Endoglin Modulates TGFβR2 Induced VEGF and Proinflammatory Cytokine Axis Mediated Angiogenesis in Prolonged DEHP-Exposed Breast Cancer Cells
Abstract
1. Introduction
2. Materials and Method
2.1. Cell Culture
2.2. Reagents and Antibodies
2.3. DEHP Exposure and Stable Clone Establishment
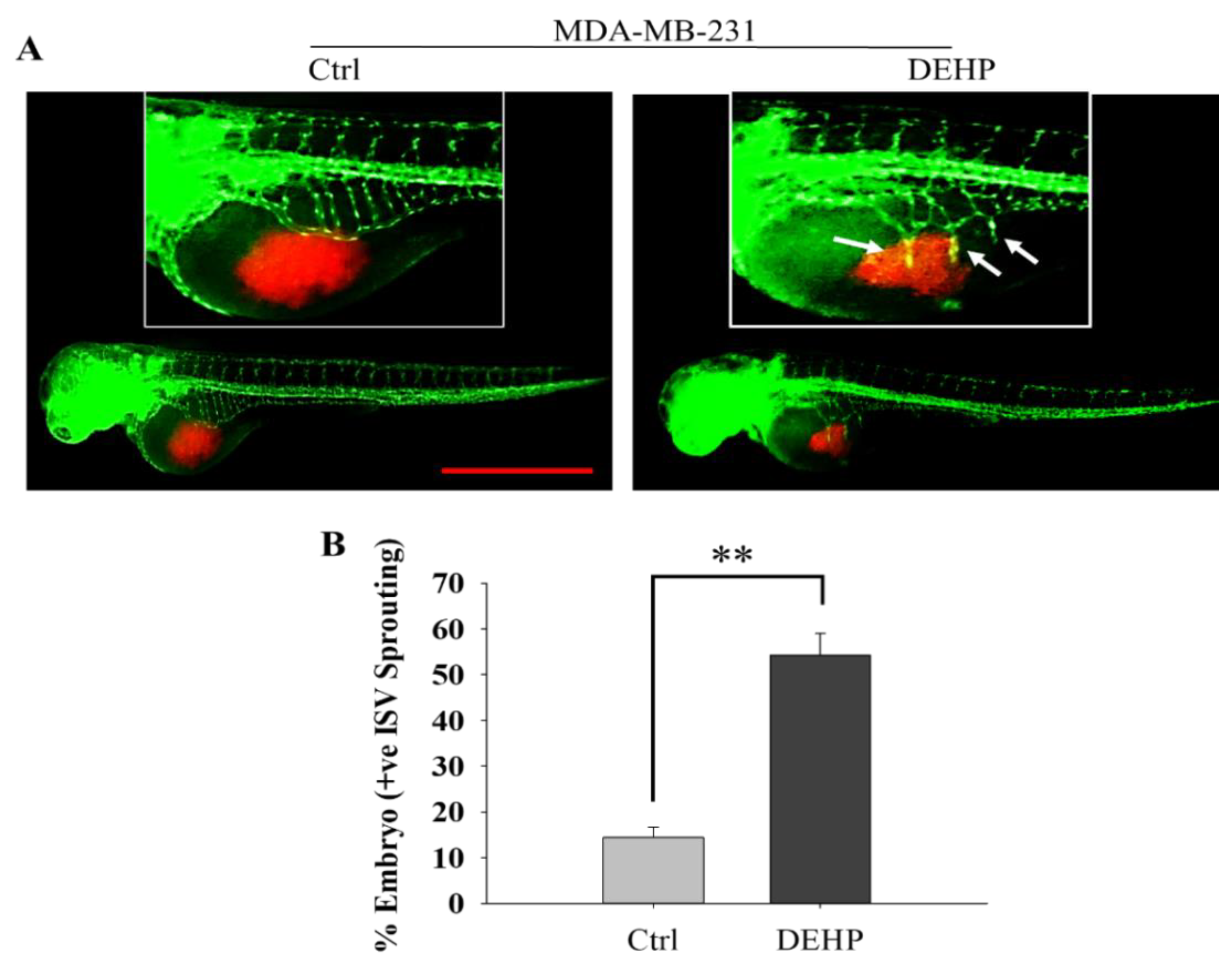
2.4. Zebrafish Xenograft
2.5. RNA Sequencing
2.6. Lentiviral Transfection
2.7. Quantitative Polymerase Chain Reaction (qPCR)
2.8. Western Blotting
2.9. In Vitro-Angiogenesis/Tube Formation Assay
2.10. Quantitative Enzyme-Linked Immunosorbent Assay (ELISA)
2.11. Immunofluorescence
2.12. Antibody Angiogenesis Array
2.13. Statistical Analysis
3. Results
3.1. Prolonged DEHP Exposure Enhances the Angiogenesis Potential of MDA-MB-231 Cells In Vivo
3.2. Endoglin Predicted as a Regulator of DEHP-Induced Angiogenesis in Breast Cancer Cells
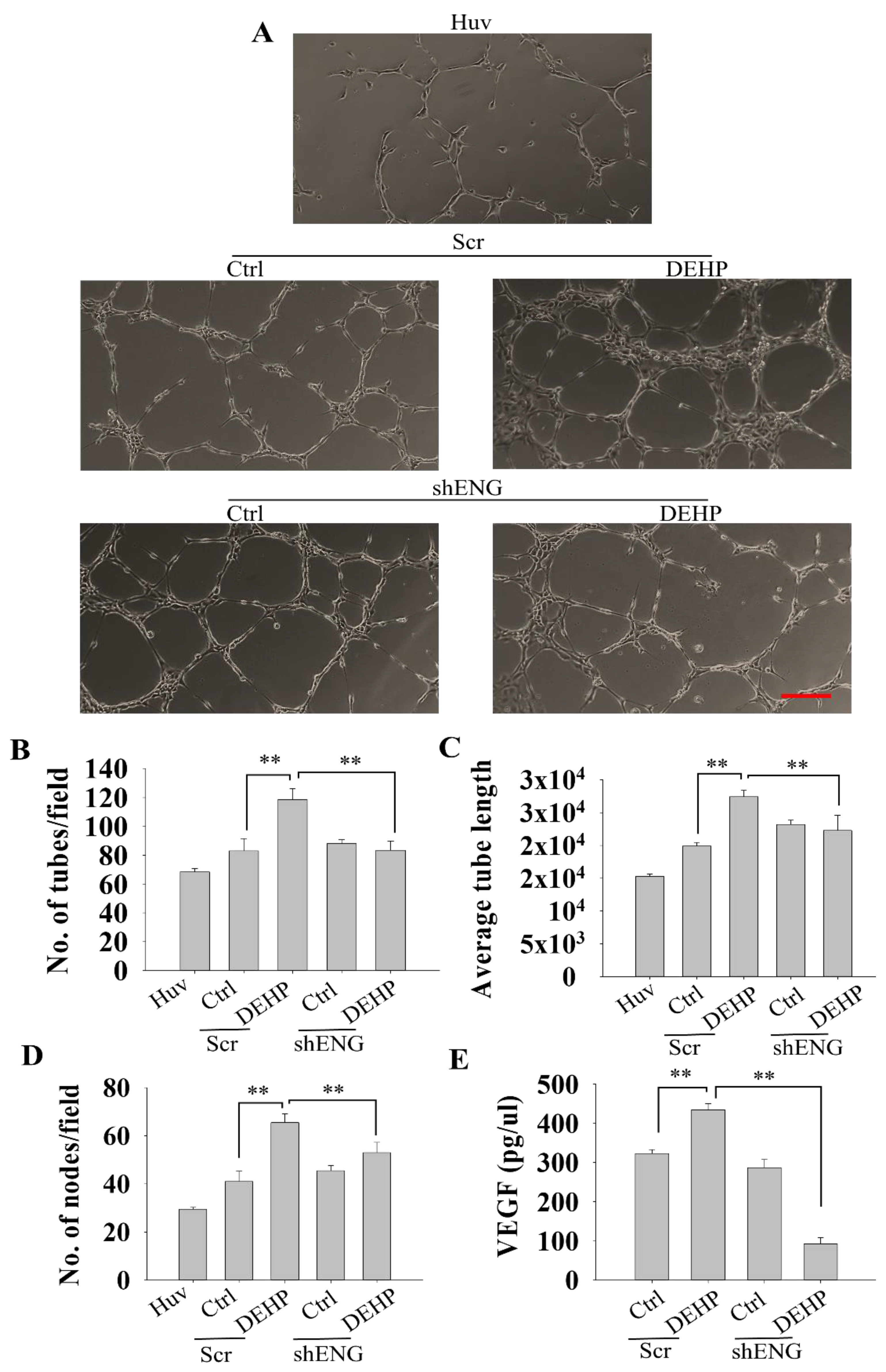
3.3. Endoglin Depletion Reverses the DEHP-Induced Angiogenic Potential of MDA-MB-231 Cells
3.4. Endoglin Regulates HUVEC Tube Formation through VEGF Production in Prolonged DEHP-Treated MDA-MB-231 Cells
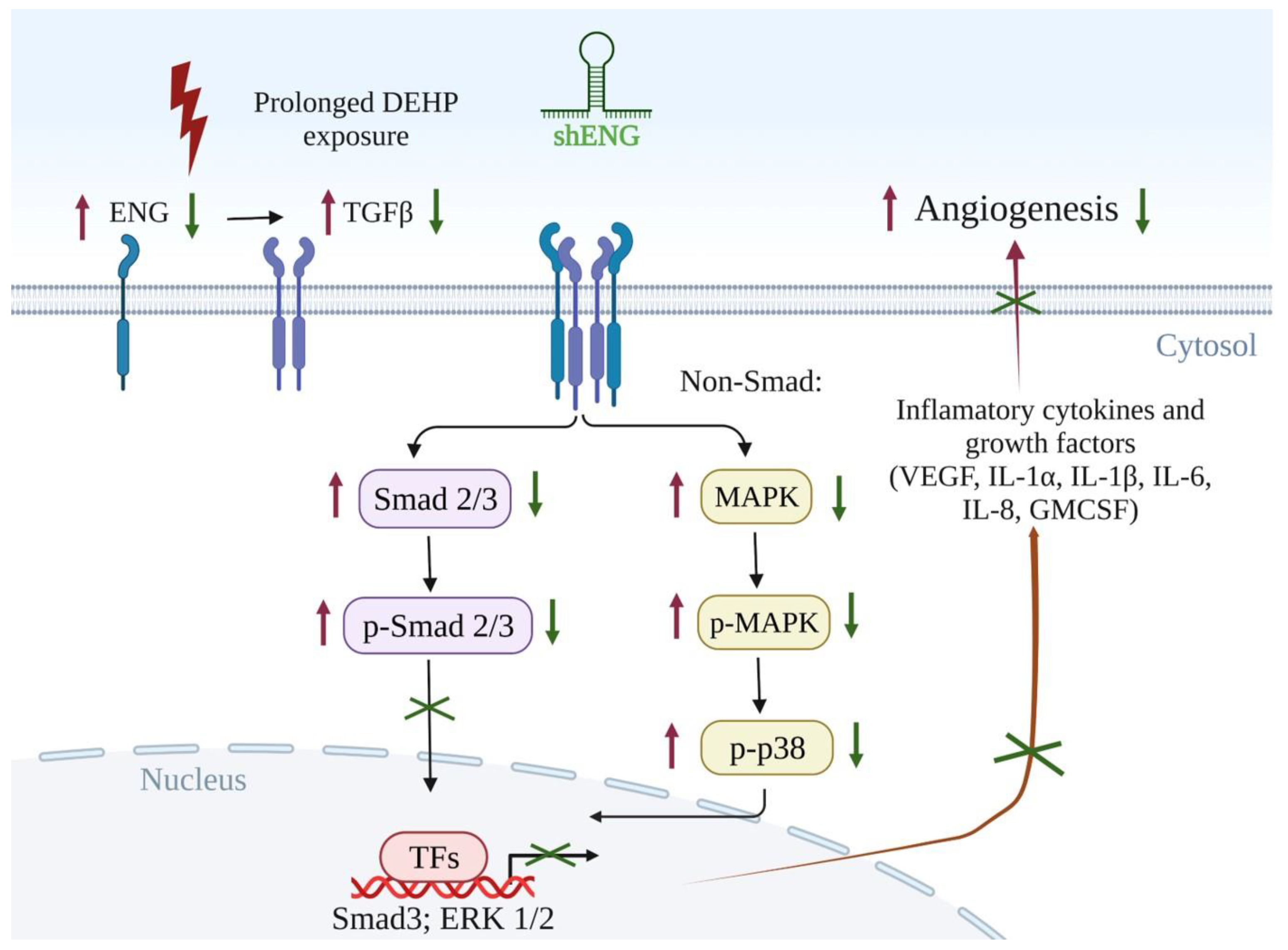
3.5. Endoglin Maintains the TGFβ/SMAD3/VEGF Signaling Axis in Prolonged DEHP-Treated MDA-MB-231 Cells
3.6. Endoglin-Mediated MAPK/p38 Signaling and Secretory Cytokine Production May Contribute to DEHP-Induced Angiogenesis in MDA-MB-231 Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carmeliet, P.; Jain, R.K. Molecular mechanisms and clinical applications of angiogenesis. Nature 2011, 473, 298–307. [Google Scholar] [CrossRef]
- Ghajar, C.M.; George, S.C.; Putnam, A.J. Matrix metalloproteinase control of capillary morphogenesis. Crit. Rev. Eukaryot. Gene Expr. 2008, 18, 251–278. [Google Scholar] [CrossRef] [PubMed]
- Bryan, B.A.; D’amore, P.A. What tangled webs they weave: Rho-GTPase control of angiogenesis. Cell. Mol. Life Sci. 2007, 64, 2053–2065. [Google Scholar] [CrossRef] [PubMed]
- Sukriti, S.; Tauseef, M.; Yazbeck, P.; Mehta, D. Mechanisms Regulating Endothelial Permeability. Pulm. Circ. 2014, 4, 535–551. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N.; Gerber, H.-P.; LeCouter, J. The biology of VEGF and its receptors. Nat. Med. 2003, 9, 669–676. [Google Scholar] [CrossRef]
- Maugeri, G.; D’Amico, A.G.; Federico, C.; Saccone, S.; Giunta, S.; Cavallaro, S.; D’Agata, V. Involvement of A3 Adenosine Receptor in Neuroblastoma Progression via Modulation of the Hypoxic/Angiogenic Pathway. J. Mol. Neurosci. 2019, 69, 166–176. [Google Scholar] [CrossRef]
