrinology 2010, 151, 3026–3037. [Google Scholar] [CrossRef]
- Lopez-Acosta, J.F.; Moreno-Amador, J.L.; Jimenez-Palomares, M.; Diaz-Marrero, A.R.; Cueto, M.; Perdomo, G.; Cozar-Castellano, I. Epoxypukalide Induces Proliferation and Protects against Cytokine-Mediated Apoptosis in Primary Cultures of Pancreatic Beta-Cells. PLoS ONE 2013, 8, e52862. [Google Scholar]
- Chamberlain, C.E.; Scheel, D.W.; McGlynn, K.; Kim, H.; Miyatsuka, T.; Wang, J.; Nguyen, V.; Zhao, S.; Mavropoulos, A.; Abraham, A.G.; et al. Menin Determines K-Ras Proliferative Outputs in Endocrine Cells. J. Clin. Investig. 2014, 124, 4093–4101. [Google Scholar] [CrossRef]
- Wang, Z.; Oh, E.; Clapp, D.W.; Chernoff, J.; Thurmond, D.C. Inhibition or Ablation of p21-activated Kinase (PAK1) Disrupts Glucose Homeostatic Mechanisms in Vivo. J. Biol. Chem. 2011, 286, 41359–41367. [Google Scholar] [CrossRef]
- Gupta, R.K.; Gao, N.; Gorski, R.K.; White, P.; Hardy, O.T.; Rafiq, K.; Brestelli, J.E.; Chen, G.; Stoeckert, C.J., Jr.; Kaestner, K.H. Expansion of Adult Beta-Cell Mass in Response to Increased Metabolic Demand Is Dependent on Hnf-4alpha. Genes Dev. 2007, 21, 756–769. [Google Scholar] [CrossRef]
- Fiaschi-Taesch, N.M.; Kleinberger, J.W.; Salim, F.G.; Troxell, R.; Wills, R.; Tanwir, M.; Casinelli, G.; Cox, A.E.; Takane, K.K.; Scott, D.K.; et al. Human Pancreatic Beta-Cell G1/S Molecule Cell Cycle Atlas. Diabetes 2013, 62, 2450–2459. [Google Scholar] [CrossRef]
- Folli, F.; Okada, T.; Perego, C.; Gunton, J.; Liew, C.W.; Akiyama, M.; D’Amico, A.; la Rosa, S.; Placidi, C.; Lupi, R.; et al. Altered Insulin Receptor Signalling and Beta-Cell Cycle Dynamics in Type 2 Diabetes Mellitus. PLoS ONE 2011, 6, e28050. [Google Scholar] [CrossRef]
- Fiaschi-Taesch, N.M.; Kleinberger, J.W.; Salim, F.G.; Troxell, R.; Wills, R.; Tanwir, M.; Casinelli, G.; Cox, A.E.; Takane, K.K.; Srinivas, H.; et al. Cytoplasmic-Nuclear Trafficking of G1/S Cell Cycle Molecules and Adult Human Beta-Cell Replication: A Revised Model of Human Beta-Cell G1/S Control. Diabetes 2013, 62, 2460–2470. [Google Scholar] [CrossRef]
- Kim, S.Y.; Lee, J.H.; Merrins, M.J.; Gavrilova, O.; Bisteau, X.; Kaldis, P.; Satin, L.S.; Rane, S.G. Loss of Cyclin-Dependent Kinase 2 in the Pancreas Links Primary Beta-Cell Dysfunction to Progressive Depletion of Beta-Cell Mass and Diabetes. J. Biol. Chem. 2017, 292, 3841–3853. [Google Scholar] [CrossRef]
- Glauser, D.A.; Schlegel, W. The Emerging Role of Foxo Transcription Factors in Pancreatic Beta Cells. J. Endocrinol. 2007, 193, 195–207. [Google Scholar] [CrossRef]
- Fiaschi-Taesch, N.; Bigatel, T.A.; Sicari, B.; Takane, K.K.; Salim, F.; Velazquez-Garcia, S.; Harb, G.; Selk, K.; Cozar-Castellano, I.; Stewart, A.F. Survey of the Human Pancreatic Beta-Cell G1/S Proteome Reveals a Potential Therapeutic Role for Cdk-6 and Cyclin D1 in Enhancing Human Beta-Cell Replication and Function in Vivo. Diabetes 2009, 58, 882–893. [Google Scholar] [CrossRef]
