Treatment with Pulsed Extremely Low Frequency Electromagnetic Field (PELF-EMF) Exhibit Anti-Inflammatory and Neuroprotective Effect in Compression Spinal Cord Injury Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Spinal Cord Compression
2.3. PELF-EMF
2.4. Treatment Protocol
- (a)
- (b)
- (c)
- Improvement of the removal of Glu+ from the damaged area (ion cyclotron resonance hypothesis), steps 4 and 5 [34].
- (d)
2.5. Immunohistochemistry
2.6. Histological Analysis
2.7. Behavioral Analyses
2.8. CatWalk
2.9. Statistical Analysis
3. Results
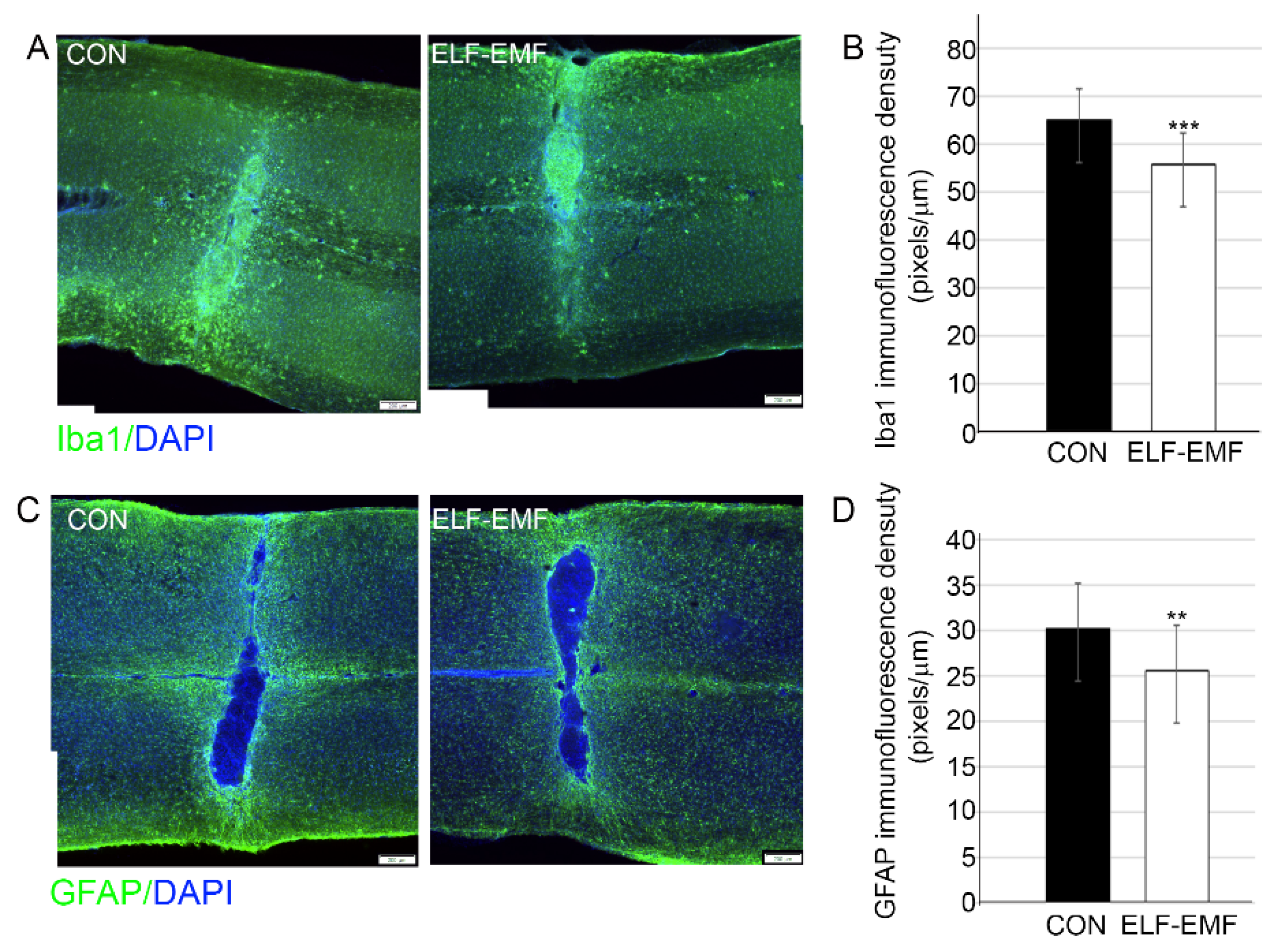
3.1. PELF-EMF Treatment Reduced Astrocyte and Microglia Reactivity
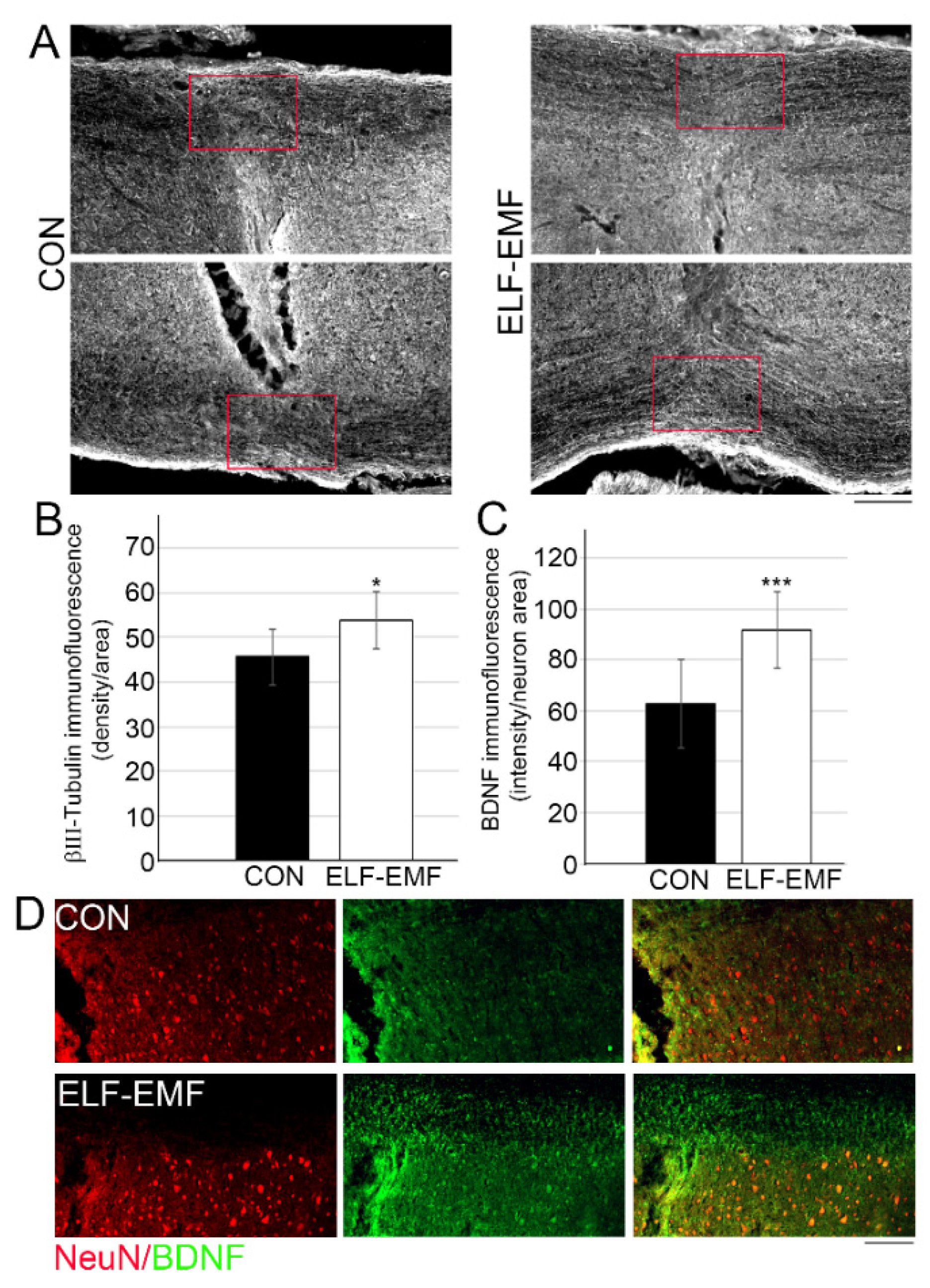
3.2. PELF-EMF Treatment Increases Axonal Survival and BDNF Expression at the Lesion Site
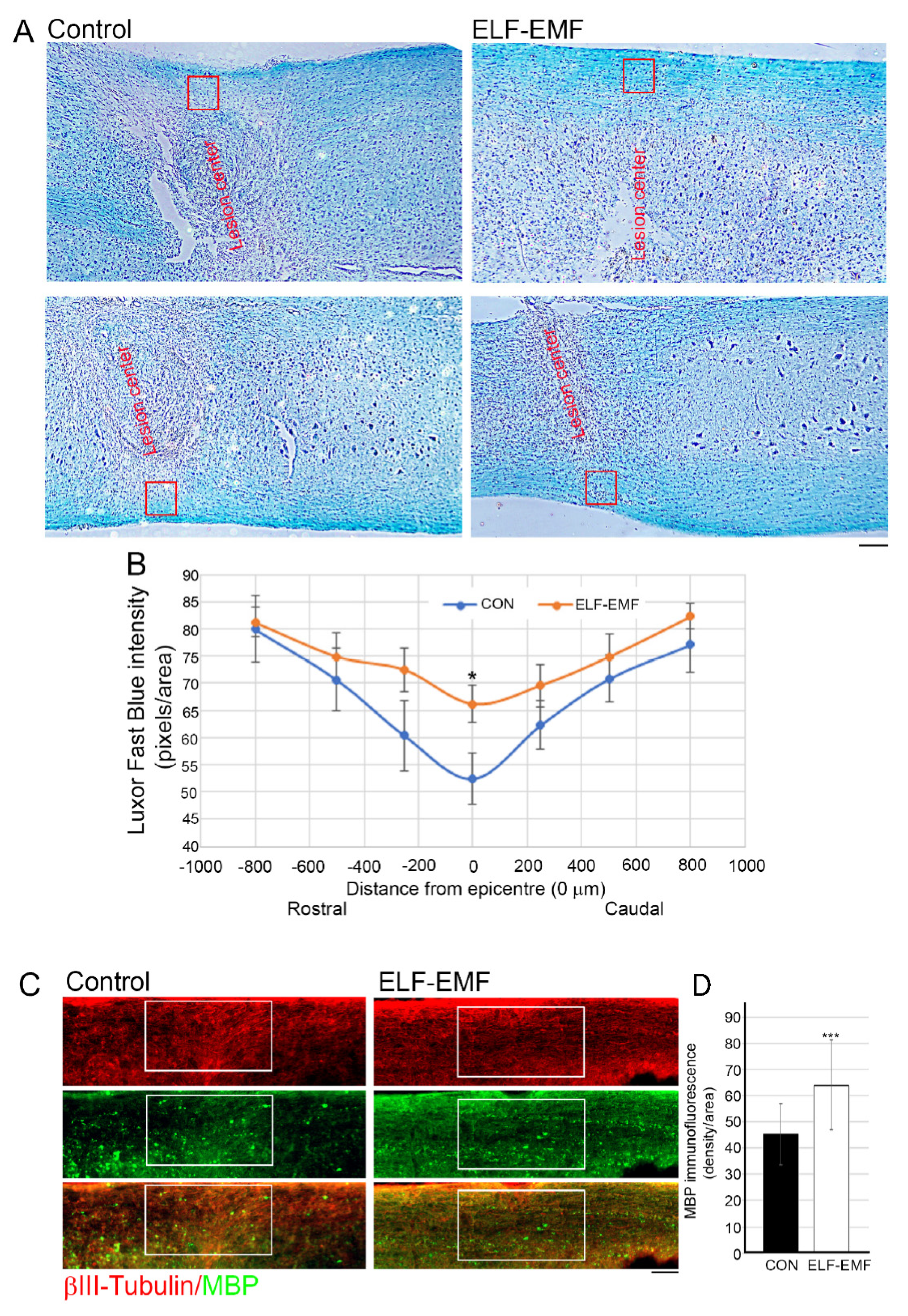
3.3. PELF-EMF Treatment Moderated the Area of Demyelination at the Lesion Site
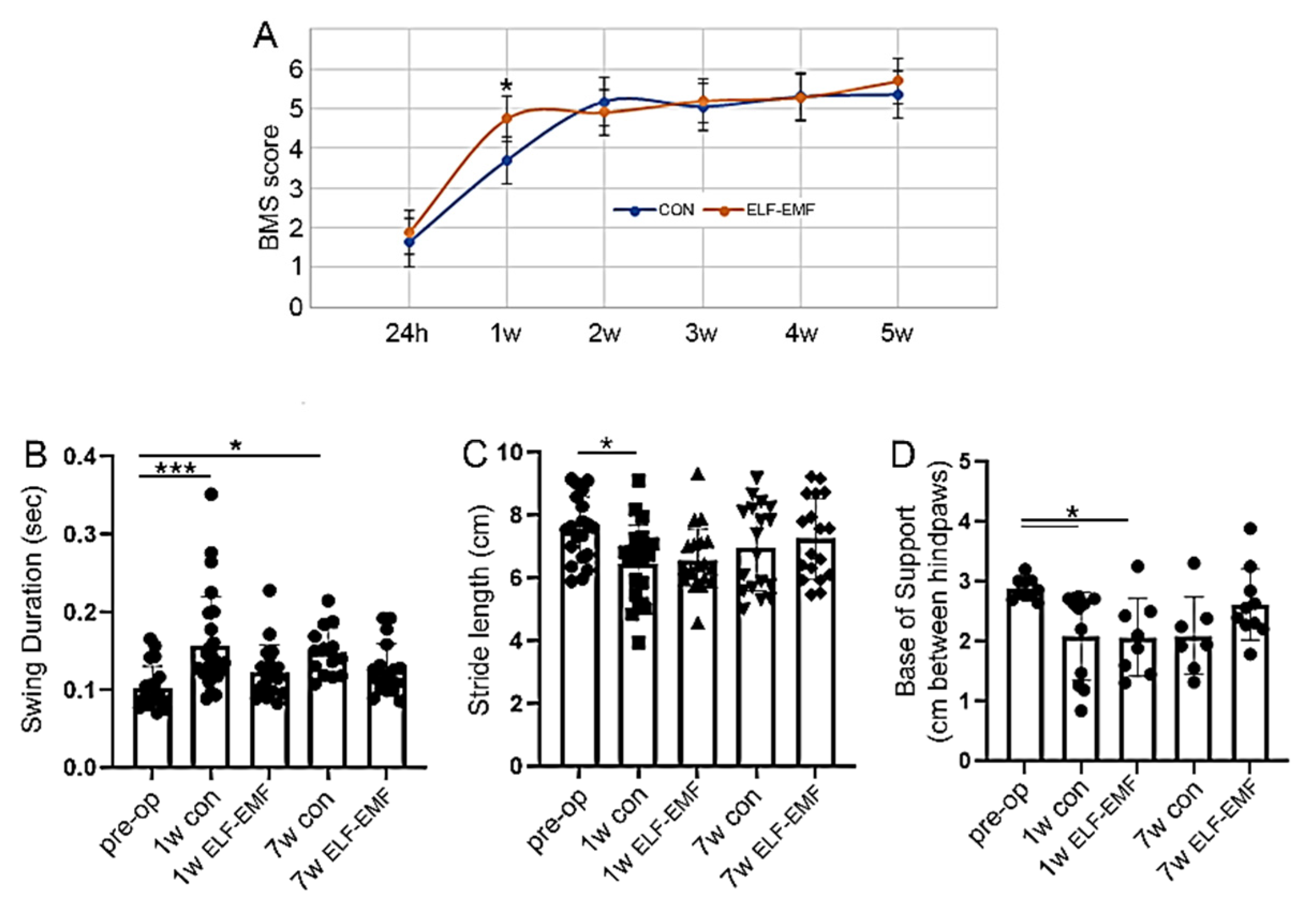
3.4. ELF-EMF Treatment Reduced Functional Deficits a Week after the Injury
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jain, N.B.; Harris, M.B.; Garshick, E. Trends in Traumatic Spinal Cord Injury-Reply. JAMA 2015, 314, 1643–1644. [Google Scholar] [CrossRef] [PubMed]
- GBD 2016 Traumatic Brain Injury and Spinal Cord Injury Collaborators. Global, regional, and national burden of traumatic brain injury and spinal cord injury, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 56–87. [Google Scholar] [CrossRef]
- Hachem, L.D.; Ahuja, C.S.; Fehlings, M.G. Assessment and management of acute spinal cord injury: From point of injury to rehabilitation. J. Spinal Cord Med. 2017, 40, 665–675. [Google Scholar] [CrossRef] [PubMed]
- Tran, A.P.; Silver, J. Systemically treating spinal cord injury. Science 2015, 348, 285–286. [Google Scholar] [CrossRef]