- Shen, W.; Li, H.-L.; Liu, L.-Z.; Cheng, J.-X. Expression levels of PTEN, HIF-1α, and VEGF as prognostic factors in ovarian cancer. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 2596–2603. [Google Scholar]
- Maugeri, G.; D’Amico, A.; Saccone, S.; Federico, C.; Rasà, D.; Caltabiano, R.; Broggi, G.; Giunta, S.; Musumeci, G.; D’Agata, V. Effect of PACAP on Hypoxia-Induced Angiogenesis and Epithelial–Mesenchymal Transition in Glioblastoma. Biomedicines 2021, 9, 965. [Google Scholar] [CrossRef]
- D’Amico, A.G.; D’Amico, A.G.; Maugeri, G.; Vanella, L.; Pittalà, V.; Reglodi, D.; D’Agata, V. Multimodal Role of PACAP in Glioblastoma. Brain Sci. 2021, 11, 994. [Google Scholar] [CrossRef]
- Maugeri, G.; D’amico, A.G.; Rasà, D.M.; Saccone, S.; Federico, C.; Magro, G.; Cavallaro, S.; D’agata, V. Caffeine Effect on HIFs/VEGF Pathway in Human Glioblastoma Cells Exposed to Hypoxia. Anti-Cancer Agents Med. Chem. 2018, 18, 1432–1439. [Google Scholar] [CrossRef]
- Wang, J.-C.; Li, G.-Y.; Li, P.-P.; Sun, X.; Li, W.-M.; Li, Y.; Lu, S.-Y.; Liu, P.-J. Suppression of hypoxia-induced excessive angiogenesis by metformin via elevating tumor blood perfusion. Oncotarget 2017, 8, 73892–73904. [Google Scholar] [CrossRef]
- Jadhao, M.; Tsai, E.-M.; Yang, H.-C.; Chen, Y.-F.; Liang, S.-S.; Wang, T.-N.; Teng, Y.-N.; Huang, H.-W.; Wang, L.-F.; Chiu, C.-C. The Long-Term DEHP Exposure Confers Multidrug Resistance of Triple-Negative Breast Cancer Cells through ABC Transporters and Intracellular ROS. Antioxidants 2021, 10, 949. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Lai, U.H.; Zhu, L.; Singh, A.; Ahmed, M.; Forsyth, N.R. Reactive Oxygen Species Formation in the Brain at Different Oxygen Levels: The Role of Hypoxia Inducible Factors. Front. Cell Dev. Biol. 2018, 6, 132. [Google Scholar] [CrossRef] [PubMed]
- Chulpanova, D.S.; Kitaeva, K.V.; Green, A.R.; Rizvanov, A.A.; Solovyeva, V.V. Molecular Aspects and Future Perspectives of Cytokine-Based Anti-cancer Immunotherapy. Front. Cell Dev. Biol. 2020, 8, 402. [Google Scholar] [CrossRef]
- Nishida, N.; Yano, H.; Nishida, T.; Kamura, T.; Kojiro, M. Angiogenesis in cancer. Vasc. Health Risk Manag. 2006, 2, 213–219. [Google Scholar] [CrossRef]
- Gore, A.C.; Chappell, V.A.; Fenton, S.E.; Flaws, J.A.; Nadal, A.; Prins, G.S.; Toppari, J.; Zoeller, R.T. EDC-2: The Endocrine Society’s Second Scientific Statement on Endocrine-Disrupting Chemicals. Endocr. Rev. 2015, 36, E1–E150. [Google Scholar] [PubMed]
- Teitelbaum, S.L.; Belpoggi, F.; Reinlib, L. Advancing research on endocrine disrupting chemicals in breast cancer: Expert panel recommendations. Reprod. Toxicol. 2015, 54, 141–147. [Google Scholar] [CrossRef]
