Impact of Adalimumab Treatment on Interleukin-17 and Interleukin-17 Receptor Expression in Skin and Synovium of Psoriatic Arthritis Patients with Mild Psoriasis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Skin and Synovial Tissue Biopsies
2.3. Skin and Synovial Biopsy Immunohistochemical Staining
2.4. Quantification of IL-17A-, IL-17F-, IL17RA- and IL-17RC-Expressing Cells in Skin and Synovium
2.5. Statistical Analysis
3. Results
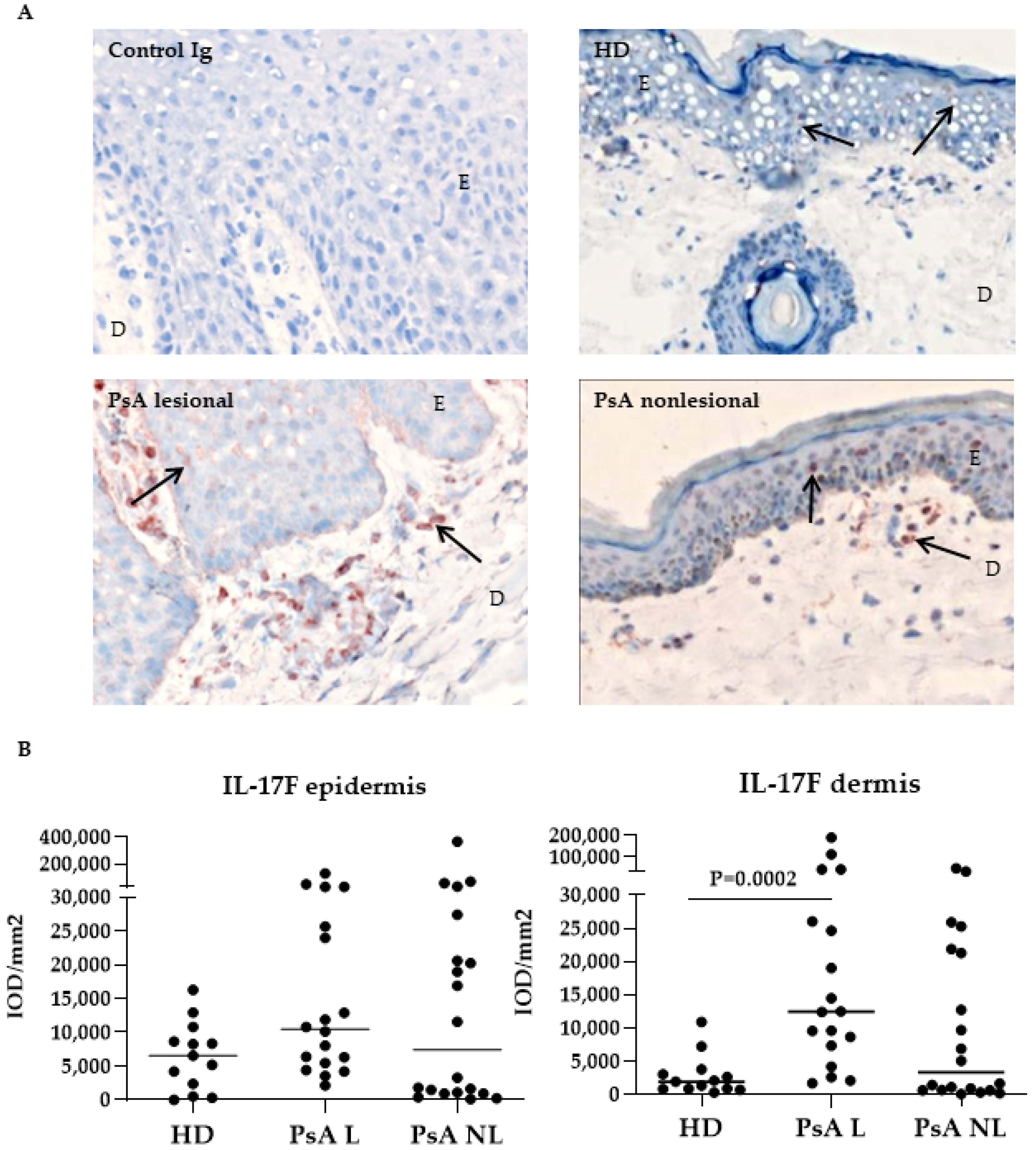
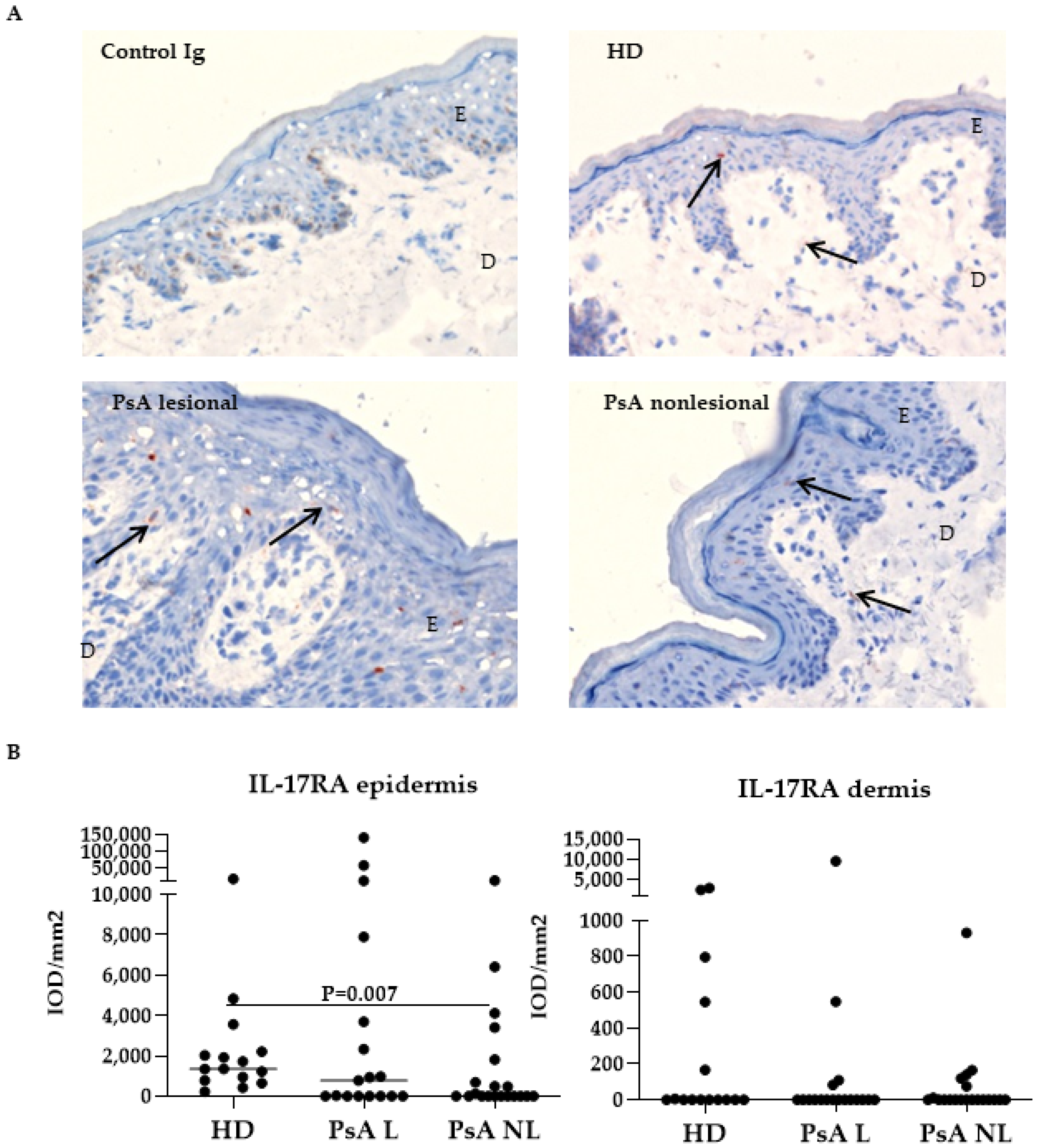
3.1. Expression of IL-17A, IL-17F and Their Receptors in Skin of Psoriatic Arthritis Patients Compared to Healthy Donors
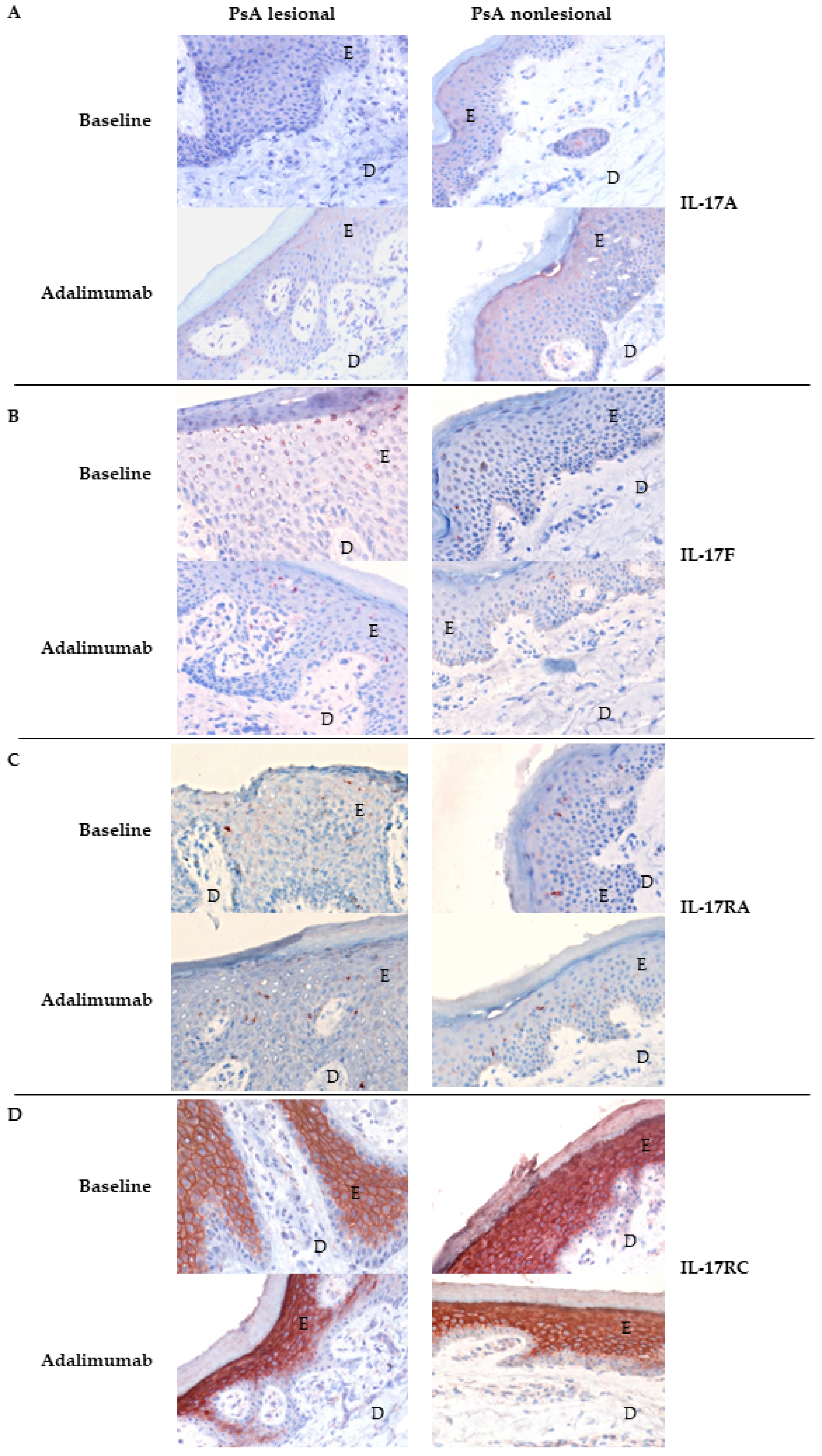
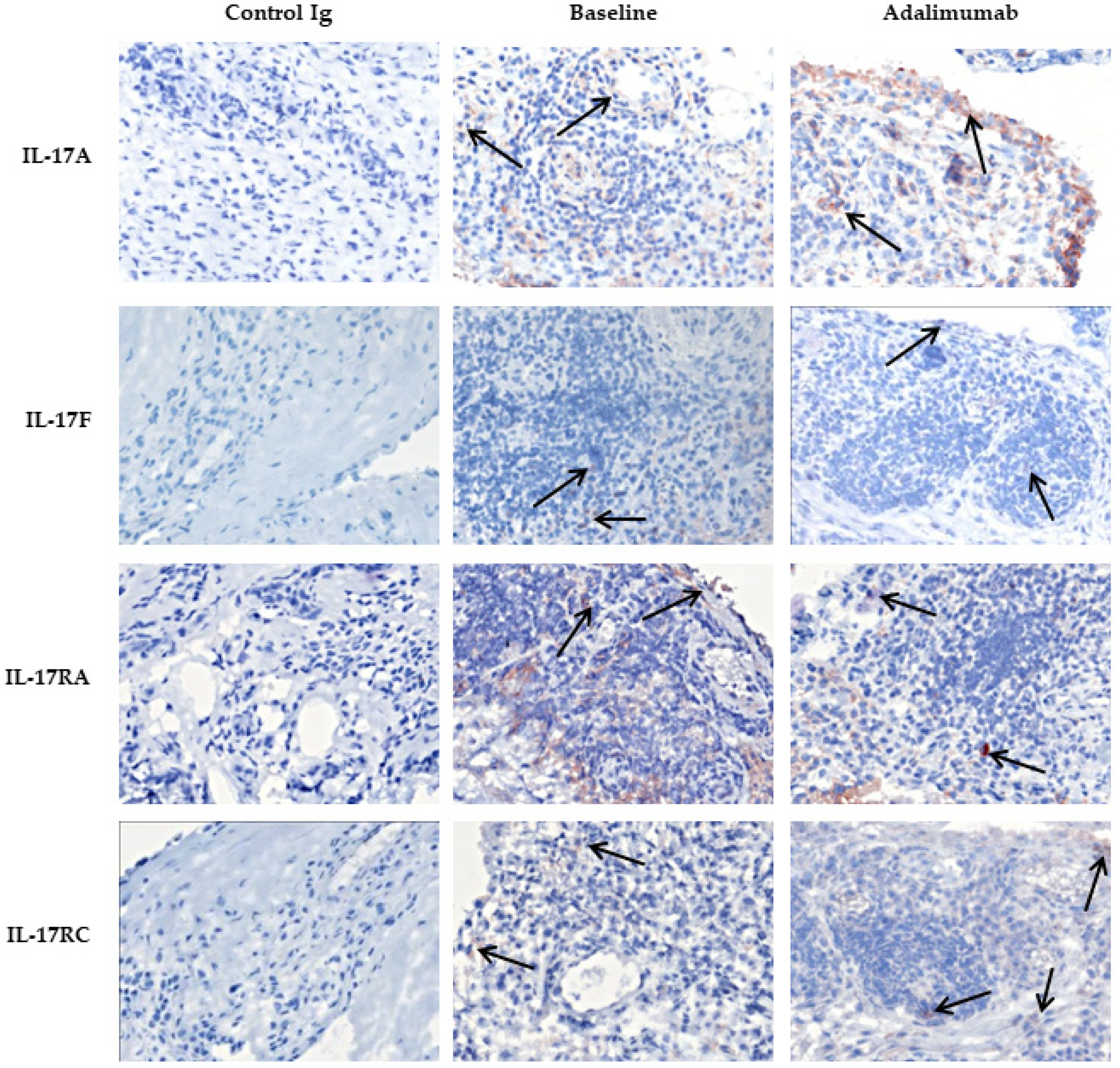
3.2. Expression of IL-17A, IL-17F and Their Receptors in Skin of Psoriatic Arthritis Patients Treated with Placebo Compared to Adalimumab
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ritchlin, C.; Colbert, R.A.; Gladman, D.D. Psoriatic Arthritis. N. Engl. J. Med. 2017, 376, 957–970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kavanaugh, A.; Helliwell, P.; Ritchlin, C.T. Psoriatic Arthritis and Burden of Disease: Patient Perspectives from the Population-Based Multinational Assessment of Psoriasis and Psoriatic Arthritis (MAPP) Survey. Rheumatol. Ther. 2016, 3, 91–102. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Davelaar, N.; Mus, A.; Asmawidjaja, P.S.; Hazes, J.M.W.; Baeten, D.L.P.; Vis, M.; Bisoendial, R.J.; Prens, E.P.; Lubberts, E. Interleukin-17A Is Produced by CD4+ but Not CD8+ T Cells in Synovial Fluid Following T Cell Receptor Activation and Regulates Different Inflammatory Mediators Compared to Tumor Necrosis Factor in a Model of Psoriatic Arthritis Synovitis. Arthritis Rheumatol. 2020, 72, 1303–1313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taams, L.S.; Steel, K.J.A.; Srenathan, U.; Burns, L.A.; Kirkham, B.W. IL-17 in the immunopathogenesis of spondyloarthritis. Nat. Rev. Rheumatol. 2018, 14, 453–466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blauvelt, A.; Chiricozzi, A. The Immunologic Role of IL-17 in Psoriasis and Psoriatic Arthritis Pathogenesis. Clin. Rev. Allergy Immunol. 2018, 55, 379–390. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.S.; Blijdorp, I.C.; Van Mens, L.J.; Bowcutt, R.; Latuhihin, T.E.; Van De Sande, M.G.; Shaw, S.; Yeremenko, N.G.; Baeten, D.L. Interleukin 17A and IL-17F Expression and Functional Responses in Rheumatoid Arthritis and Peripheral Spondyloarthritis. J. Rheumatol. 2020, 47, 1606–1613. [Google Scholar] [CrossRef] [PubMed]