- Karsy, M.; Hawryluk, G. Modern Medical Management of Spinal Cord Injury. Curr. Neurol. Neurosci. Rep. 2019, 19, 65. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Young, W.; Skaper, S.; Chen, L.; Moviglia, G.; Saberi, H.; Al-Zoubi, Z.; Sharma, H.S.; Muresanu, D. Clinical Neurorestorative Therapeutic Guidelines for Spinal Cord Injury (IANR/CANR version 2019). J. Orthop. Transl. 2020, 20, 14–24. [Google Scholar] [CrossRef]
- Ahuja, C.S.; Wilson, J.R.; Nori, S.; Kotter, M.R.; Druschel, C.; Curt, A.; Fehlings, M.G. Traumatic spinal cord injury. Nat. Rev. Dis. Primers 2017, 3, 17018. [Google Scholar] [CrossRef]
- Loy, K.; Schmalz, A.; Höche, T.; Jacobi, A.; Kreutzfeldt, M.; Merkler, D.; Bareyre, F.M. Enhanced Voluntary Exercise Improves Functional Recovery following Spinal Cord Injury by Impacting the Local Neuroglial Injury Response and Supporting the Rewiring of Supraspinal Circuits. J. Neurotrauma 2018, 35, 2904–2915. [Google Scholar] [CrossRef]
- Elbasiouny, S.M.; Moroz, D.; Bakr, M.M.; Mushahwar, V.K. Management of spasticity after spinal cord injury: Current techniques and future directions. Neurorehabil. Neural Repair. 2010, 24, 23–33. [Google Scholar] [CrossRef]
- Mortazavi, M.M.; Verma, K.; Harmon, O.A.; Griessenauer, C.J.; Adeeb, N.; Theodore, N.; Tubbs, R.S. The microanatomy of spinal cord injury: A review. Clin. Anat. 2015, 28, 27–36. [Google Scholar] [CrossRef]
- Ahmed, A.; Patil, A.A.; Agrawal, D.K. Immunobiology of spinal cord injuries and potential therapeutic approaches. Mol. Cell. Biochem. 2018, 441, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Gensel, J.C.; Zhang, B. Macrophage activation and its role in repair and pathology after spinal cord injury. Brain Res. 2015, 1619, 1–11. [Google Scholar] [CrossRef]
- Okada, S.; Hara, M.; Kobayakawa, K.; Matsumoto, Y.; Nakashima, Y. Astrocyte reactivity and astrogliosis after spinal cord injury. Neurosci. Res. 2018, 126, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Beck, K.D.; Nguyen, H.X.; Galvan, M.D.; Salazar, D.L.; Woodruff, T.M.; Anderson, A.J. Quantitative analysis of cellular inflammation after traumatic spinal cord injury: Evidence for a multiphasic inflammatory response in the acute to chronic environment. Brain 2010, 133 Pt 2, 433–447. [Google Scholar] [CrossRef]
