Host Defense Peptides LL-37 and Lactoferrin Trigger ET Release from Blood-Derived Circulating Monocytes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Isolating CD14+ Monocytes from the PBMC Fraction
2.3. TNFα and Nucleosome Release
2.4. Oxidative Burst
2.5. Monocyte ET Induction and Immunofluorescence Microscopy
2.6. Collection and Analysis of Neutrophil-derived Granulation Content (NGC)
2.7. Sample Preparation for Mass Spectrometry
2.8. Liquid Chromatography Tandem Mass Spectrometry (LC-MS/MS)
2.9. Data Analysis
2.10. Statistical Analysis
3. Results
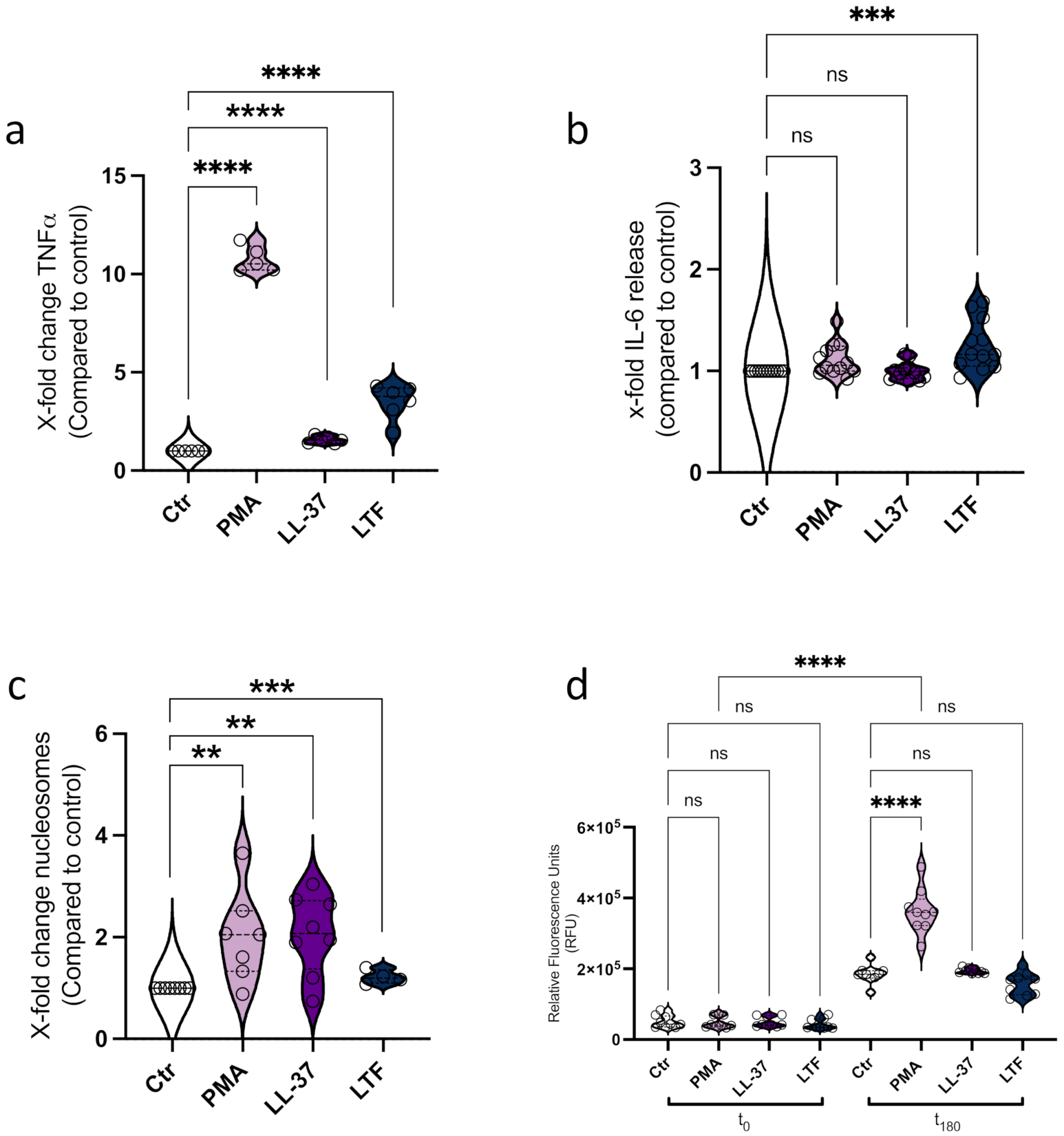
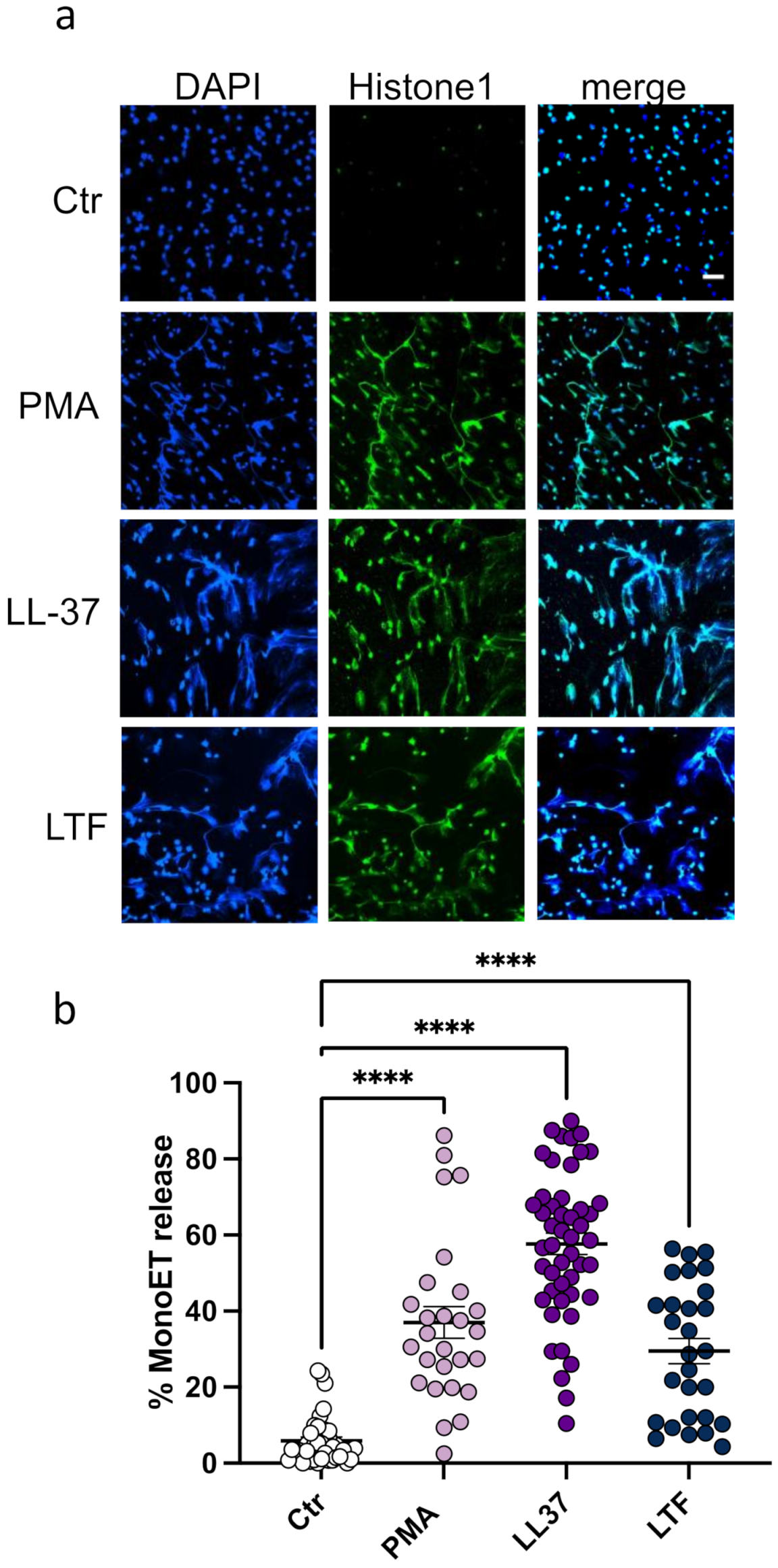
3.1. LL-37 Triggers Secretion of TNFα, Nucleosomes, and ETs from CD14+ Monocytes
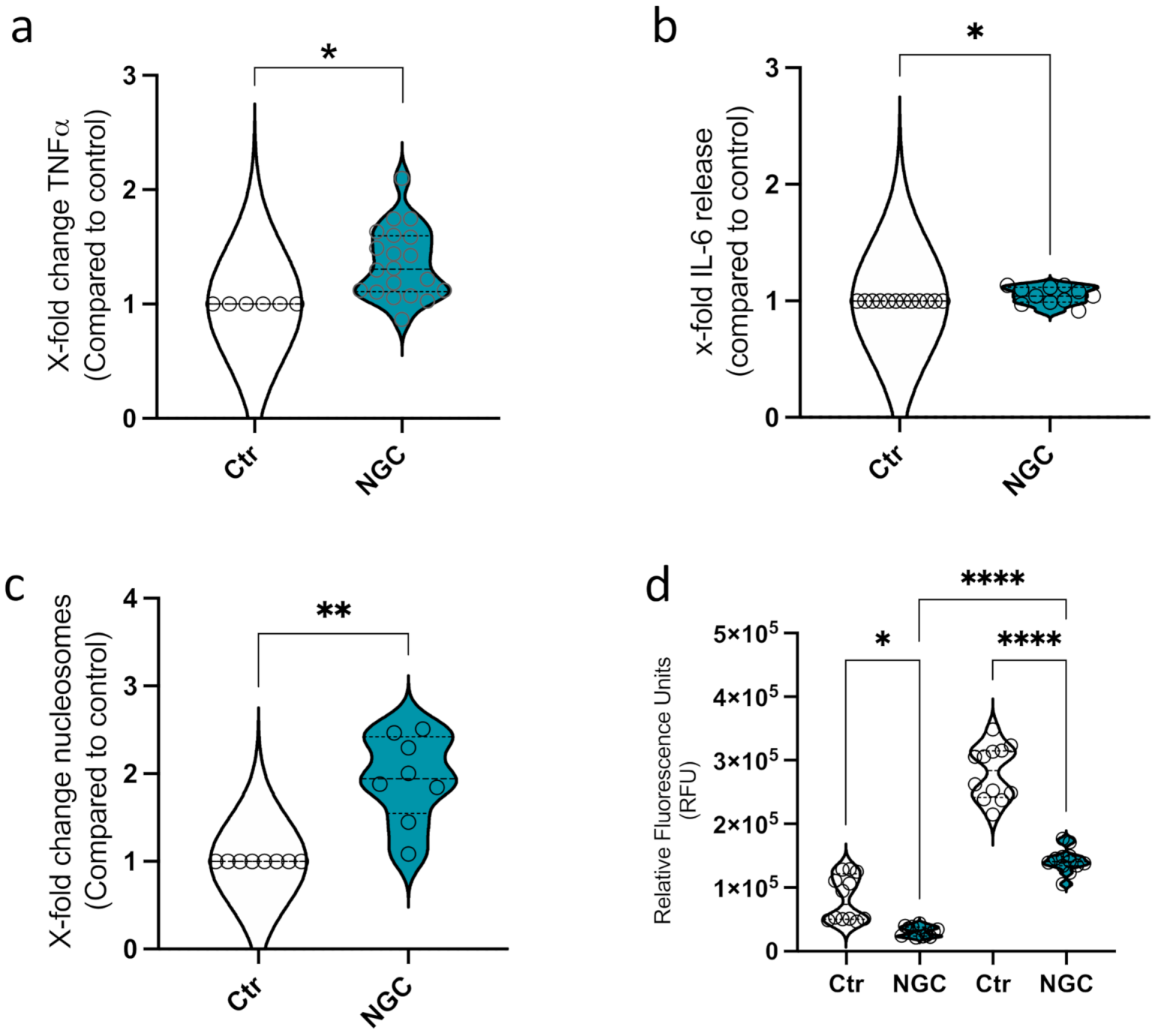
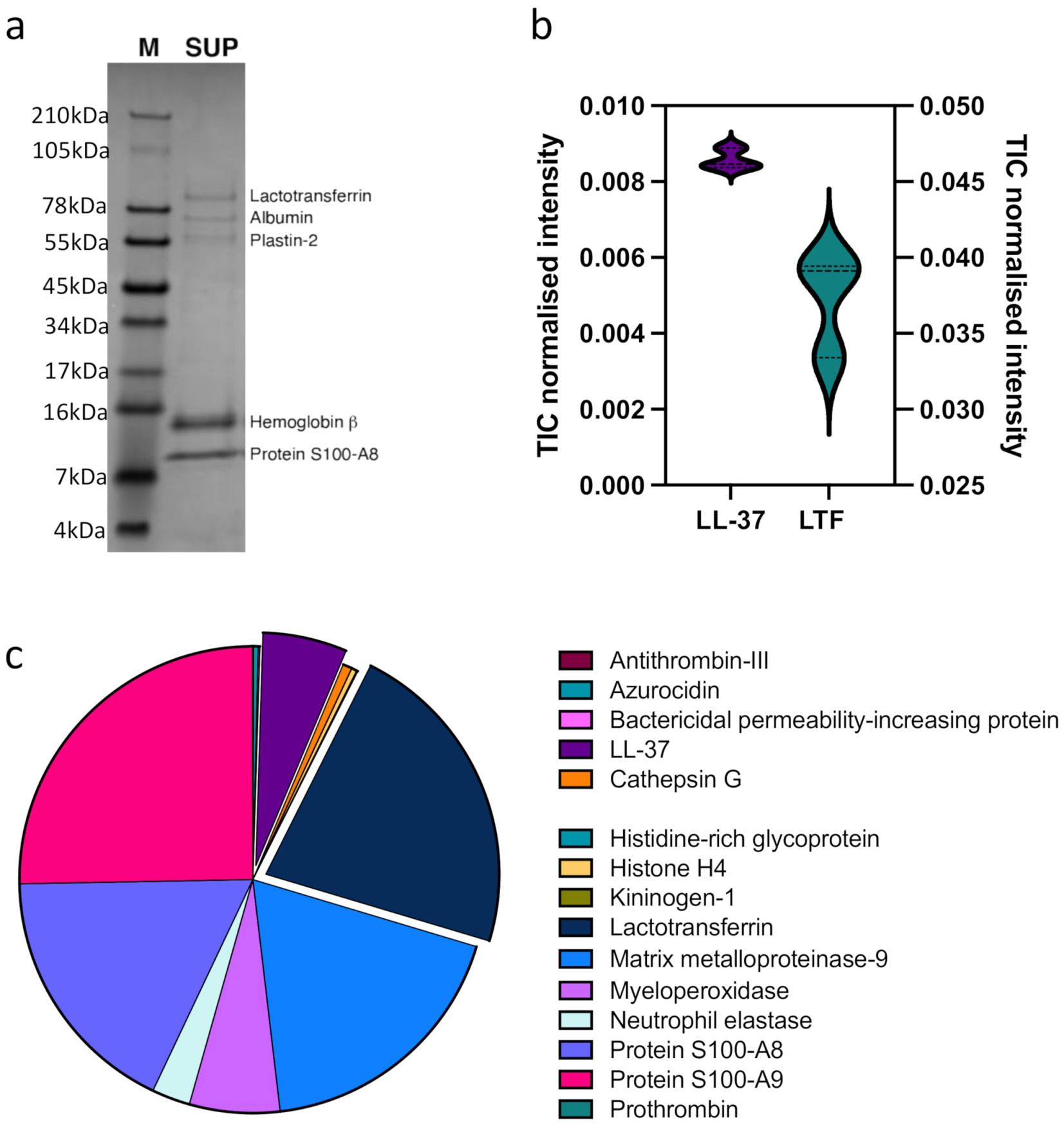
3.2. Neutrophil Granular Content Triggers Monocyte ET Formation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Borregaard, N.; Lollike, K.; Kjeldsen, L.; Sengeløv, H.; Bastholm, L.; Nielsen, M.H.; Bainton, D.F. Human neutrophil granules and secretory vesicles. Eur. J. Haematol. 2009, 51, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Silvestre-Roig, C.; Hidalgo, A.; Soehnlein, O. Neutrophil heterogeneity: Implications for homeostasis and pathogenesis. Blood 2016, 127, 2173–2181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teh, Y.C.; Ding, J.L.; Ng, L.G.; Chong, S.Z. Capturing the Fantastic Voyage of Monocytes Through Time and Space. Front. Immunol. 2019, 10, 834. [Google Scholar] [CrossRef] [PubMed]
- Chong, S.Z.; Evrard, M.; Goh, C.C.; Ng, L.G. Illuminating the covert mission of mononuclear phagocytes in their regional niches. Curr. Opin. Immunol. 2018, 50, 94–101. [Google Scholar] [CrossRef]
- Thomas, G.D.; Hamers, A.A.; Nakao, C.; Marcovecchio, P.; Taylor, A.M.; McSkimming, C.; Nguyen, A.T.; McNamara, C.A.; Hedrick, C.C. Human Blood Monocyte Subsets. Arter. Thromb. Vasc. Biol. 2017, 37, 1548–1558. [Google Scholar] [CrossRef] [Green Version]
- Kapellos, T.S.; Bonaguro, L.; Gemünd, I.; Reusch, N.; Saglam, A.; Hinkley, E.R.; Schultze, J.L. Human Monocyte Subsets and Phenotypes in Major Chronic Inflammatory Diseases. Front. Immunol. 2019, 10, 2035. [Google Scholar] [CrossRef] [Green Version]
- Boyette, L.B.; Macedo, C.; Hadi, K.; Elinoff, B.D.; Walters, J.; Ramaswami, B.; Chalasani, G.; Taboas, J.M.; Lakkis, F.G.; Metes, D.M. Phenotype, function, and differentiation potential of human monocyte subsets. PLoS ONE 2017, 12, e0176460. [Google Scholar] [CrossRef]