- Chiricozzi, A.; Suarez-Farinas, M.; Fuentes-Duculan, J.; Cueto, I.; Li, K.; Tian, S.; Brodmerkel, C.; Krueger, J. Increased expression of interleukin-17 pathway genes in nonlesional skin of moderate-to-severe psoriasis vulgaris. Br. J. Dermatol. 2015, 174, 136–145. [Google Scholar] [CrossRef] [Green Version]
- Yeremenko, N. Out of the shadow of interleukin-17A: The role of interleukin-17F and other interleukin-17 family cytokines in spondyloarthritis. Curr. Opin. Rheumatol. 2021, 33, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Van Kuijk, A.W.R.; Reinders-Blankert, P.; Smeets, T.J.M.; Dijkmans, B.A.C.; Tak, P.P. Detailed analysis of the cell infiltrate and the expression of mediators of synovial inflammation and joint destruction in the synovium of patients with psoriatic arthritis: Implications for treatment. Ann. Rheum. Dis. 2006, 65, 1551–1557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fiocco, U.; Sfriso, P.; Oliviero, F.; Roux-Lombard, P.; Scagliori, E.; Cozzi, L.; Lunardi, F.; Calabrese, F.; Vezzù, M.; Dainese, S.; et al. Synovial effusion and synovial fluid biomarkers in psoriatic arthritis to assess intraarticular tumor necrosis factor-α blockade in the knee joint. Arthritis Res. Ther. 2010, 12, R148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfeffer, K. Biological functions of tumor necrosis factor cytokines and their receptors. Cytokine Growth Factor Rev. 2003, 14, 185–191. [Google Scholar] [CrossRef]
- Singh, T.P.; Zhang, H.H.; Borek, I.; Wolf, P.; Hedrick, M.N.; Singh, S.P.; Kelsall, B.L.; Clausen, B.; Farber, J.M. Monocyte-derived inflammatory Langerhans cells and dermal dendritic cells mediate psoriasis-like inflammation. Nat. Commun. 2016, 7, 13581. [Google Scholar] [CrossRef] [Green Version]
- Zaba, L.C.; Cardinale, I.; Gilleaudeau, P.; Sullivan-Whalen, M.; Suárez-Fariñas, M.; Fuentes-Duculan, J.; Novitskaya, I.; Khatcherian, A.; Bluth, M.J.; Lowes, M.A.; et al. Amelioration of epidermal hyperplasia by TNF inhibition is associated with reduced Th17 responses. J. Exp. Med. 2007, 204, 3183–3194. [Google Scholar] [CrossRef]