- Shultz, R.B.; Zhong, Y. Minocycline targets multiple secondary injury mechanisms in traumatic spinal cord injury. Neural Regen. Res. 2017, 12, 702–713. [Google Scholar] [PubMed]
- Prüss, H.; A Kopp, M.; Brommer, B.; Gatzemeier, N.; Laginha, I.; Dirnagl, U.; Schwab, J.M. Non-resolving aspects of acute inflammation after spinal cord injury (SCI): Indices and resolution plateau. Brain Pathol. 2011, 21, 652–660. [Google Scholar] [CrossRef] [PubMed]
- Fleming, J.C.; Norenberg, M.D.; Ramsay, D.A.; Dekaban, G.A.; Marcillo, A.E.; Saenz, A.D.; Pasquale-Styles, M.; Dietrich, W.D.; Weaver, L.C. The cellular inflammatory response in human spinal cords after injury. Brain 2006, 129 Pt 12, 3249–3269. [Google Scholar] [CrossRef] [PubMed]
- Anwar, M.A.; Al Shehabi, T.S.; Eid, A.H. Inflammogenesis of Secondary Spinal Cord Injury. Front. Cell. Neurosci. 2016, 10, 98. [Google Scholar] [CrossRef] [PubMed]
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Münch, A.E.; Chung, W.-S.; Peterson, T.C.; et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 2017, 541, 481–487. [Google Scholar] [CrossRef]
- Thuret, S.; Moon, L.D.; Gage, F.H. Therapeutic interventions after spinal cord injury. Nat. Rev. Neurosci. 2006, 7, 628–643. [Google Scholar] [CrossRef] [PubMed]
- Pineau, I.; Sun, L.; Bastien, D.; Lacroix, S. Astrocytes initiate inflammation in the injured mouse spinal cord by promoting the entry of neutrophils and inflammatory monocytes in an IL-1 receptor/MyD88-dependent fashion. Brain Behav. Immun. 2010, 24, 540–553. [Google Scholar] [CrossRef] [PubMed]
- Ramer, L.M.; Ramer, M.S.; Bradbury, E.J. Restoring function after spinal cord injury: Towards clinical translation of experimental strategies. Lancet Neurol. 2014, 13, 1241–1256. [Google Scholar] [CrossRef]