- Kyathanahalli, S.J.; Janardhan, K.S.; Sandhu, S.K.; Singh, B. Neutrophil depletion inhibits early and late monocyte/macrophage increase in lung inflammation. Front. Biosci. 2006, 11, 1569–1576. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Stohlman, S.A.; Hinton, D.R.; Marten, N.W. Neutrophils Promote Mononuclear Cell Infiltration During Viral-Induced Encephalitis. J. Immunol. 2003, 170, 3331–3336. [Google Scholar] [CrossRef]
- Soehnlein, O.; Zernecke, A.; Eriksson, E.E.; Rothfuchs, A.G.; Pham, C.T.; Herwald, H.; Bidzhekov, K.; Rottenberg, M.E.; Weber, C.; Lindbom, L. Neutrophil secretion products pave the way for inflammatory monocytes. Blood 2008, 112, 1461–1471. [Google Scholar] [CrossRef] [Green Version]
- De La Rosa, G.; Yang, D.; Tewary, P.; Varadhachary, A.; Oppenheim, J.J. Lactoferrin Acts as an Alarmin to Promote the Recruitment and Activation of APCs and Antigen-Specific Immune Responses. J. Immunol. 2008, 180, 6868–6876. [Google Scholar] [CrossRef]
- Soehnlein, O.; Weber, C.; Lindbom, L. Neutrophil granule proteins tune monocytic cell function. Trends Immunol. 2009, 30, 538–546. [Google Scholar] [CrossRef] [PubMed]
- Påhlman, L.I.; Mörgelin, M.; Eckert, J.; Johansson, L.; Russell, W.; Riesbeck, K.; Soehnlein, O.; Lindbom, L.; Norrby-Teglund, A.; Schumann, R.R.; et al. Streptococcal M Protein: A Multipotent and Powerful Inducer of Inflammation. J. Immunol. 2006, 177, 1221–1228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wantha, S.; Alard, J.-E.; Megens, R.T.; van der Does, A.M.; Döring, Y.; Drechsler, M.; Pham, C.T.; Wang, M.-W.; Wang, J.-M.; Gallo, R.L.; et al. Neutrophil-Derived Cathelicidin Promotes Adhesion of Classical Monocytes. Circ. Res. 2013, 112, 792–801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soehnlein, O.; Xie, X.; Ulbrich, H.; Kenne, E.; Rotzius, P.; Flodgaard, H.; Eriksson, E.E.; Lindbom, L. Neutrophil-Derived Heparin-Binding Protein (HBP/CAP37) Deposited on Endothelium Enhances Monocyte Arrest under Flow Conditions. J. Immunol. 2005, 174, 6399–6405. [Google Scholar] [CrossRef]
- Neumann, A.; Berends, E.T.M.; Nerlich, A.; Molhoek, E.M.; Gallo, R.; Meerloo, T.; Nizet, V.; Naim, H.Y.; Von Köckritz-Blickwede, M. The antimicrobial peptide LL-37 facilitates the formation of neutrophil extracellular traps. Biochem. J. 2014, 464, 3–11. [Google Scholar] [CrossRef]