- Chabaud, M.; Page, G.; Miossec, P. Enhancing Effect of IL-1, IL-17, and TNF-α on Macrophage Inflammatory Protein-3α Production in Rheumatoid Arthritis: Regulation by Soluble Receptors and Th2 Cytokines. J. Immunol. 2001, 167, 6015–6020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lowes, M.A.; Russell, C.; Martin, D.A.; Towne, J.E.; Krueger, J.G. The IL-23/T17 pathogenic axis in psoriasis is amplified by keratinocyte responses. Trends Immunol. 2013, 34, 174–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silvagni, E.; Missiroli, S.; Perrone, M.; Patergnani, S.; Boncompagni, C.; Bortoluzzi, A.; Govoni, M.; Giorgi, C.; Alivernini, S.; Pinton, P.; et al. From Bed to Bench and Back: TNF-α, IL-23/IL-17A, and JAK-Dependent Inflammation in the Pathogenesis of Psoriatic Synovitis. Front. Pharmacol. 2021, 12, 672515. [Google Scholar] [CrossRef]
- Zrioual, S.; Ecochard, R.; Tournadre, A.; Lenief, V.; Cazalis, M.-A.; Miossec, P. Genome-Wide Comparison between IL-17A- and IL-17F-Induced Effects in Human Rheumatoid Arthritis Synoviocytes. J. Immunol. 2009, 182, 3112–3120. [Google Scholar] [CrossRef] [PubMed]
- Osta, B.; Lavocat, F.; Eljaafari, A.; Miossec, P. Effects of Interleukin-17A on Osteogenic Differentiation of Isolated Human Mesenchymal Stem Cells. Front. Immunol. 2014, 5, 425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gladman, D.D.; Mease, P.J.; Ritchlin, C.; Choy, E.H.S.; Sharp, J.T.; Ory, P.A.; Perdok, R.J.; Sasso, E.H. Adalimumab for long-term treatment of psoriatic arthritis: Forty-eight week data from the adalimumab effectiveness in psoriatic arthritis trial. Arthritis Care Res. 2007, 56, 476–488. [Google Scholar] [CrossRef] [PubMed]
- Nash, P.; Kirkham, B.; Okada, M.; Rahman, P.; Combe, B.; Burmester, G.-R.; Adams, D.H.; Kerr, L.; Lee, C.; Shuler, C.L.; et al. Ixekizumab for the treatment of patients with active psoriatic arthritis and an inadequate response to tumour necrosis factor inhibitors: Results from the 24-week randomised, double-blind, placebo-controlled period of the SPIRIT-P2 phase 3 trial. Lancet 2017, 389, 2317–2327. [Google Scholar] [CrossRef]
- McInnes, I.B.; Mease, P.J.; Kirkham, B.; Kavanaugh, A.; Ritchlin, C.; Rahman, P.; van der Heijde, D.; Landewé, R.; Conaghan, P.; Gottlieb, A.B.; et al. Secukinumab, a human anti-interleukin-17A monoclonal antibody, in patients with psoriatic arthritis (FUTURE 2): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2015, 386, 1137–1146. [Google Scholar] [CrossRef] [Green Version]