- David, S.; Lopez-Vales, R.; Wee Yong, V. Harmful and beneficial effects of inflammation after spinal cord injury: Potential therapeutic implications. Handb. Clin. Neurol. 2012, 109, 485–502. [Google Scholar] [PubMed]
- Orr, M.B.; Gensel, J.C. Spinal Cord Injury Scarring and Inflammation: Therapies Targeting Glial and Inflammatory Responses. Neurotherapeutics 2018, 15, 541–553. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yao, F.; Cheng, L.; Cheng, W.; Qi, L.; Yu, S.; Zhang, L.; Zha, X.; Jing, J. Low frequency pulsed electromagnetic field promotes the recovery of neurological function after spinal cord injury in rats. J. Orthop. Res. 2019, 37, 449–456. [Google Scholar] [CrossRef]
- Segal, Y.; Segal, L.; Blumenfeld-Katzir, T.; Sasson, E.; Poliansky, V.; Loeb, E.; Levy, A.; Alter, A.; Bregman, N. The Effect of Electromagnetic Field Treatment on Recovery from Ischemic Stroke in a Rat Stroke Model: Clinical, Imaging, and Pathological Findings. Stroke Res. Treat. 2016, 2016, 6941946. [Google Scholar] [CrossRef][Green Version]
- Urnukhsaikhan, E.; Mishig-Ochir, T.; Kim, S.C.; Park, J.K.; Seo, Y.K. Neuroprotective Effect of Low Frequency-Pulsed Electromagnetic Fields in Ischemic Stroke. Appl. Biochem. Biotechnol. 2017, 181, 1360–1371. [Google Scholar] [CrossRef]
- Lisi, A.; Ledda, M.; Rosola, E.; Pozzi, D.; Emilia, E.D.; Giuliani, L.; Foletti, A.; Modesti, A.; Morris, S.J.; Grimaldi, S. Extremely low frequency electromagnetic field exposure promotes differentiation of pituitary corticotrope-derived AtT20 D16V cells. Bioelectromagnetics 2006, 27, 641–651. [Google Scholar] [CrossRef]
- Ross, C.L.; Harrison, B.S. Effect of pulsed electromagnetic field on inflammatory pathway markers in RAW 264.7 murine macrophages. J. Inflamm. Res. 2013, 6, 45–51. [Google Scholar] [CrossRef]
- Oda, T.; Koike, T. Magnetic field exposure saves rat cerebellar granule neurons from apoptosis in vitro. Neurosci. Lett. 2004, 365, 83–86. [Google Scholar] [CrossRef]