- Granger, V.; Faille, D.; Marani, V.; Noël, B.; Gallais, Y.; Szely, N.; Flament, H.; Pallardy, M.; Chollet-Martin, S.; De Chaisemartin, L. Human blood monocytes are able to form extracellular traps. J. Leukoc. Biol. 2017, 102, 775–781. [Google Scholar] [CrossRef] [Green Version]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil extracellular traps kill bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef]
- Neumann, A.; Brogden, G.; Von Köckritz-Blickwede, M. Extracellular Traps: An Ancient Weapon of Multiple Kingdoms. Biology 2020, 9, 34. [Google Scholar] [CrossRef] [Green Version]
- Webster, S.J.; Daigneault, M.; Bewley, M.A.; Preston, J.A.; Marriott, H.M.; Walmsley, S.R.; Read, R.C.; Whyte, M.K.B.; Dockrell, D.H. Distinct Cell Death Programs in Monocytes Regulate Innate Responses Following Challenge with Common Causes of Invasive Bacterial Disease. J. Immunol. 2010, 185, 2968–2979. [Google Scholar] [CrossRef] [Green Version]
- Pérez, D.; Muñoz, M.; Molina, J.; Muñoz-Caro, T.; Silva, L.; Taubert, A.; Hermosilla, C.; Ruiz, A. Eimeria ninakohlyakimovae induces NADPH oxidase-dependent monocyte extracellular trap formation and upregulates IL-12 and TNF-α, IL-6 and CCL2 gene transcription. Veter. Parasitol. 2016, 227, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Caro, T.; Silva, L.M.R.; Ritter, C.; Taubert, A.; Hermosilla, C. Besnoitia besnoiti tachyzoites induce monocyte extracellular trap formation. Parasitol. Res. 2014, 113, 4189–4197. [Google Scholar] [CrossRef]
- Halder, L.D.; Abdelfatah, M.A.; Jo, E.A.H.; Jacobsen, I.D.; Westermann, M.; Beyersdorf, N.; Lorkowski, S.; Zipfel, P.F.; Skerka, C. Factor H Binds to Extracellular DNA Traps Released from Human Blood Monocytes in Response to Candida albicans. Front. Immunol. 2017, 7, 671. [Google Scholar] [CrossRef] [Green Version]
- Neumann, A.; Björck, L.; Frick, I.-M. Finegoldia magna, an Anaerobic Gram-Positive Bacterium of the Normal Human Microbiota, Induces Inflammation by Activating Neutrophils. Front. Microbiol. 2020, 11, 65. [Google Scholar] [CrossRef] [PubMed]
- Shevchenko, A.; Tomas, H.; Havlis, J.; Olsen, J.; Mann, M.J. In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nat. Protoc. 2007, 1, 2856–2860. [Google Scholar] [CrossRef] [PubMed]