- Mease, P.J.; Kivitz, A.J.; Burch, F.X.; Siegel, E.L.; Cohen, S.B.; Ory, P.; Salonen, D.; Rubenstein, J.; Sharp, J.T.; Tsuji, W. Etanercept treatment of psoriatic arthritis: Safety, efficacy, and effect on disease progression. Arthritis Care Res. 2004, 50, 2264–2272. [Google Scholar] [CrossRef] [PubMed]
- Kavanaugh, A.; Van Der Heijde, D.; McInnes, I.B.; Mease, P.; Krueger, G.G.; Gladman, D.D.; Gómez-Reino, J.; Papp, K.; Baratelle, A.; Xu, W.; et al. Golimumab in psoriatic arthritis: One-year clinical efficacy, radiographic, and safety results from a phase III, randomized, placebo-controlled trial. Arthritis Care Res. 2012, 64, 2504–2517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coates, L.C.; Kavanaugh, A.; Mease, P.J.; Soriano, E.R.; Acosta-Felquer, M.L.; Armstrong, A.W.; Bautista-Molano, W.; Boehncke, W.-H.; Campbell, W.; Cauli, A.; et al. Group for Research and Assessment of Psoriasis and Psoriatic Arthritis 2015 Treatment Recommendations for Psoriatic Arthritis. Arthritis Rheumatol. 2016, 68, 1060–1071. [Google Scholar] [CrossRef] [Green Version]
- Gossec, L.; Baraliakos, X.; Kerschbaumer, A.; de Wit, M.; McInnes, I.; Dougados, M.; Primdahl, J.; McGonagle, D.G.; Aletaha, D.; Balanescu, A.; et al. EULAR recommendations for the management of psoriatic arthritis with pharmacological therapies: 2019 update. Ann. Rheum. Dis. 2020, 79, 700–712. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.A.; Guyatt, G.; Ogdie, A.; Gladman, D.D.; Deal, C.; Deodhar, A.; Dubreuil, M.; Dunham, J.; Husni, M.E.; Kenny, S.; et al. 2018 American College of Rheumatology/National Psoriasis Foundation Guideline for the Treatment of Psoriatic Arthritis. Arthritis Care Res. 2019, 71, 2–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McInnes, I.B.; Behrens, F.; Mease, P.J.; Kavanaugh, A.; Ritchlin, C.; Nash, P.; Masmitja, J.G.; Goupille, P.; Korotaeva, T.; Gottlieb, A.B.; et al. Secukinumab versus adalimumab for treatment of active psoriatic arthritis (EXCEED): A double-blind, parallel-group, randomised, active-controlled, phase 3b trial. Lancet 2020, 395, 1496–1505. [Google Scholar] [CrossRef]
- Mease, P.J.; Smolen, J.S.; Behrens, F.; Nash, P.; Leage, S.L.; Li, L.; Tahir, H.; Gooderham, M.; Krishnan, E.; Liu-Seifert, H.; et al. A head-to-head comparison of the efficacy and safety of ixekizumab and adalimumab in biological-naïve patients with active psoriatic arthritis: 24-week results of a randomised, open-label, blinded-assessor trial. Ann. Rheum. Dis. 2019, 79, 123–131. [Google Scholar] [CrossRef] [Green Version]