- Takane, K.K.; Kleinberger, J.W.; Salim, F.G.; Fiaschi-Taesch, N.M.; Stewart, A.F. Regulated and Reversible Induction of Adult Human Beta-Cell Replication. Diabetes 2012, 61, 418–424. [Google Scholar] [CrossRef][Green Version]
- Fiaschi-Taesch, N.M.; Salim, F.; Kleinberger, J.; Troxell, R.; Cozar-Castellano, I.; Selk, K.; Cherok, E.; Takane, K.K.; Scott, D.K.; Stewart, A.F. Induction of Human Beta-Cell Proliferation and Engraftment Using a Single G1/S Regulatory Molecule, Cdk6. Diabetes 2010, 59, 1926–1936. [Google Scholar] [CrossRef]
- Gupta, V. Glucagon-Like Peptide-1 Analogues: An Overview. Indian J. Endocrinol. Metab. 2013, 17, 413–421. [Google Scholar] [CrossRef]
- Parnaud, G.; Bosco, D.; Berney, T.; Pattou, F.; Kerr-Conte, J.; Donath, M.Y.; Bruun, C.; Mandrup-Poulsen, T.; Billestrup, N.; Halban, P.A. Proliferation of Sorted Human and Rat Beta Cells. Diabetologia 2008, 51, 91–100. [Google Scholar] [CrossRef]
- Ackeifi, C.; Wang, P.; Karakose, E.; Fox, J.E.M.; Gonzalez, B.J.; Liu, H.; Wilson, J.; Swartz, E.; Berrouet, C.; Li, Y.; et al. Glp-1 Receptor Agonists Synergize with Dyrk1a Inhibitors to Potentiate Functional Human Beta Cell Regeneration. Sci. Transl. Med. 2020, 12, eaaw9996. [Google Scholar] [CrossRef]
- Rutti, S.; Sauter, N.S.; Bouzakri, K.; Prazak, R.; Halban, P.A.; Donath, M.Y. In Vitro Proliferation of Adult Human Beta-Cells. PLoS ONE 2012, 7, e35801. [Google Scholar] [CrossRef]
- Dai, C.; Hang, Y.; Shostak, A.; Poffenberger, G.; Hart, N.; Prasad, N.; Phillips, N.; Levy, S.E.; Greiner, D.L.; Shultz, L.D.; et al. Age-Dependent Human Beta Cell Proliferation Induced by Glucagon-Like Peptide 1 and Calcineurin Signaling. J. Clin. Investig. 2017, 127, 3835–3844. [Google Scholar] [CrossRef]
- Bole-Feysot, C.; Goffin, V.; Edery, M.; Binart, N.; Kelly, P.A. Prolactin (Prl) and Its Receptor: Actions, Signal Transduction Pathways and Phenotypes Observed in Prl Receptor Knockout Mice. Endocr. Rev. 1998, 19, 225–268. [Google Scholar] [CrossRef]
- Shengold, L. An Attempt at Soul Murder. Rudyard Kipling’s Early Life and Work. Psychoanal. Study Child 1975, 30, 683–724. [Google Scholar] [CrossRef]
- Chen, H.; Kleinberger, J.W.; Takane, K.K.; Salim, F.; Fiaschi-Taesch, N.; Pappas, K.; Parsons, R.; Jiang, J.; Zhang, Y.; Liu, H.; et al. Augmented Stat5 Signaling Bypasses Multiple Impediments to Lactogen-Mediated Proliferation in Human Beta-Cells. Diabetes 2015, 64, 3784–3797. [Google Scholar] [CrossRef]
- Butler, A.E.; Cao-Minh, L.; Galasso, R.; Rizza, R.A.; Corradin, A.; Cobelli, C.; Butler, P.C. Adaptive Changes in Pancreatic Beta Cell Fractional Area and Beta Cell Turnover in Human Pregnancy. Diabetologia 2010, 53, 2167–2176. [Google Scholar] [CrossRef]