- Li, X.; Xu, H.; Lei, T.; Yang, Y.; Jing, D.; Dai, S.; Luo, P.; Xu, Q. A Pulsed Electromagnetic Field Protects against Glutamate-Induced Excitotoxicity by Modulating the Endocannabinoid System in HT22 Cells. Front. Neurosci. 2017, 11, 42. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Comisso, N.; Del Giudice, E.; De Ninno, A.; Fleischmann, M.; Giuliani, L.; Mengoli, G.; Merlo, F.; Talpo, G. Dynamics of the ion cyclotron resonance effect on amino acids adsorbed at the interfaces. Bioelectromagnetics 2006, 27, 16–25. [Google Scholar] [CrossRef]
- Zou, J.; Chen, Y.; Qian, J.; Yang, H. Effect of a low-frequency pulsed electromagnetic field on expression and secretion of IL-1beta and TNF-alpha in nucleus pulposus cells. J. Int. Med. Res. 2017, 45, 462–470. [Google Scholar] [CrossRef] [PubMed]
- Liboff, A.R. ION cyclotron resonance: Geomagnetic strategy for living systems? Electromagn. Biol. Med. 2019, 38, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Bragin, D.E.; Statom, G.L.; Hagberg, S.; Nemoto, E.M. Increases in microvascular perfusion and tissue oxygenation via pulsed electromagnetic fields in the healthy rat brain. J. Neurosurg. 2015, 122, 1239–1247. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Dai, Y.; Zhu, X.; Xu, H.; Cai, P.; Xia, R.; Mao, L.; Zhao, B.-Q.; Fan, W. Extremely low-frequency electromagnetic fields enhance the proliferation and differentiation of neural progenitor cells cultured from ischemic brains. Neuroreport 2015, 26, 896–902. [Google Scholar] [CrossRef]
- Das, S.; Kumar, S.; Jain, S.; Avelev, V.D.; Mathur, R. Exposure to ELF-magnetic field promotes restoration of sensori-motor functions in adult rats with hemisection of thoracic spinal cord. Electromagn. Biol. Med. 2012, 31, 180–194. [Google Scholar] [CrossRef]
- Goldshmit, Y.; Banyas, E.; Bens, N.; Yakovchuk, A.; Ruban, A. Blood glutamate scavengers and exercises as an effective neuroprotective treatment in mice with spinal cord injury. J. Neurosurg. Spine 2020, 33, 692–704. [Google Scholar] [CrossRef]