- Van Kuijk, A.W.R.; Gerlag, D.M.; Vos, K.; Wolbink, G.J.; De Groot, M.; De Rie, M.A.; Zwinderman, A.H.; Dijkmans, B.A.C.; Tak, P.P. A prospective, randomised, placebo-controlled study to identify biomarkers associated with active treatment in psoriatic arthritis: Effects of adalimumab treatment on synovial tissue. Ann. Rheum. Dis. 2008, 68, 1303–1309. [Google Scholar] [CrossRef]
- De Groot, M.; Picavet, D.I.; Van Kuijk, A.W.; Tak, P.P.; Bos, J.D.; De Rie, M.A.; Teunissen, M.B. A Prospective, Randomized, Placebo-Controlled Study to Identify Biomarkers Associated with Active Treatment in Psoriatic Arthritis: Effects of Adalimumab Treatment on Lesional and Nonlesional Skin. Dermatology 2012, 225, 298–303. [Google Scholar] [CrossRef]
- Chandran, V.; Schentag, C.T.; Gladman, D.D. Sensitivity of the classification of psoriatic arthritis criteria in early psoriatic arthritis. Arthritis Rheum. 2007, 57, 1560–1563. [Google Scholar] [CrossRef] [PubMed]
- Taylor, W.; Gladman, D.; Helliwell, P.; Marchesoni, A.; Mease, P.; Mielants, H. CASPAR Study Group Classification criteria for psoriatic arthritis: Development of new criteria from a large international study. Arthritis Care Res. 2006, 54, 2665–2673. [Google Scholar] [CrossRef]
- Smeets, T.J.M.; Kraan, M.C.; Galjaard, S.; Youssef, P.P.; Smith, M.D.; Tak, P.P. Analysis of the cell infiltrate and expression of matrix metalloproteinases and granzyme B in paired synovial biopsy specimens from the cartilage-pannus junction in patients with RA. Ann. Rheum. Dis. 2001, 60, 561–565. [Google Scholar] [CrossRef]
- Conover, J.W.; Iman, R.L. Analysis of covariance using the rank transformation. Biometrics 1982, 38, 715–724. [Google Scholar] [CrossRef]
- Johansen, C.; Usher, P.; Kjellerup, R.; Lundsgaard, D.; Iversen, L.; Kragballe, K. Characterization of the interleukin-17 isoforms and receptors in lesional psoriatic skin. Br. J. Dermatol. 2009, 160, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Harper, E.G.; Guo, C.; Rizzo, H.; Lillis, J.V.; Kurtz, S.E.; Skorcheva, I.; Purdy, D.; Fitch, E.; Iordanov, M.; Blauvelt, A. Th17 Cytokines Stimulate CCL20 Expression in Keratinocytes In Vitro and In Vivo: Implications for Psoriasis Pathogenesis. J. Investig. Dermatol. 2009, 9, 2175–2183. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Chen, X.; Liu, Z.; Yue, Q.; Liu, H. Expression of Th17 cytokines in skin lesions of patients with psoriasis. Acta Acad. Med. Wuhan 2007, 27, 330–332. [Google Scholar] [CrossRef]