- Wang, T.; Lu, J.; Xu, Y.; Li, M.; Sun, J.; Zhang, J.; Xu, B.; Xu, M.; Chen, Y.; Bi, Y.; et al. Circulating Prolactin Associates with Diabetes and Impaired Glucose Regulation: A Population-Based Study. Diabetes Care 2013, 36, 1974–1980. [Google Scholar] [CrossRef]
- Yamamoto, T.; Mita, A.; Ricordi, C.; Messinger, S.; Miki, A.; Sakuma, Y.; Timoneri, F.; Barker, S.; Fornoni, A.; Molano, R.D.; et al. Prolactin Supplementation to Culture Medium Improves Beta-Cell Survival. Transplantation 2010, 89, 1328–1335. [Google Scholar] [CrossRef]
- Freemark, M.; Driscoll, P.; Maaskant, R.; Petryk, A.; Kelly, P.A. Ontogenesis of Prolactin Receptors in the Human Fetus in Early Gestation Implications for Tissue Differentiation and Development. J. Clin. Investig. 1997, 99, 1107–1117. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, F.; Chai, Y.; Wang, L.; Yu, B. The Role of Bone-Derived Pdgf-Aa in Age-Related Pancreatic Beta Cell Proliferation and Function. Biochem. Biophys. Res. Commun. 2020, 524, 22–27. [Google Scholar] [CrossRef]
- Welsh, M.; Claesson-Welsh, L.; Hallberg, A.; Welsh, N.; Betsholtz, C.; Arkhammar, P.; Nilsson, T.; Heldin, C.H.; Berggren, P.O. Coexpression of the platelet-derived growth factor (PDGF) B chain and the PDGF beta receptor in isolated pancreatic islet cells stimulates DNA synthesis. Proc. Natl. Acad. Sci. USA 1990, 87, 5807–5811. [Google Scholar] [CrossRef]
- Chen, H.; Gu, X.; Liu, Y.; Wang, J.; Wirt, S.E.; Bottino, R.; Schorle, H.; Sage, J.; Kim, S.K. Pdgf Signalling Controls Age-Dependent Proliferation in Pancreatic Beta-Cells. Nature 2011, 478, 349–355. [Google Scholar] [CrossRef]
- Fernandez-Ruiz, R.; García-Alamán, A.; Esteban, Y.; Mir-Coll, J.; Serra-Navarro, B.; Fontcuberta-PiSunyer, M.; Broca, C.; Armanet, M.; Wojtusciszyn, A.; Kram, V.; et al. Wisp1 is a circulating factor that stimulates proliferation of adult mouse and human beta cells. Nat. Commun. 2020, 11, 5982. [Google Scholar] [CrossRef]
- Takebayashi, K.; Inukai, T. Effect of proton pump inhibitors on glycemic control in patients with diabetes. World J. Diabetes 2015, 6, 1122–1131. [Google Scholar] [CrossRef]
- Boj-Carceller, D.; Bocos-Terraz, P.; Moreno-Vernis, M.; Sanz-Paris, A.; Trincado-Aznar, P.; Albero-Gamboa, R. Are Proton Pump Inhibitors a New Antidiabetic Drug? A Cross Sectional Study. World J. Diabet. 2011, 2, 217–220. [Google Scholar] [CrossRef]
- Peng, C.C.; Tu, Y.K.; Lee, G.Y.; Chang, R.H.; Huang, Y.; Bukhari, K.; Tsai, Y.C.; Fu, Y.; Huang, H.K.; Munir, K.M. Effects of Proton Pump Inhibitors on Glycemic Control and Incident Diabetes: A Systematic Review and Meta-Analysis. J. Clin. Endocrinol. Metab. 2021, 106, 3354–3366. [Google Scholar] [CrossRef]
- Rooman, I.; Lardon, J.; Bouwens, L. Gastrin Stimulates Beta-Cell Neogenesis and Increases Islet Mass from Transdifferentiated but Not from Normal Exocrine Pancreas Tissue. Diabetes 2002, 51, 686–690. [Google Scholar] [CrossRef][Green Version]