- Vizcaíno, J.A.; Deutsch, E.W.; Wang, R.; Csordas, A.; Reisinger, F.; Ríos, D.; Dianes, J.A.; Sun, Z.; Farrah, T.; Bandeira, N.; et al. ProteomeXchange provides globally coordinated proteomics data submission and dissemination. Nat. Biotechnol. 2014, 32, 223–226. [Google Scholar] [CrossRef] [PubMed]
- Prame Kumar, K.; Nicholls, A.J.; Wong, C.H.Y. Partners in crime: Neutrophils and monocytes/macrophages in inflammation and disease. Cell Tissue Res. 2018, 371, 551–565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hemshekhar, M.; Choi, K.-Y.G.; Mookherjee, N. Host Defense Peptide LL-37-Mediated Chemoattractant Properties, but Not Anti-Inflammatory Cytokine IL-1RA Production, Is Selectively Controlled by Cdc42 Rho GTPase via G Protein-Coupled Receptors and JNK Mitogen-Activated Protein Kinase. Front. Immunol. 2018, 9, 1871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stallmann, H.P.; Faber, C.; Bronckers, A.L.; de Blieck-Hogervorst, J.M.; Brouwer, C.P.; Amerongen, A.V.N.; Wuisman, P.I. Histatin and lactoferrin derived peptides: Antimicrobial properties and effects on mammalian cells. Peptides 2005, 26, 2355–2359. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.D.; Gonzalez, M.L.; Kumar, P.; Grammas, P.; Pereira, H. CAP37, a neutrophil-derived inflammatory mediator, augments leukocyte adhesion to endothelial monolayers. Microvasc. Res. 2003, 66, 38–48. [Google Scholar] [CrossRef]
- Guimaraes-Costa, A.B.; Nascimento, M.T.C.; Froment, G.S.; Soares, R.P.P.; Morgado, F.N.; Conceicao-Silva, F.; Saraiva, E.M. Leishmania amazonensis promastigotes induce and are killed by neutrophil extracellular traps. Proc. Natl. Acad. Sci. USA 2009, 106, 6748–6753. [Google Scholar] [CrossRef] [Green Version]
- Fuchs, T.A.; Abed, U.; Goosmann, C.; Hurwitz, R.; Schulze, I.; Wahn, V.; Weinrauch, Y.; Brinkmann, V.; Zychlinsky, A. Novel cell death program leads to neutrophil extracellular traps. J. Cell Biol. 2007, 176, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Neumann, A.; Brogden, G.; Jerjomiceva, N.; Brodesser, S.; Naim, H.Y.; von Köckritz-Blickwede, M. Lipid alterations in human blood-derived neutrophils lead to formation of neutrophil extracellular traps. Eur. J. Cell Biol. 2014, 93, 347–354. [Google Scholar] [CrossRef]
- Ponath, V.; Kaina, B. Death of Monocytes through Oxidative Burst of Macrophages and Neutrophils: Killing in Trans. PLoS ONE 2017, 12, e0170347. [Google Scholar] [CrossRef]