- Wang, Y.; Qian, H. Phthalates and Their Impacts on Human Health. Healthcare 2021, 9, 603. [Google Scholar] [CrossRef]
- Wormuth, M.; Scheringer, M.; Vollenweider, M.; Hungerbühler, K. What are the sources of exposure to eight frequently used phthalic acid esters in Europeans? Risk Anal. 2006, 26, 803–824. [Google Scholar] [CrossRef]
- Panagiotou, E.M.; Ojasalo, V.; Damdimopoulou, P. Phthalates, ovarian function and fertility in adulthood. Best Pract. Res. Clin. Endocrinol. Metab. 2021, 35, 101552. [Google Scholar] [CrossRef]
- Tanay Das, M.; Kumar, M.; Thakur, I.S. Differential toxicological endpoints of di(2-ethylhexyl) phthalate (DEHP) exposure in MCF-7 and MDA-MB-231 cell lines: Possible estrogen receptor alpha (ERalpha) independent modulations. Indian J. Exp. Biol. 2014, 52, 1052–1061. [Google Scholar] [PubMed]
- Rowdhwal, S.S.S.; Chen, J. Toxic Effects of Di-2-ethylhexyl Phthalate: An Overview. BioMed Res. Int. 2018, 2018, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zarean, M.; Keikha, M.; Poursafa, P.; Khalighinejad, P.; Amin, M.; Kelishadi, R. A systematic review on the adverse health effects of di-2-ethylhexyl phthalate. Environ. Sci. Pollut. Res. 2016, 23, 24642–24693. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H. Analysis of the in vitro effects of di-(2-ethylhexyl) phthalate exposure on human uterine leiomyoma cells. Exp. Ther. Med. 2018, 15, 4972–4978. [Google Scholar] [CrossRef]
- Kim, J.H. Di(2-ethylhexyl) phthalate promotes lung cancer cell line A549 progression via Wnt/β-catenin signaling. J. Toxicol. Sci. 2019, 44, 237–244. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, T.-L.; Zong, T.; Chen, Y.-L.; Ren, M.; Yu, X.-C.; Kuang, H.-B. Effect of di-(2-ethylhexyl) phthalate exposure on placental development in pregnant mice. J. South. Med. Univ. 2016, 36, 467–471. [Google Scholar]
- Ferguson, K.K.; McElrath, T.F.; Cantonwine, D.E.; Mukherjee, B.; Meeker, J.D. Phthalate metabolites and bisphenol-A in association with circulating angiogenic biomarkers across pregnancy. Placenta 2015, 36, 699–703. [Google Scholar] [CrossRef]
- Zhu, M.; Huang, C.; Ma, X.; Wu, R.; Zhu, W.; Li, X.; Liang, Z.; Deng, F.; Wu, J.; Geng, S.; et al. Phthalates promote prostate cancer cell proliferation through activation of ERK5 and p38. Environ. Toxicol. Pharmacol. 2018, 63, 29–33. [Google Scholar] [CrossRef]