- Wang, M.; Racine, J.J.; Song, X.; Li, X.; Nair, I.; Liu, H.; Avakian-Mansoorian, A.; Johnston, H.F.; Liu, C.; Shen, C.; et al. Mixed Chimerism and Growth Factors Augment Beta Cell Regeneration and Reverse Late-Stage Type 1 Diabetes. Sci. Transl. Med. 2012, 4, 133ra59. [Google Scholar] [CrossRef]
- Khan, D.; Vasu, S.; Moffett, R.C.; Irwin, N.; Flatt, P.R. Expression of Gastrin Family Peptides in Pancreatic Islets and Their Role in Beta-Cell Function and Survival. Pancreas 2018, 47, 190–199. [Google Scholar] [CrossRef]
- Meier, J.J.; Butler, A.E.; Galasso, R.; Rizza, R.A.; Butler, P.C. Increased Islet Beta Cell Replication Adjacent to Intrapancreatic Gastrinomas in Humans. Diabetologia 2006, 49, 2689–2696. [Google Scholar] [CrossRef]
- Breuer, T.G.K.; Borker, L.; Quast, D.R.; Tannapfel, A.; Schmidt, W.E.; Uhl, W.; Meier, J.J. Impact of proton pump inhibitor treatment on pancreatic beta-cell area and beta-cell proliferation in humans. Eur. J. Endocrinol. 2016, 175, 467–476. [Google Scholar] [CrossRef]
- Suarez-Pinzon, W.L.; Lakey, J.R.; Brand, S.J.; Rabinovitch, A. Combination Therapy with Epidermal Growth Factor and Gastrin Induces Neogenesis of Human Islet {Beta}-Cells from Pancreatic Duct Cells and an Increase in Functional {Beta}-Cell Mass. J. Clin. Endocrinol. Metab. 2005, 90, 3401–3409. [Google Scholar] [CrossRef]
- Lenz, A.; Lenz, G.; Ku, H.; Ferreri, K.; Kandeel, F. Islets from human donors with higher but not lower hemoglobin A1c levels respond to gastrin treatment in vitro. PLoS ONE 2019, 14, e0221456. [Google Scholar] [CrossRef]
- Hove, K.D.; Færch, K.; Bödvarsdóttir, T.B.; Karlsen, A.E.; Petersen, J.S.; Vaag, A.A. Treatment with a proton pump inhibitor improves glycaemic control in type 2 diabetic patients—A retrospective analysis. Diabet. Res. Clin. Pract. 2010, 90, e72–e74. [Google Scholar] [CrossRef]
- Griffin, K.J.; Thompson, P.A.; Gottschalk, M.; Kyllo, J.H.; Rabinovitch, A. Combination Therapy with Sitagliptin and Lansoprazole in Patients with Recent-Onset Type 1 Diabetes (Repair-T1d): 12-Month Results of a Multicentre, Randomised, Placebo-Controlled, Phase 2 Trial. Lancet Diabet. Endocrinol. 2014, 2, 710–718. [Google Scholar] [CrossRef]
- Shen, W.; Taylor, B.; Jin, Q.; Nguyen-Tran, V.; Meeusen, S.; Zhang, Y.Q.; Kamireddy, A.; Swafford, A.; Powers, A.F.; Walker, J.; et al. Inhibition of Dyrk1a and Gsk3b Induces Human Beta-Cell Proliferation. Nat. Commun. 2015, 6, 8372. [Google Scholar] [CrossRef]
- Kumar, K.; Ung, P.M.; Wang, P.; Wang, H.; Li, H.; Andrews, M.K.; Stewart, A.F.; Schlessinger, A.; DeVita, R.J. Novel Selective Thiadiazine Dyrk1a Inhibitor Lead Scaffold with Human Pancreatic Beta-Cell Proliferation Activity. Eur. J. Med. Chem. 2018, 157, 1005–1016. [Google Scholar] [CrossRef]
- Dirice, E.; Walpita, D.; Vetere, A.; Meier, B.C.; Kahraman, S.; Hu, J.; Dancik, V.; Burns, S.M.; Gilbert, T.J.; Olson, D.E.; et al. Inhibition of Dyrk1a Stimulates Human Beta-Cell Proliferation. Diabetes 2016, 65, 1660–1671. [Google Scholar] [CrossRef]