- Kozlov, S.V. Inflammation and cancer. Methods and protocols. Volume 1: Experimental models and practical approaches. Preface. Methods Mol. Biol. 2009, 511, v–viii. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnston, A.; Guzman, A.M.; Swindell, W.R.; Wang, F.; Kang, S.; Gudjonsson, J.E. Early tissue responses in psoriasis to the anti-TNF-α biologic etanercept suggest reduced IL-17R expression and signalling. Br. J. Dermatol. 2014, 171, 97–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frleta, M.; Siebert, S.; McInnes, I.B.; Frleta-Gilchrist, M. The Interleukin-17 Pathway in Psoriasis and Psoriatic Arthritis: Disease Pathogenesis and Possibilities of Treatment. Curr. Rheumatol. Rep. 2014, 16, 414. [Google Scholar] [CrossRef] [PubMed]
- Mease, P.P.; Gladman, D.D.; Ritchlin, C.; Ruderman, E.E.; Steinfeld, S.D.; Choy, E.H.S.; Sharp, J.J.; Ory, P.P.; Perdok, R.R.; Weinberg, M.M.; et al. Adalimumab for the treatment of patients with moderately to severely active psoriatic arthritis: Results of a double-blind, randomized, placebo-controlled trial. Arthritis Care Res. 2005, 52, 3279–3289. [Google Scholar] [CrossRef]
- Milanez, F.M.; Saad, C.G.S.; Viana, V.T.; Moraes, J.C.B.; Périco, G.V.; Sampaio-Barros, P.D.; Goncalves, C.R.; Bonfá, E. IL-23/Th17 axis is not influenced by TNF-blocking agents in ankylosing spondylitis patients. Arthritis Res. Ther. 2016, 18, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walters, H.M.; Pan, N.; Lehman, T.J.; Adams, A.; Kalliolias, G.D.; Zhu, Y.; Santiago, F.; Nguyen, J.; Sitaras, L.; Cunningham-Rundles, S.; et al. The impact of disease activity and tumour necrosis factor-α inhibitor therapy on cytokine levels in juvenile idiopathic arthritis. Clin. Exp. Immunol. 2016, 184, 308–317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Mens, L.J.J.; Van De Sande, M.G.H.; Menegatti, S.; Chen, J.; Blijdorp, I.C.J.; De Jong, H.M.; Fluri, I.A.; Latuhihin, T.E.; Van Kuijk, A.W.R.; Rogge, L.; et al. Brief Report: Interleukin-17 Blockade with Secukinumab in Peripheral Spondyloarthritis Impacts Synovial Immunopathology Without Compromising Systemic Immune Responses. Arthritis Rheumatol. 