- Bhattacharya, N.; Dufour, J.M.; Vo, M.-N.; Okita, J.; Okita, R.; Kim, K.H. Differential Effects of Phthalates on the Testis and the Liver1. Biol. Reprod. 2005, 72, 745–754. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, W.; Zhang, J.; Li, H. Role of JNK and ERK1/2 MAPK signaling pathway in testicular injury of rats induced by di-N-butyl-phthalate (DBP). Biol. Res. 2019, 52, 41. [Google Scholar] [CrossRef]
- Gougos, A.; Letarte, M. Primary structure of endoglin, an RGD-containing glycoprotein of human endothelial cells. J. Biol. Chem. 1990, 265, 8361–8364. [Google Scholar] [CrossRef]
- Miller, D.W.; Graulich, W.; Karges, B.; Stahl, S.; Ernst, M.; Ramaswamy, A.; Sedlacek, H.H.; Müller, R.; Adamkiewicz, J. Elevated expression of endoglin, a component of the TGF-beta-receptor complex, correlates with proliferation of tumor endothelial cells. Int. J. Cancer 1999, 81, 568–572. [Google Scholar] [CrossRef]
- Cheifetz, S.; Bellón, T.; Calés, C.; Vera, S.; Bernabeu, C.; Massagué, J.; Letarte, M. Endoglin is a component of the transforming growth factor-beta receptor system in human endothelial cells. J. Biol. Chem. 1992, 267, 19027–19030. [Google Scholar] [CrossRef]
- Koleva, R.I.; Conley, B.A.; Romero, D.; Riley, K.S.; Marto, J.A.; Lux, A.; Vary, C.P. Endoglin structure and function: Determinants of endoglin phosphorylation by transforming growth factor-beta receptors. J. Biol. Chem. 2006, 281, 25110–25123. [Google Scholar] [CrossRef]
- Liu, Z.; Lebrin, F.; Maring, J.A.; Driesche, S.V.D.; Van Der Brink, S.; van Dinther, M.; Thorikay, M.; Martin, S.; Kobayashi, K.; Hawinkels, L.J.A.C.; et al. ENDOGLIN Is Dispensable for Vasculogenesis, but Required for Vascular Endothelial Growth Factor-Induced Angiogenesis. PLoS ONE 2014, 9, e86273. [Google Scholar] [CrossRef]
- Barbara, N.P.; Wrana, J.L.; Letarte, M. Endoglin is an accessory protein that interacts with the signaling receptor complex of multiple members of the transforming growth factor-beta superfamily. J. Biol. Chem. 1999, 274, 584–594. [Google Scholar] [CrossRef] [PubMed]
- Hata, A.; Chen, Y.G. TGF-beta Signaling from Receptors to Smads. Cold Spring Harb. Perspect. Biol. 2016, 8, a022061. [Google Scholar] [CrossRef] [PubMed]
- Nassiri, F.; Cusimano, M.D.; Scheithauer, B.W.; Rotondo, F.; Fazio, A.; Yousef, G.M.; Syro, L.V.; Kovacs, K.; Lloyd, R.V. Endoglin (CD105): A review of its role in angiogenesis and tumor diagnosis, progression and therapy. Anticancer Res. 2011, 31, 2283–2290. [Google Scholar] [PubMed]
- Beresford, M.; Harris, A.L.; Ah-See, M.; Daley, F.; Padhani, A.R.; Makris, A. The relationship of the neo-angiogenic marker, endoglin, with response to neoadjuvant chemotherapy in breast cancer. Br. J. Cancer 2006, 95, 1683–1688. [Google Scholar] [CrossRef] [PubMed]