- Heit, J.J.; Apelqvist, A.A.; Gu, X.; Winslow, M.M.; Neilson, J.R.; Crabtree, G.R.; Kim, S.K. Calcineurin/Nfat Signalling Regulates Pancreatic Beta-Cell Growth and Function. Nature 2006, 443, 345–349. [Google Scholar] [CrossRef]
- Wang, P.; Alvarez-Perez, J.C.; Felsenfeld, D.P.; Liu, H.; Sivendran, S.; Bender, A.; Kumar, A.; Sanchez, R.; Scott, D.K.; Garcia-Ocana, A.; et al. A High-Throughput Chemical Screen Reveals That Harmine-Mediated Inhibition of Dyrk1a Increases Human Pancreatic Beta Cell Replication. Nat. Med. 2015, 21, 383–388. [Google Scholar] [CrossRef]
- Wang, P.; Karakose, E.; Liu, H.; Swartz, E.; Ackeifi, C.; Zlatanic, V.; Wilson, J.; González, B.J.; Bender, A.; Takane, K.K.; et al. Combined Inhibition of DYRK1A, SMAD, and Trithorax Pathways Synergizes to Induce Robust Replication in Adult Human Beta Cells. Cell Metab. 2018, 29, 638–652.e5. [Google Scholar] [CrossRef]
- Allegretti, P.A.; Horton, T.M.; Abdolazimi, Y.; Moeller, H.P.; Yeh, B.; Caffet, M.; Michel, G.; Smith, M.; Annes, J.P. Generation of Highly Potent Dyrk1a-Dependent Inducers of Human Beta-Cell Replication Via Multi-Dimensional Compound Optimization. Bioorg. Med. Chem. 2020, 28, 115193. [Google Scholar] [CrossRef]
- Kumar, K.; Wang, P.; Wilson, J.; Zlatanic, V.; Berrouet, C.; Khamrui, S.; Secor, C.; Swartz, E.A.; Lazarus, M.; Sanchez, R.; et al. Synthesis and Biological Validation of a Harmine-Based, Central Nervous System (Cns)-Avoidant, Selective, Human Beta-Cell Regenerative Dual-Specificity Tyrosine Phosphorylation-Regulated Kinase a (Dyrk1a) Inhibitor. J. Med. Chem. 2020, 63, 2986–3003. [Google Scholar] [CrossRef]
- Liu, Y.A.; Jin, Q.; Zou, Y.; Ding, Q.; Yan, S.; Wang, Z.; Hao, X.; Nguyen, B.; Zhang, X.; Pan, J.; et al. Selective Dyrk1a Inhibitor for the Treatment of Type 1 Diabetes: Discovery of 6-Azaindole Derivative Gnf2133. J. Med. Chem. 2020, 63, 2958–2973. [Google Scholar] [CrossRef]
- Kumar, K.; Wang, P.; Sanchez, R.; Swartz, E.A.; Stewart, A.F.; DeVita, R.J. Development of Kinase-Selective, Harmine-Based Dyrk1a Inhibitors That Induce Pancreatic Human Beta-Cell Proliferation. J. Med. Chem. 2018, 61, 7687–7699. [Google Scholar] [CrossRef]
- Korol, S.V.; Jin, Z.; Jin, Y.; Bhandage, A.K.; Tengholm, A.; Gandasi, N.R.; Barg, S.; Espes, D.; Carlsson, P.O.; Laver, D.; et al. Functional Characterization of Native, High-Affinity Gabaa Receptors in Human Pancreatic Beta Cells. EBioMedicine 2018, 30, 273–282. [Google Scholar] [CrossRef]
- Soltani, N.; Qiu, H.; Aleksic, M.; Glinka, Y.; Zhao, F.; Liu, R.; Li, Y.; Zhang, N.; Chakrabarti, R.; Ng, T.; et al. Gaba Exerts Protective and Regenerative Effects on Islet Beta Cells and Reverses Diabetes. Proc. Natl. Acad. Sci. USA 2011, 108, 11692–11697. [Google Scholar] [CrossRef]