2018, 70, 1994–2002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khatri, A.; Klünder, B.; Peloso, P.M.; Othman, A. Exposure–response analyses demonstrate no evidence of interleukin 17A contribution to efficacy of ABT-122 in rheumatoid or psoriatic arthritis. Rheumatology 2018, 58, 352–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Adalimumab (n = 12) | Placebo (n = 12) | |||
|---|---|---|---|---|
| Sex, female/male (n) | 9/3 | 6/6 | ||
| Age, years | 42.8 | (21–61) | 47.2 | (25–78) |
| Patients on MTX, number (%) | 7 | (58%) | 5 | (42) |
| MTX dose, mg/week | 18.2 | (10–25) | 19 | (15–25) |
| Duration of psoriasis, years | 8.8 | (0.1–27.7) | 20.8 | (1.9–53.2) |
| Duration of PsA, years | 5.5 | (0.4–14.1) | 8.4 | (1.9–18.2) |
| DAS28 score | 4.67 | (3.0–5.78) | 5.07 | (2.21–6.83) |
| VAS disease activity (0–100 mm) | 73 | (45–94) | 62.8 | (18–92) |
| VAS pain (0–100 mm) | 72.8 | (55–91) | 67.4 | (11–89) |
| CRP, mg/liter | 19.9 | (2.3–81.6) | 9.9 | (1.3–26.7) |
| ESR mm/h | 24.2 | (4–66) | 22.4 | (3–66) |
| PASI | 5.89 | (0–14.0) | 4.72 | (0–7.0) |
| Skin Tissue | Adalimumab (n = 10) | Placebo (n = 11) | ANCOVA p Value | |||||
|---|---|---|---|---|---|---|---|---|
| IOD/mm2 Baseline | IOD/mm2 Change upon Treatment | IOD/mm2 Baseline | IOD/mm2 Change upon Treatment | |||||
| IL-17A | epidermis dermis | 130,024 7261 | (1421–406,897) (62–12,426) | 1621 43 | 32,938 687 | (10,501–75,100) (110–11,503) | 2351 440 | 0.818 0.990 |
| IL-17F | epidermis dermis | 8127 13,436 | (4276–20,978) (2979–25,674) | 1583 9257 | 10,155 9591 | (6351–30,021) (7997–29,477) | −6139 1379 | 0.945 0.812 |
| IL-17RA | epidermis dermis | 942 0 | (0–3695) (0–42) | 893 447 | 37 0 | (0–4439) (0–0) | 0 1 | 0.010 0.215 |
| IL-17RC | epidermis dermis | 1,473,790 5762 | (726,625–1,679,188) (389–7648) | 293,761 −3496 | 1,824,572 2679 | (1,118,472–2,115,390) (247–5577) | 26,775 1270 | 0.237 0.332 |
| IL-17A | epidermis dermis | 21,892 3297 | (1015–20,420) (385–11,211) | 323 189 | 35,249 1719 | (5714–91,372) (461–7907) | 3513 2436 | 0.945 0.083 |
| IL-17F | epidermis dermis | 1646 893 | (0–1255) (0–44) | 415 969 | 10,093 5960 | (365–39,274) (970–22,719) | 2372 644 | 0.760 0.914 |
| IL-17RA | epidermis dermis | 129 0 | (773,173–1,689,893) (2378–25,450) | 0 0 | 0 0 | (0–1399) (0–30) | 321 0 | 0.194 0.454 |