- Brewer, C.A.; Setterdahl, J.J.; Li, M.J.; Johnston, J.M.; Mann, J.L.; McAsey, M.E. Endoglin expression as a measure of microvessel density in cervical cancer. Obstet. Gynecol. 2000, 96, 224–228. [Google Scholar]
- Wikström, P.; Lissbrant, I.F.; Stattin, P.; Egevad, L.; Bergh, A. Endoglin (CD105) is expressed on immature blood vessels and is a marker for survival in prostate cancer. Prostate 2002, 51, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Jonker, L.; Arthur, H.M. Endoglin expression in early development is associated with vasculogenesis and angiogenesis. Mech. Dev. 2002, 110, 193–196. [Google Scholar] [CrossRef]
- Torsney, E.; Charlton, R.; Parums, D.; Collis, M.; Arthur, H.M. Inducible expression of human endoglin during inflammation and wound healing in vivo. Agents Actions 2002, 51, 464–470. [Google Scholar] [CrossRef]
- Li, D.Y.; Sorensen, L.K.; Brooke, B.S.; Urness, L.D.; Davis, E.C.; Taylor, D.G.; Boak, B.B.; Wendel, D.P. Defective Angiogenesis in Mice Lacking Endoglin. Science 1999, 284, 1534–1537. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, D.; Zhao, L.-M.; Zhao, X.-L.; Shen, J.-J.; Xie, Y.; Cao, L.-L.; Chen, Z.-B.; Luo, Y.-M.; Bao, B.-H.; et al. Endoglin is necessary for angiogenesis in human ovarian carcinoma-derived primary endothelial cells. Cancer Biol. Ther. 2013, 14, 937–948. [Google Scholar] [CrossRef][Green Version]
- Dolinsek, T.; Markelc, B.; Sersa, G.; Coer, A.; Stimac, M.; Lavrencak, J.; Brozic, A.; Kranjc, S.; Cemazar, M. Multiple Delivery of siRNA against Endoglin into Murine Mammary Adenocarcinoma Prevents Angiogenesis and Delays Tumor Growth. PLoS ONE 2013, 8, e58723. [Google Scholar] [CrossRef]
- Tian, H.; Huang, J.J.; Golzio, C.; Gao, X.; Hector-Greene, M.; Katsanis, N.; Blobe, G.C. Endoglin interacts with VEGFR2 to promote angiogenesis. FASEB J. 2018, 32, 2934–2949. [Google Scholar] [CrossRef]
- Nellinger, S.; Schmidt, I.; Heine, S.; Volz, A.; Kluger, P.J. Adipose stem cell-derived extracellular matrix represents a promising biomaterial by inducing spontaneous formation of prevascular-like structures by mvECs. Biotechnol. Bioeng. 2020, 117, 3160–3172. [Google Scholar] [CrossRef]
- Folkman, J. Role of angiogenesis in tumor growth and metastasis. Semin. Oncol. 2002, 29, 15–18. [Google Scholar] [CrossRef]
- Lugano, R.; Ramachandran, M.; Dimberg, A. Tumor angiogenesis: Causes, consequences, challenges and opportunities. Cell. Mol. Life Sci. 2020, 77, 1745–1770. [Google Scholar] [CrossRef]
- Sun, Y.; Shen, J.; Zeng, L.; Yang, D.; Shao, S.; Wang, J.; Wei, J.; Xiong, J.; Chen, J. Role of autophagy in di-2-ethylhexyl phthalate (DEHP)-induced apoptosis in mouse Leydig cells. Environ. Pollut. 2018, 243, 563–572. [Google Scholar] [CrossRef]