- Tian, J.; Dang, H.; Chen, Z.; Guan, A.; Jin, Y.; Atkinson, M.A.; Kaufman, D.L. Gamma-Aminobutyric Acid Regulates Both the Survival and Replication of Human Beta-Cells. Diabetes 2013, 62, 3760–3765. [Google Scholar] [CrossRef]
- Purwana, I.; Zheng, J.; Li, X.; Deurloo, M.; Son, D.O.; Zhang, Z.; Liang, C.; Shen, E.; Tadkase, A.; Feng, Z.P.; et al. Gaba Promotes Human Beta-Cell Proliferation and Modulates Glucose Homeostasis. Diabetes 2014, 63, 4197–4205. [Google Scholar] [CrossRef]
- Sparrow, E.L.; James, S.; Hussain, K.; Beers, S.A.; Cragg, M.S.; Bogdanov, Y.D. Activation of Gaba(a) Receptors Inhibits T Cell Proliferation. PLoS ONE 2021, 16, e0251632. [Google Scholar] [CrossRef]
- Choat, H.M.; Martin, A.; Mick, G.J.; Heath, K.E.; Tse, H.M.; McGwin, G., Jr.; McCormick, K.L. Effect of Gamma Aminobutyric Acid (Gaba) or Gaba with Glutamic Acid Decarboxylase (Gad) on the Progression of Type 1 Diabetes Mellitus in Children: Trial Design and Methodology. Contemp. Clin. Trials 2019, 82, 93–100. [Google Scholar] [CrossRef]
- Soltani, N.; Rezazadeh, H.; Sharifi, M.R. Insulin resistance and the role of gamma-aminobutyric acid. J. Res. Med. Sci. 2021, 26, 39. [Google Scholar] [CrossRef]
- Tian, J.; Dang, H.N.; Yong, J.; Chui, W.S.; Dizon, M.P.; Yaw, C.K.; Kaufman, D.L. Oral Treatment with Gamma-Aminobutyric Acid Improves Glucose Tolerance and Insulin Sensitivity by Inhibiting Inflammation in High Fat Diet-Fed Mice. PLoS ONE 2011, 6, e25338. [Google Scholar] [CrossRef]
- El Ouaamari, A.; Dirice, E.; Gedeon, N.; Hu, J.; Zhou, J.Y.; Shirakawa, J.; Hou, L.; Goodman, J.; Karampelias, C.; Qiang, G.; et al. Serpinb1 Promotes Pancreatic Beta Cell Proliferation. Cell Metab. 2016, 23, 194–205. [Google Scholar] [CrossRef]
- Shen, W.; Tremblay, M.S.; Deshmukh, V.A.; Wang, W.; Filippi, C.M.; Harb, G.; Zhang, Y.Q.; Kamireddy, A.; Baaten, J.E.; Jin, Q.; et al. Small-Molecule Inducer of Beta Cell Proliferation Identified by High-Throughput Screening. J. Am. Chem. Soc. 2013, 135, 1669–1672. [Google Scholar] [CrossRef]
- Liu, H.; Remedi, M.S.; Pappan, K.L.; Kwon, G.; Rohatgi, N.; Marshall, C.A.; McDaniel, M.L. Glycogen Synthase Kinase-3 and Mammalian Target of Rapamycin Pathways Contribute to DNA Synthesis, Cell Cycle Progression, and Proliferation in Human Islets. Diabetes 2009, 58, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Pahlavanneshan, S.; Behmanesh, M.; Oropeza, D.; Furuyama, K.; Tahamtani, Y.; Basiri, M.; Herrera, P.L.; Baharvand, H. Combined inhibition of menin-MLL interaction and TGF-β signaling induces replication of human pancreatic beta cells. Eur. J. Cell Biol. 2020, 99, 151094. [Google Scholar] [CrossRef]
- Dhawan, S.; Dirice, E.; Kulkarni, R.N.; Bhushan, A. Inhibition of Tgf-Beta Signaling Promotes Human Pancreatic Beta-Cell Replication. Diabetes 2016, 65, 1208–1218. [Google Scholar] [CrossRef]
- Freeman, A.M.; Pennings, N. Insulin Resistance; Statpearls: Treasure Island, FL, USA, 2021. [Google Scholar]