- Carmona-Rivera, C.; Kaplan, M.J. Induction and Quantification of NETosis. Curr. Protoc. Immunol. 2016, 115, 14–41. [Google Scholar] [CrossRef] [PubMed]
- Neumann, A.; Völlger, L.; Berends, E.T.; Molhoek, E.M.; Stapels, D.A.; Midon, M.; Friães, A.; Pingoud, A.; Rooijakkers, S.H.; Gallo, R.; et al. Novel Role of the Antimicrobial Peptide LL-37 in the Protection of Neutrophil Extracellular Traps against Degradation by Bacterial Nucleases. J. Innate Immun. 2014, 6, 860–868. [Google Scholar] [CrossRef] [PubMed]
- Alarcón, P.; Manosalva, C.; Conejeros, I.; Carretta, M.D.; Muñoz-Caro, T.; Silva, L.M.R.; Taubert, A.; Hermosilla, C.; Hidalgo, M.A.; Burgos, R.A. d(−) Lactic Acid-Induced Adhesion of Bovine Neutrophils onto Endothelial Cells Is Dependent on Neutrophils Extracellular Traps Formation and CD11b Expression. Front. Immunol. 2017, 8, 975. [Google Scholar] [CrossRef] [Green Version]
- Muñoz-Caro, T.; Mena Huertas, S.J.; Conejeros, I.; Alarcon, P.; Hidalgo, M.A.; Burgos, R.A.; Hermosilla, C.; Taubert, A. Eimeria bovis-triggered neutrophil extracellular trap formation is CD11b-, ERK 1/2-, p38 MAP kinase- and SOCE-dependent. Vet. Res. 2015, 46, 23. [Google Scholar] [CrossRef] [Green Version]
- Behrendt, J.H.; Ruiz, A.; Zahner, H.; Taubert, A.; Hermosilla, C. Neutrophil extracellular trap formation as innate immune reactions against the apicomplexan parasite Eimeria bovis. Veter- Immunol. Immunopathol. 2010, 133, 1–8. [Google Scholar] [CrossRef]
- Han, Z.; Zhang, Y.; Wang, C.; Liu, X.; Jiang, A.; Liu, Z.; Wang, J.; Yang, Z.; Wei, Z. Ochratoxin A-Triggered Chicken Heterophil Extracellular Traps Release through Reactive Oxygen Species Production Dependent on Activation of NADPH Oxidase, ERK, and p38 MAPK Signaling Pathways. J. Agric. Food Chem. 2019, 67, 11230–11235. [Google Scholar] [CrossRef]
- Clerk, A.; Sugden, P.H. The p38-MAPK inhibitor, SB203580, inhibits cardiac stress-activated protein kinases/c-Jun N-terminal kinases (SAPKs/JNKs). FEBS Lett. 1998, 426, 93–96. [Google Scholar] [CrossRef] [Green Version]
- Okubo, K.; Kamiya, M.; Urano, Y.; Nishi, H.; Herter, J.M.; Mayadas, T.; Hirohama, D.; Suzuki, K.; Kawakami, H.; Tanaka, M.; et al. Lactoferrin Suppresses Neutrophil Extracellular Traps Release in Inflammation. eBioMedicine 2016, 10, 204–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kenny, E.F.; Herzig, A.; Krüger, R.; Muth, A.; Mondal, S.; Thompson, P.R.; Brinkmann, V.; Von Bernuth, H.; Zychlinsky, A. Diverse stimuli engage different neutrophil extracellular trap pathways. eLife 2017, 6, e24437. [Google Scholar] [CrossRef]