- Wang, W.; Leung, A.O.W.; Chu, L.H.; Wong, M.H. Phthalates contamination in China: Status, trends and human exposure-with an emphasis on oral intake. Environ. Pollut. 2018, 238, 771–782. [Google Scholar] [CrossRef] [PubMed]
- Yen, P.L.; How, C.M.; Liao, V.H.-C. Early-life and chronic exposure to di(2-ethylhexyl) phthalate enhances amyloid-beta toxicity associated with an autophagy-related gene in Caenorhabditis elegans Alzheimer’s disease models. Chemosphere 2021, 273, 128594. [Google Scholar] [CrossRef] [PubMed]
- Khasin, L.G.; Della Rosa, J.; Petersen, N.; Moeller, J.; Kriegsfeld, L.J.; Lishko, P.V. The Impact of Di-2-Ethylhexyl Phthalate on Sperm Fertility. Front. Cell Dev. Biol. 2020, 8, 426. [Google Scholar] [CrossRef] [PubMed]
- Crobeddu, B.; Ferraris, E.; Kolasa, E.; Plante, I. Di(2-ethylhexyl) phthalate (DEHP) increases proliferation of epithelial breast cancer cells through progesterone receptor dysregulation. Environ. Res. 2019, 173, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Barakat, R.; Lin, P.-C.P.; Rattan, S.; Brehm, E.; Canisso, I.F.; Abosalum, M.E.; Flaws, J.A.; Hess, R.; Ko, C. Prenatal Exposure to DEHP Induces Premature Reproductive Senescence in Male Mice. Toxicol. Sci. 2017, 156, 96–108. [Google Scholar] [CrossRef] [PubMed]
- Brehm, E.; Rattan, S.; Gao, L.; Flaws, J.A. Prenatal Exposure to Di(2-Ethylhexyl) Phthalate Causes Long-Term Transgenerational Effects on Female Reproduction in Mice. Endocrinology 2017, 159, 795–809. [Google Scholar] [CrossRef]
- Martínez-Razo, L.D.; Martínez-Ibarra, A.; Vázquez-Martínez, E.R.; Cerbón, M. The impact of Di-(2-ethylhexyl) Phthalate and Mono(2-ethylhexyl) Phthalate in placental development, function, and pathophysiology. Environ. Int. 2020, 146, 106228. [Google Scholar] [CrossRef]
- Tsai, C.F.; Hsieh, T.H.; Lee, J.N.; Hsu, C.Y.; Wang, Y.C.; Lai, F.J.; Kuo, K.K.; Wu, H.L.; Tsai, E.M.; Kuo, P.L. Benzyl butyl phthalate induces migration, invasion, and angiogenesis of Huh7 hepatocellular carcinoma cells through nongenomic AhR/G-protein signaling. BMC Cancer 2014, 14, 556. [Google Scholar] [CrossRef]
- Rossi, E.; Bernabeu, C.; Smadja, D.M. Endoglin as an Adhesion Molecule in Mature and Progenitor Endothelial Cells: A Function Beyond TGF-beta. Front. Med. 2019, 6, 10. [Google Scholar] [CrossRef]
- Kasprzak, A.; Adamek, A. Role of Endoglin (CD105) in the Progression of Hepatocellular Carcinoma and Anti-Angiogenic Therapy. Int. J. Mol. Sci. 2018, 19, 3887. [Google Scholar] [CrossRef] [PubMed]