- Eguchi, N.; Vaziri, N.D.; Dafoe, D.C.; Ichii, H. The Role of Oxidative Stress in Pancreatic Beta Cell Dysfunction in Diabetes. Int. J. Mol. Sci. 2021, 22, 1509. [Google Scholar] [CrossRef] [PubMed]
- Miki, A.; Ricordi, C.; Sakuma, Y.; Yamamoto, T.; Misawa, R.; Mita, A.; Molano, R.D.; Vaziri, N.D.; Pileggi, A.; Ichii, H. Divergent Antioxidant Capacity of Human Islet Cell Subsets: A Potential Cause of Beta-Cell Vulnerability in Diabetes and Islet Transplantation. PLoS ONE 2018, 13, e0196570. [Google Scholar] [CrossRef] [PubMed]
- Masuda, Y.; Vaziri, N.D.; Li, S.; Le, A.; Hajighasemi-Ossareh, M.; Robles, L.; Foster, C.E.; Stamos, M.J.; Al-Abodullah, I.; Ricordi, C.; et al. The Effect of Nrf2 Pathway Activation on Human Pancreatic Islet Cells. PLoS ONE 2015, 10, e0131012. [Google Scholar] [CrossRef] [PubMed]
- Den Hartogh, D.J.; Gabriel, A.; Tsiani, E. Antidiabetic Properties of Curcumin I: Evidence from In Vitro Studies. Nutrients 2020, 12, 118. [Google Scholar] [CrossRef]
- Schultheis, J.; Beckmann, D.; Mulac, D.; Müller, L.; Esselen, M.; Düfer, M. Nrf2 Activation Protects Mouse Beta Cells from Glucolipotoxicity by Restoring Mitochondrial Function and Physiological Redox Balance. Oxidative Med. Cell. Longev. 2019, 2019, 7518510. [Google Scholar] [CrossRef]
- Song, M.Y.; Kim, E.K.; Moon, W.S.; Park, J.W.; Kim, H.J.; So, H.S.; Park, R.; Kwon, K.B.; Park, B.H. Sulforaphane Protects against Cytokine- and Streptozotocin-Induced Beta-Cell Damage by Suppressing the Nf-Kappab Pathway. Toxicol. Appl. Pharmacol. 2009, 235, 57–67. [Google Scholar] [CrossRef]
- Ganesan, K.; Ramkumar, K.M.; Xu, B. Vitexin Restores Pancreatic Beta-Cell Function and Insulin Signaling through Nrf2 and Nf-Kappab Signaling Pathways. Eur. J. Pharmacol. 2020, 888, 173606. [Google Scholar] [CrossRef]
- Kumar, A.; Katz, L.S.; Schulz, A.M.; Kim, M.; Honig, L.B.; Li, L.; Davenport, B.; Homann, D.; Garcia-Ocana, A.; Herman, M.A.; et al. Activation of Nrf2 Is Required for Normal and Chrebpalpha-Augmented Glucose-Stimulated Beta-Cell Proliferation. Diabetes 2018, 67, 1561–1575. [Google Scholar] [CrossRef] [PubMed]
- Baumel-Alterzon, S.; Katz, L.S.; Brill, G.; Garcia-Ocaña, A.; Scott, D.K. Nrf2: The Master and Captain of Beta Cell Fate. Trends Endocrinol. Metab. 2020, 32, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Herold, K.C.; Bundy, B.N.; Long, S.A.; Bluestone, J.A.; DiMeglio, L.A.; Dufort, M.J.; Gitelman, S.E.; Gottlieb, P.A.; Krischer, J.P.; Linsley, P.S.; et al. An Anti-Cd3 Antibody, Teplizumab, in Relatives at Risk for Type 1 Diabetes. N. Engl. J. Med. 2019, 381, 603–613. [Google Scholar] [CrossRef] [PubMed]
- Bluestone, J.A.; Buckner, J.H.; Herold, K.C. Immunotherapy: Building a Bridge to a Cure for Type 1 Diabetes. Science 2021, 373, 510–516. [Google Scholar] [CrossRef] [PubMed]