- Turner, J.; Cho, Y.; Dinh, N.-N.; Waring, A.J.; Lehrer, R.I. Activities of LL-37, a Cathelin-Associated Antimicrobial Peptide of Human Neutrophils. Antimicrob. Agents Chemother. 1998, 42, 2206–2214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dürr, U.H.; Sudheendra, U.; Ramamoorthy, A. LL-37, the only human member of the cathelicidin family of antimicrobial peptides. Biochim. et Biophys. Acta (BBA) Biomembr. 2006, 1758, 1408–1425. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Hou, L.; Lu, C.; Wang, Q.; Pan, B.; Wang, Q.; Tian, J.; Ge, L. Enteral Lactoferrin Supplementation for Preventing Sepsis and Necrotizing Enterocolitis in Preterm Infants: A Meta-Analysis with Trial Sequential Analysis of Randomized Controlled Trials. Front. Pharmacol. 2020, 11, 1186. [Google Scholar] [CrossRef] [PubMed]
- Wolf, Y.; Shemer, A.; Polonsky, M.; Gross, M.; Mildner, A.; Yona, S.; David, E.; Kim, K.-W.; Goldmann, T.; Amit, I.; et al. Autonomous TNF is critical for in vivo monocyte survival in steady state and inflammation. J. Exp. Med. 2017, 214, 905–917. [Google Scholar] [CrossRef] [PubMed]
- Jovinge, S.; Ares, M.P.; Kallin, B.; Nilsson, J. Human Monocytes/Macrophages Release TNF-α in Response to Ox-LDL. Arter. Thromb. Vasc. Biol. 1996, 16, 1573–1579. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.; Poon, G.F.T.; Birkenhead, D.; Pena, O.M.; Falsafi, R.; Dahlgren, C.; Karlsson, A.; Bylund, J.; Hancock, R.; Johnson, P. Host Defense Peptide LL-37 Selectively Reduces Proinflammatory Macrophage Responses. J. Immunol. 2011, 186, 5497–5505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alalwani, S.M.; Sierigk, J.; Herr, C.; Pinkenburg, O.; Gallo, R.; Vogelmeier, C.; Bals, R. The antimicrobial peptide LL-37 modulates the inflammatory and host defense response of human neutrophils. Eur. J. Immunol. 2010, 40, 1118–1126. [Google Scholar] [CrossRef]
- Zhang, G.-H.; Mann, D.M.; Tsai, C.-M. Neutralization of Endotoxin In Vitro and In Vivo by a Human Lactoferrin-Derived Peptide. Infect. Immun. 1999, 67, 1353–1358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lactoferrin in Health and Disease-PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/17507874/ (accessed on 2 February 2022).