| IL-17RC | epidermis dermis | 1,496,622 10,226 | (1,421–406,897) (62–12,426) | 48,011 −3942 | 1,508,035 7371 | (1,100,136–2,156,572) (1554–14,607) | 213,162 −1483 | 0.339 0.375 |
| Synovial Tissue | Adalimumab (n = 11) | Placebo (n = 10) | ANCOVA p Value | ||||
|---|---|---|---|---|---|---|---|
| IOD/mm2 Baseline | IOD/mm2 Change upon Treatment | IOD/mm2 Baseline | IOD/mm2 Change upon Treatment | ||||
| IL-17A | 7322 | (1464–11,314) | 12,211 | 2082 | (228–17,070) | 1581 | 0.333 |
| IL-17F | 19,297 | (795–43,043) | 4509 | 9146 | (1015–21,304) | 5495 | 0.811 |
| IL-17RA | 18,562 | (2596–107,978) | −3676 | 682 | (470–37,101) | 2337 | 0.451 |
| IL-17RC | 3330 | (1337–14,102) | 283 | 3934 | (800–5249) | 230 | 0.385 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bolt, J.W.; van Kuijk, A.W.; Teunissen, M.B.M.; van der Coelen, D.; Aarrass, S.; Gerlag, D.M.; Tak, P.P.; van de Sande, M.G.; Lebre, M.C.; van Baarsen, L.G.M. Impact of Adalimumab Treatment on Interleukin-17 and Interleukin-17 Receptor Expression in Skin and Synovium of Psoriatic Arthritis Patients with Mild Psoriasis. Biomedicines 2022, 10, 324. https://doi.org/10.3390/biomedicines10020324
Bolt JW, van Kuijk AW, Teunissen MBM, van der Coelen D, Aarrass S, Gerlag DM, Tak PP, van de Sande MG, Lebre MC, van Baarsen LGM. Impact of Adalimumab Treatment on Interleukin-17 and Interleukin-17 Receptor Expression in Skin and Synovium of Psoriatic Arthritis Patients with Mild Psoriasis. Biomedicines. 2022; 10(2):324. https://doi.org/10.3390/biomedicines10020324
Chicago/Turabian StyleBolt, Janne W., Arno W. van Kuijk, Marcel B. M. Teunissen, Dennis van der Coelen, Saïda Aarrass, Daniëlle M. Gerlag, Paul P. Tak, Marleen G. van de Sande, Maria C. Lebre, and Lisa G. M. van Baarsen. 2022. "Impact of Adalimumab Treatment on Interleukin-17 and Interleukin-17 Receptor Expression in Skin and Synovium of Psoriatic Arthritis Patients with Mild Psoriasis" Biomedicines 10, no. 2: 324. https://doi.org/10.3390/biomedicines10020324
APA StyleBolt, J. W., van Kuijk, A. W., Teunissen, M. B. M., van der Coelen, D., Aarrass, S., Gerlag, D. M., Tak, P. P., van de Sande, M. G., Lebre, M. C., & van Baarsen, L. G. M. (2022). Impact of Adalimumab Treatment on Interleukin-17 and Interleukin-17 Receptor Expression in Skin and Synovium of Psoriatic Arthritis Patients with Mild Psoriasis. Biomedicines, 10(2), 324. https://doi.org/10.3390/biomedicines10020324