- Chatenoud, L.; Warncke, K.; Ziegler, A.-G. Clinical Immunologic Interventions for the Treatment of Type 1 Diabetes. Cold Spring Harb. Perspect. Med. 2012, 2, a007716. [Google Scholar] [CrossRef]
- Fu, J.; Zheng, H.; Wang, H.; Yang, B.; Zhao, R.; Lu, C.; Liu, Z.; Hou, Y.; Xu, Y.; Zhang, Q.; et al. Protective Role of Nuclear Factor E2-Related Factor 2 against Acute Oxidative Stress-Induced Pancreatic Beta -Cell Damage. Oxid. Med. Cell. Longev. 2015, 2015, 639191. [Google Scholar] [CrossRef]
- Marastoni, D.; Buriani, A.; Pisani, A.I.; Crescenzo, F.; Zuco, C.; Fortinguerra, S.; Sorrenti, V.; Marenda, B.; Romualdi, C.; Magliozzi, R.; et al. Increased Nk Cell Count in Multiple Sclerosis Patients Treated with Dimethyl Fumarate: A 2-Year Longitudinal Study. Front. Immunol. 2019, 10, 1666. [Google Scholar] [CrossRef]
- Hosseini, A.; Masjedi, A.; Baradaran, B.; Hojjat-Farsangi, M.; Ghalamfarsa, G.; Anvari, E.; Jadidi-Niaragh, F. Dimethyl fumarate: Regulatory effects on the immune system in the treatment of multiple sclerosis. J. Cell. Physiol. 2018, 234, 9943–9955. [Google Scholar] [CrossRef]
- Holm Hansen, R.; Hojsgaard Chow, H.; Sellebjerg, F.; Rode von Essen, M. Dimethyl fumarate therapy suppresses B cell responses and follicular helper T cells in relapsing-remitting multiple sclerosis. Mult. Scler. J. 2019, 25, 1289–1297. [Google Scholar] [CrossRef]
- Li, S.; Vaziri, N.; Swentek, L.; Takasu, C.; Vo, K.; Stamos, M.; Ricordi, C.; Ichii, H. Prevention of Autoimmune Diabetes in NOD Mice by Dimethyl Fumarate. Antioxidants 2021, 10, 193. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eguchi, N.; Toribio, A.J.; Alexander, M.; Xu, I.; Whaley, D.L.; Hernandez, L.F.; Dafoe, D.; Ichii, H. Dysregulation of β-Cell Proliferation in Diabetes: Possibilities of Combination Therapy in the Development of a Comprehensive Treatment. Biomedicines 2022, 10, 472. https://doi.org/10.3390/biomedicines10020472
Eguchi N, Toribio AJ, Alexander M, Xu I, Whaley DL, Hernandez LF, Dafoe D, Ichii H. Dysregulation of β-Cell Proliferation in Diabetes: Possibilities of Combination Therapy in the Development of a Comprehensive Treatment. Biomedicines. 2022; 10(2):472. https://doi.org/10.3390/biomedicines10020472
Chicago/Turabian StyleEguchi, Natsuki, Arvin John Toribio, Michael Alexander, Ivana Xu, David Lee Whaley, Luis F. Hernandez, Donald Dafoe, and Hirohito Ichii. 2022. "Dysregulation of β-Cell Proliferation in Diabetes: Possibilities of Combination Therapy in the Development of a Comprehensive Treatment" Biomedicines 10, no. 2: 472. https://doi.org/10.3390/biomedicines10020472
APA StyleEguchi, N., Toribio, A. J., Alexander, M., Xu, I., Whaley, D. L., Hernandez, L. F., Dafoe, D., & Ichii, H. (2022). Dysregulation of β-Cell Proliferation in Diabetes: Possibilities of Combination Therapy in the Development of a Comprehensive Treatment. Biomedicines, 10(2), 472. https://doi.org/10.3390/biomedicines10020472