- Oyarzún, C.P.M.; Carestia, A.; Lev, P.R.; Glembotsky, A.C.; Ríos, M.A.C.; Moiraghi, B.; Molinas, F.C.; Marta, R.F.; Schattner, M.; Heller, P.G. Neutrophil extracellular trap formation and circulating nucleosomes in patients with chronic myeloproliferative neoplasms. Sci. Rep. 2016, 6, 38738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pilsczek, F.H.; Salina, D.; Poon, K.K.H.; Fahey, C.; Yipp, B.G.; Sibley, C.D.; Robbins, S.M.; Green, F.H.Y.; Surette, M.G.; Sugai, M.; et al. A Novel Mechanism of Rapid Nuclear Neutrophil Extracellular Trap Formation in Response to Staphylococcus aureus. J. Immunol. 2010, 185, 7413–7425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rochael, N.C.; Guimarães-Costa, A.B.; Nascimento, M.T.C.; DeSouza-Vieira, T.S.; De Oliveira, M.P.; Souza, L.F.G.E.; Oliveira, M.F.; Saraiva, E.M. Classical ROS-dependent and early/rapid ROS-independent release of Neutrophil Extracellular Traps triggered by Leishmania parasites. Sci. Rep. 2015, 5, 18302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arai, Y.; Nishinaka, Y.; Arai, T.; Morita, M.; Mizugishi, K.; Adachi, S.; Takaori-Kondo, A.; Watanabe, T.; Yamashita, K. Uric acid induces NADPH oxidase-independent neutrophil extracellular trap formation. Biochem. Biophys. Res. Commun. 2014, 443, 556–561. [Google Scholar] [CrossRef] [PubMed]
- Villagra-Blanco, R.; Silva, L.; Gärtner, U.; Wagner, H.; Failing, K.; Wehrend, A.; Taubert, A.; Hermosilla, C. Molecular analyses on Neospora caninum -triggered NETosis in the caprine system. Dev. Comp. Immunol. 2017, 72, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Hakkim, A.; Fuchs, T.A.; Martinez, N.E.; Hess, S.; Prinz, H.; Zychlinsky, A.; Waldmann, H. Activation of the Raf-MEK-ERK pathway is required for neutrophil extracellular trap formation. Nat. Chem. Biol. 2011, 7, 75–77. [Google Scholar] [CrossRef]
- Wei, Z.; Hermosilla, C.; Taubert, A.; He, X.; Wang, X.; Gong, P.; Li, J.; Yang, Z.; Zhang, X. Canine Neutrophil Extracellular Traps Release Induced by the Apicomplexan Parasite Neospora caninum In Vitro. Front. Immunol. 2016, 7, 436. [Google Scholar] [CrossRef] [Green Version]
- Clemens, R.A.; Lowell, C.A. Store-operated calcium signaling in neutrophils. J. Leukoc. Biol. 2015, 98, 497–502. [Google Scholar] [CrossRef] [Green Version]
- Villagra-Blanco, R.; Silva, L.M.R.; Muñoz-Caro, T.; Yang, Z.; Li, J.; Gärtner, U.; Taubert, A.; Zhang, X.; Hermosilla, C. Bovine Polymorphonuclear Neutrophils Cast Neutrophil Extracellular Traps against the Abortive Parasite Neospora caninum. Front. Immunol. 2017, 8, 606. [Google Scholar] [CrossRef]
- Zhang, Z.; Cherryholmes, G.; Chang, F.; Rose, D.M.; Schraufstatter, I.; Shively, J.E. Evidence that cathelicidin peptide LL-37 may act as a functional ligand for CXCR2 on human neutrophils. Eur. J. Immunol. 2009, 39, 3181–3194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomasinsig, L.; Pizzirani, C.; Skerlavaj, B.; Pellegatti, P.; Gulinelli, S.; Tossi, A.; Di Virgilio, F.; Zanetti, M. The Human Cathelicidin LL-37 Modulates the Activities of the P2X7 Receptor in a Structure-dependent Manner. J. Biol. Chem. 2008, 283, 30471–30481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grigorieva, D.V.; Gorudko, I.V.; Shamova, E.V.; Terekhova, M.S.; Maliushkova, E.V.; Semak, I.V.; Cherenkevich, S.N.; Sokolov, A.V.; Timoshenko, A.V. Effects of recombinant human lactoferrin on calcium signaling and functional responses of human neutrophils. Arch. Biochem. Biophys. 2019, 675, 108122. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Tu, Y.; Xie, S.; Liu, X.S.; Song, Y.; Wang, S.; Chen, X.; Lu, L. A Role for Receptor-Interacting Protein Kinase-1 in Neutrophil Extracellular Trap Formation in Patients with Systemic Lupus Erythematosus: A Preliminary Study. Cell. Physiol. Biochem. 2018, 45, 2317–2328. [Google Scholar] [CrossRef] [PubMed]