- Grace, P.M.; Wang, X.; Strand, K.A.; Baratta, M.V.; Zhang, Y.; Galer, E.L.; Yin, H.; Maier, S.F.; Watkins, L.R. DREADDed microglia in pain: Implications for spinal inflammatory signaling in male rats. Exp. Neurol. 2018, 304, 125–131. [Google Scholar] [CrossRef]
- Chen, Z.; Duan, R.-S.; Quezada, H.C.; Mix, E.; Nennesmo, I.; Adem, A.; Winblad, B.; Zhu, J. Increased microglial activation and astrogliosis after intranasal administration of kainic acid in C57BL/6 mice. J. Neurobiol. 2005, 62, 207–218. [Google Scholar] [CrossRef]
- Hodgetts, S.I.; Harvey, A.R. Neurotrophic Factors Used to Treat Spinal Cord Injury. Vitam. Horm. 2017, 104, 405–457. [Google Scholar] [PubMed]
- Linker, R.; Gold, R.; Luhder, F. Function of neurotrophic factors beyond the nervous system: Inflammation and autoimmune demyelination. Crit. Rev. Immunol. 2009, 29, 43–68. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-Y.; Chen, C.; Xu, H.-H.; Zhang, Y.-S.; Zhong, L.; Hu, N.; Jia, X.-L.; Wang, Y.-W.; Zhong, K.-H.; Liu, C.; et al. Integrated printed BDNF/collagen/chitosan scaffolds with low temperature extrusion 3D printer accelerated neural regeneration after spinal cord injury. Regen. Biomater. 2021, 8, rbab047. [Google Scholar] [CrossRef] [PubMed]
- Bradbury, E.J.; Burnside, E.R. Moving beyond the glial scar for spinal cord repair. Nat. Commun. 2019, 10, 3879. [Google Scholar] [CrossRef] [PubMed]
- Oyinbo, C.A. Secondary injury mechanisms in traumatic spinal cord injury: A nugget of this multiply cascade. Acta Neurobiol. Exp. (Wars) 2011, 71, 281–299. [Google Scholar] [PubMed]
- Hara, M.; Kobayakawa, K.; Ohkawa, Y.; Kumamaru, H.; Yokota, K.; Saito, T.; Kijima, K.; Yoshizaki, S.; Harimaya, K.; Nakashima, Y.; et al. Interaction of reactive astrocytes with type I collagen induces astrocytic scar formation through the integrin-N-cadherin pathway after spinal cord injury. Nat. Med. 2017, 23, 818–828. [Google Scholar] [CrossRef]