- Paauwe, M.; Schoonderwoerd, M.J.; Helderman, R.F.; Harryvan, T.J.; Groenewoud, A.; Van Pelt, G.W.; Bor, R.; Hemmer, D.M.; Versteeg, H.H.; Snaar-Jagalska, B.E.; et al. Endoglin Expression on Cancer-Associated Fibroblasts Regulates Invasion and Stimulates Colorectal Cancer Metastasis. Clin. Cancer Res. 2018, 24, 6331–6344. [Google Scholar] [CrossRef] [PubMed]
- Perez-Gomez, E.; Del Castillo, G.; Santibáñez, J.F.; Lêpez-Novoa, J.M.; Bernabéu, C.; Quintanilla, M. The role of the TGF-beta coreceptor endoglin in cancer. Sci. World J. 2010, 10, 2367–2384. [Google Scholar] [CrossRef] [PubMed]
- Frick, C.L.; Yarka, C.; Nunns, H.; Goentoro, L. Sensing relative signal in the Tgf-beta/Smad pathway. Proc. Natl. Acad. Sci. USA 2017, 114, E2975–E2982. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Guo, L.W.; Seedial, S.M.; Si, Y.; Wang, B.; Takayama, T.; Suwanabol, P.A.; Ghosh, S.; DiRenzo, D.; Liu, B.; et al. TGF-beta/Smad3 inhibit vascular smooth muscle cell apoptosis through an autocrine signaling mechanism involving VEGF-A. Cell Death Dis. 2014, 5, e1317. [Google Scholar] [CrossRef]
- Chang, W.-T.; Bow, Y.-D.; Fu, P.-J.; Li, C.-Y.; Wu, C.-Y.; Chang, Y.-H.; Teng, Y.-N.; Li, R.-N.; Lu, M.-C.; Liu, Y.-C.; et al. A Marine Terpenoid, Heteronemin, Induces Both the Apoptosis and Ferroptosis of Hepatocellular Carcinoma Cells and Involves the ROS and MAPK Pathways. Oxid. Med. Cell. Longev. 2021, 2021, 1–12. [Google Scholar] [CrossRef]
- Guo, Y.J.; Pan, W.W.; Liu, S.B.; Shen, Z.F.; Xu, Y.; Hu, L.L. ERK/MAPK signalling pathway and tumorigenesis. Exp. Ther. Med. 2020, 19, 1997–2007. [Google Scholar] [CrossRef]
- Luangdilok, S.; Box, C.; Harrington, K.; Rhŷs-Evans, P.; Eccles, S. MAPK and PI3K signalling differentially regulate angiogenic and lymphangiogenic cytokine secretion in squamous cell carcinoma of the head and neck. Eur. J. Cancer 2011, 47, 520–529. [Google Scholar] [CrossRef]
- Koga, Y.; Hisada, T.; Ishizuka, T.; Utsugi, M.; Ono, A.; Yatomi, M.; Kamide, Y.; Aoki-Saito, H.; Tsurumaki, H.; Dobashi, K.; et al. CREB regulates TNF-α-induced GM-CSF secretion via p38 MAPK in human lung fibroblasts. Allergol. Int. 2016, 65, 406–413. [Google Scholar] [CrossRef]
- Klemm, C.; Bruchhagen, C.; Van Krüchten, A.; Niemann, S.; Löffler, B.; Peters, G.; Ludwig, S.; Ehrhardt, C. Mitogen-activated protein kinases (MAPKs) regulate IL-6 over-production during concomitant influenza virus and Staphylococcus aureus infection. Sci. Rep. 2017, 7, srep42473. [Google Scholar] [CrossRef]