- Gan, T.; Yang, Y.; Hu, F.; Chen, X.; Zhou, J.; Li, Y.; Xu, Y.; Wang, H.; Chen, Y.; Zhang, M. TLR3 Regulated Poly I:C-Induced Neutrophil Extracellular Traps and Acute Lung Injury Partly Through p38 MAP Kinase. Front. Microbiol. 2018, 9, 3174. [Google Scholar] [CrossRef]
- Neeli, I.; Radic, M. Opposition between PKC isoforms regulates histone deimination and neutrophil extracellular chromatin release. Front. Immunol. 2013, 4, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wan, M.; Soehnlein, O.; Tang, X.; van der Does, A.M.; Smedler, E.; Uhlén, P.; Lindbom, L.; Agerberth, B.; Haeggström, J.Z. Cathelicidin LL-37 induces time-resolved release of LTB 4 and TXA 2 by human macrophages and triggers eicosanoid generation in vivo. FASEB J. 2014, 28, 3456–3467. [Google Scholar] [CrossRef] [Green Version]
- Ikoma-Seki, K.; Nakamura, K.; Morishita, S.; Ono, T.; Sugiyama, K.; Nishino, H.; Hirano, H.; Murakoshi, M. Role of LRP1 and ERK and cAMP Signaling Pathways in Lactoferrin-Induced Lipolysis in Mature Rat Adipocytes. PLoS ONE 2015, 10, e0141378. [Google Scholar] [CrossRef] [Green Version]
- Neeli, I.; Dwivedi, N.; Khan, S.; Radic, M. Regulation of Extracellular Chromatin Release from Neutrophils. J. Innate Immun. 2009, 1, 194–201. [Google Scholar] [CrossRef]
- Neubert, E.; Meyer, D.; Rocca, F.; Günay, G.; Kwaczala-Tessmann, A.; Grandke, J.; Senger-Sander, S.; Geisler, C.; Egner, A.; Schön, M.P.; et al. Chromatin swelling drives neutrophil extracellular trap release. Nat. Commun. 2018, 9, 1–13. [Google Scholar] [CrossRef]
- Hou, R.-Q.; Scharnagl, N.; Willumeit, R.; Feyerabend, F. Different effects of single protein vs. protein mixtures on magnesium degradation under cell culture conditions. Acta Biomater. 2019, 98, 256–268. [Google Scholar] [CrossRef] [PubMed]
- Urban, C.F.; Ermert, D.; Schmid, M.; Abu-Abed, U.; Goosmann, C.; Nacken, W.; Brinkmann, V.; Jungblut, P.R.; Zychlinsky, A. Neutrophil Extracellular Traps Contain Calprotectin, a Cytosolic Protein Complex Involved in Host Defense against Candida albicans. PLoS Pathog. 2009, 5, e1000639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thammavongsa, V.; Missiakas, D.M.; Schneewind, O. Staphylococcus aureus Degrades Neutrophil Extracellular Traps to Promote Immune Cell Death. Science 2013, 342, 863–866. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schwäbe, F.V.; Happonen, L.; Ekestubbe, S.; Neumann, A. Host Defense Peptides LL-37 and Lactoferrin Trigger ET Release from Blood-Derived Circulating Monocytes. Biomedicines 2022, 10, 469. https://doi.org/10.3390/biomedicines10020469
Schwäbe FV, Happonen L, Ekestubbe S, Neumann A. Host Defense Peptides LL-37 and Lactoferrin Trigger ET Release from Blood-Derived Circulating Monocytes. Biomedicines. 2022; 10(2):469. https://doi.org/10.3390/biomedicines10020469
Chicago/Turabian StyleSchwäbe, Frederic V., Lotta Happonen, Sofie Ekestubbe, and Ariane Neumann. 2022. "Host Defense Peptides LL-37 and Lactoferrin Trigger ET Release from Blood-Derived Circulating Monocytes" Biomedicines 10, no. 2: 469. https://doi.org/10.3390/biomedicines10020469
APA StyleSchwäbe, F. V., Happonen, L., Ekestubbe, S., & Neumann, A. (2022). Host Defense Peptides LL-37 and Lactoferrin Trigger ET Release from Blood-Derived Circulating Monocytes. Biomedicines, 10(2), 469. https://doi.org/10.3390/biomedicines10020469