- Zamanian, J.L.; Xu, L.; Foo, L.C.; Nouri, N.; Zhou, L.; Giffard, R.G.; Barres, B.A. Genomic analysis of reactive astrogliosis. J. Neurosci. 2012, 32, 6391–6410. [Google Scholar] [CrossRef]
- Gris, D.; Marsh, D.R.; Oatway, M.A.; Chen, Y.; Hamilton, E.F.; Dekaban, G.A.; Weaver, L.C. Transient blockade of the CD11d/CD18 integrin reduces secondary damage after spinal cord injury, improving sensory, autonomic, and motor function. J. Neurosci. 2004, 24, 4043–4051. [Google Scholar] [CrossRef]
- Cichon, N.; Saluk-Bijak, J.; Miller, E.; Sliwinski, T.; Synowiec, E.; Wigner, P.; Bijak, M. Evaluation of the effects of extremely low frequency electromagnetic field on the levels of some inflammatory cytokines in post-stroke patients. J. Rehabil. Med. 2019, 51, 854–860. [Google Scholar] [CrossRef]
- Ross, C.L.; Pettenati, M.J.; Procita, J.; Cathey, L.; George, S.K.; Almeida-Porada, G. Evaluation of Cytotoxic and Genotoxic Effects of Extremely Low-frequency Electromagnetic Field on Mesenchymal Stromal Cells. Glob. Adv. Health Med. 2018, 7, 2164956118777472. [Google Scholar] [CrossRef]
- Cherry, J.D.; Olschowka, J.A.; O’Banion, M.K. Neuroinflammation and M2 microglia: The good, the bad, and the inflamed. J Neuroinflamm. 2014, 11, 98. [Google Scholar] [CrossRef] [PubMed]
- Kigerl, K.A.; Gensel, J.C.; Ankeny, D.P.; Alexander, J.K.; Donnelly, D.J.; Popovich, P.G. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J. Neurosci. 2009, 29, 13435–13444. [Google Scholar] [CrossRef] [PubMed]
- Greenhalgh, A.D.; David, S. Differences in the phagocytic response of microglia and peripheral macrophages after spinal cord injury and its effects on cell death. J. Neurosci. 2014, 34, 6316–6322. [Google Scholar] [CrossRef]
- Tran, A.P.; Warren, P.M.; Silver, J. The Biology of Regeneration Failure and Success After Spinal Cord Injury. Physiol Rev. 2018, 98, 881–917. [Google Scholar] [CrossRef] [PubMed]
- Bilchak, J.N.; Caron, G.; Cote, M.P. Exercise-Induced Plasticity in Signaling Pathways Involved in Motor Recovery after Spinal Cord Injury. Int. J. Mol. Sci. 2021, 22, 4858. [Google Scholar] [CrossRef] [PubMed]