- Sinfield, J.K.; Das, A.; O’Regan, D.J.; Ball, S.G.; Porter, K.E.; Turner, N.A. p38 MAPK alpha mediates cytokine-induced IL-6 and MMP-3 expression in human cardiac fibroblasts. Biochem. Biophys. Res. Commun. 2013, 430, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Mohr, T.; Haudek-Prinz, V.; Slany, A.; Grillari, J.; Micksche, M.; Gerner, C. Proteome profiling in IL-1beta and VEGF-activated human umbilical vein endothelial cells delineates the interlink between inflammation and angiogenesis. PLoS ONE 2017, 12, e0179065. [Google Scholar] [CrossRef] [PubMed]
- Fahey, E.; Doyle, S.L. IL-1 Family Cytokine Regulation of Vascular Permeability and Angiogenesis. Front. Immunol. 2019, 10, 1426. [Google Scholar] [CrossRef]
- Middleton, K.; Jones, J.; Lwin, Z.; Coward, J.I. Interleukin-6: An angiogenic target in solid tumours. Crit. Rev. Oncol. Hematol. 2014, 89, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Gopinathan, G.; Milagre, C.; Pearce, O.; Reynolds, L.E.; Hodivala-Dilke, K.; Leinster, D.A.; Zhong, H.; Hollingsworth, R.E.; Thompson, R.G.; Whiteford, J.R.; et al. Interleukin-6 Stimulates Defective Angiogenesis. Cancer Res. 2015, 75, 3098–3107. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jadhao, M.; Chen, C.-L.; Liu, W.; Deshmukh, D.; Liao, W.-T.; Chen, J.Y.-F.; Urade, R.; Tsai, E.-M.; Hsu, S.-K.; Wang, L.-F.; et al. Endoglin Modulates TGFβR2 Induced VEGF and Proinflammatory Cytokine Axis Mediated Angiogenesis in Prolonged DEHP-Exposed Breast Cancer Cells. Biomedicines 2022, 10, 417. https://doi.org/10.3390/biomedicines10020417
Jadhao M, Chen C-L, Liu W, Deshmukh D, Liao W-T, Chen JY-F, Urade R, Tsai E-M, Hsu S-K, Wang L-F, et al. Endoglin Modulates TGFβR2 Induced VEGF and Proinflammatory Cytokine Axis Mediated Angiogenesis in Prolonged DEHP-Exposed Breast Cancer Cells. Biomedicines. 2022; 10(2):417. https://doi.org/10.3390/biomedicines10020417
Chicago/Turabian StyleJadhao, Mahendra, Chun-Lin Chen, Wangta Liu, Dhanashri Deshmukh, Wei-Ting Liao, Jeff Yi-Fu Chen, Ritesh Urade, Eing-Mei Tsai, Sheng-Kai Hsu, Li-Fang Wang, and et al. 2022. "Endoglin Modulates TGFβR2 Induced VEGF and Proinflammatory Cytokine Axis Mediated Angiogenesis in Prolonged DEHP-Exposed Breast Cancer Cells" Biomedicines 10, no. 2: 417. https://doi.org/10.3390/biomedicines10020417
APA StyleJadhao, M., Chen, C.-L., Liu, W., Deshmukh, D., Liao, W.-T., Chen, J. Y.-F., Urade, R., Tsai, E.-M., Hsu, S.-K., Wang, L.-F., & Chiu, C.-C. (2022). Endoglin Modulates TGFβR2 Induced VEGF and Proinflammatory Cytokine Axis Mediated Angiogenesis in Prolonged DEHP-Exposed Breast Cancer Cells. Biomedicines, 10(2), 417. https://doi.org/10.3390/biomedicines10020417