- Seanez, I.; Capogrosso, M. Motor improvements enabled by spinal cord stimulation combined with physical training after spinal cord injury: Review of experimental evidence in animals and humans. Bioelectron. Med. 2021, 7, 16. [Google Scholar] [CrossRef] [PubMed]

| Step | Frequency (Hz) | Field Intensity (µT) | T-On (min) | T-Off (min) | Duration (min) |
|---|---|---|---|---|---|
| 1 | 15 | 20 | 2 | 3 | 14 |
| 2 | 75 | 20 | 4 | 1 | 12 |
| 3 | 2 | 20 | 2 | 3 | 14 |
| 4 | 20 | 20 | 3 | 1 | 14 |
| 5 | 12 | 20 | 3 | 1 | 14 |
| 6 | 3 | 20 | 3 | 1 | 14 |
| 7 | 50 | 20 | 2 | 1 | 12 |
| 8 | 25 | 20 | 5 | 2 | 12 |
| 9 | 10 | 20 | 5 | 1 | 14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goldshmit, Y.; Shalom, M.; Ruban, A. Treatment with Pulsed Extremely Low Frequency Electromagnetic Field (PELF-EMF) Exhibit Anti-Inflammatory and Neuroprotective Effect in Compression Spinal Cord Injury Model. Biomedicines 2022, 10, 325. https://doi.org/10.3390/biomedicines10020325
Goldshmit Y, Shalom M, Ruban A. Treatment with Pulsed Extremely Low Frequency Electromagnetic Field (PELF-EMF) Exhibit Anti-Inflammatory and Neuroprotective Effect in Compression Spinal Cord Injury Model. Biomedicines. 2022; 10(2):325. https://doi.org/10.3390/biomedicines10020325
Chicago/Turabian StyleGoldshmit, Yona, Moshe Shalom, and Angela Ruban. 2022. "Treatment with Pulsed Extremely Low Frequency Electromagnetic Field (PELF-EMF) Exhibit Anti-Inflammatory and Neuroprotective Effect in Compression Spinal Cord Injury Model" Biomedicines 10, no. 2: 325. https://doi.org/10.3390/biomedicines10020325
APA StyleGoldshmit, Y., Shalom, M., & Ruban, A. (2022). Treatment with Pulsed Extremely Low Frequency Electromagnetic Field (PELF-EMF) Exhibit Anti-Inflammatory and Neuroprotective Effect in Compression Spinal Cord Injury Model. Biomedicines, 10(2), 325. https://doi.org/10.3390/biomedicines10020325







