Radiosensitization and Radioprotection by Curcumin in Glioblastoma and Other Cancers
Abstract
:1. Introduction
2. Radiosensitization by Curcumin
3. Curcumin as a Radiosensitizer in Various Malignant Tumors and Glioblastoma
4. Radioprotection by Curcumin
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Kargiotis, O.; Geka, A.; Rao, J.S.; Kyritsis, A.P. Effects of irradiation on tumor cell survival, invasion and angiogenesis. J. Neuro-Oncol. 2010, 100, 323–338. [Google Scholar] [CrossRef] [PubMed]
- Blyth, B.J.; Cole, A.J.; MacManus, M.P.; Martin, O.A. Radiation therapy-induced metastasis: Radiobiology and clinical implications. Clin. Exp. Metastasis 2018, 35, 223–236. [Google Scholar] [CrossRef] [Green Version]
- Lyhne, N.M.; Primdahl, H.; Kristensen, C.A.; Andersen, E.; Johansen, J.; Andersen, L.J.; Evensen, J.; Mortensen, H.R.; Overgaard, J. The DAHANCA 6 randomized trial: Effect of 6 vs. 5 weekly fractions of radiotherapy in patients with glottic squamous cell carcinoma. Radiother. Oncol. 2015, 117, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Bennardo, L.; Passante, M.; Cameli, N.; Cristaudo, A.; Patruno, C.; Nisticò, S.P.; Silvestri, M. Skin Manifestations after Ionizing Radiation Exposure: A Systematic Review. Bioengineering 2021, 8, 153. [Google Scholar] [CrossRef] [PubMed]
- Levin, V.A.; Maor, M.H.; Thall, P.F.; Yung, W.K.; Bruner, J.; Sawaya, R.; Kyritsis, A.P.; Leeds, N.; Woo, S.; Rodriguez, L.; et al. Phase II study of accelerated fractionation radiation therapy with carboplatin followed by vincristine chemotherapy for the treatment of glioblastoma multiforme. Int. J. Radiat. Oncol. Biol. Phys. 1995, 33, 357–364. [Google Scholar] [CrossRef]
- Pham-Huy, L.A.; He, H.; Pham-Huy, C. Free radicals, antioxidants in disease and health. Int. J. Biomed. Sci. 2008, 4, 89–96. [Google Scholar] [PubMed]
- Gupta, J.K.; Upmanyu, N.; Patnaik, A.K.; Mazumder, P.M. Evaluation of antiulcer activity of Leucas lavandulifolia on mucosal lesion in rat. Asian, J. Pharm. Clin. Res. 2010, 3, 118–120. [Google Scholar]
- Emami, B.; Lyman, J.; Brown, A.; Coia, L.; Goitein, M.; Munzenrider, J.E.; Shank, B.; Solin, L.J.; Wesson, M. Tolerance of normal tissue to therapeutic irradiation. Int. J. Radiat. Oncol. Biol. Phys. 1991, 21, 109–122. [Google Scholar] [CrossRef]
- Groves, M.D.; Maor, M.H.; Meyers, C.; Kyritsis, A.P.; Jaeckle, K.A.; Yung, W.K.; Sawaya, R.E.; Hess, K.; Bruner, J.M.; Peterson, P.; et al. A phase II trial of high-dose bromodeoxyuridine with accelerated fractionation radiotherapy followed by procarbazine, lomustine, and vincristine for glioblastoma multiforme. Int. J. Radiat. Oncol. Biol. Phys. 1999, 45, 127–135. [Google Scholar] [CrossRef]
- Maier, P.; Hartmann, L.; Wenz, F.; Herskind, C. Cellular Pathways in Response to Ionizing Radiation and Their Targetability for Tumor Radiosensitization. Int. J. Mol. Sci. 2016, 17, 102. [Google Scholar] [CrossRef] [Green Version]
- Zoi, V.; Galani, V.; Lianos, G.D.; Voulgaris, S.; Kyritsis, A.P.; Alexiou, G.A. The Role of Curcumin in Cancer Treatment. Biomedicines 2021, 9, 1086. [Google Scholar] [CrossRef] [PubMed]
- Nistico, S.; Tamburi, F.; Bennardo, L.; Dastoli, S.; Schipani, G.; Caro, G.; Fortuna, F.C.; Rossi, A. Treatment of telogen effluvium using a dietary supplement containing Boswellia serrata, Curcuma longa, and Vitis vinifera: Results of an observational study. Dermatol. Ther. 2019, 32, 3. [Google Scholar] [CrossRef] [PubMed]
- Vollono, L.; Falconi, M.; Gaziano, R.; Iacovelli, F.; Dika, E.; Terracciano, C.; Bianchi, L.; Campione, E. Potential of Curcumin in Skin Disorders. Nutrients 2019, 11, 2169. [Google Scholar]
- Dhandapani, K.M.; Mahesh, V.B.; Brann, D.W. Curcumin suppresses growth and chemoresistance of human glioblastoma cells via AP-1 and NFkappaB transcription factors. J. Neurochem. 2007, 102, 522–538. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ding, X.; Huang, J.; Jiang, C.; Cao, B.; Qian, Y.; Cheng, C.; Dai, M.; Guo, X.; Shao, J. In vivo Radiosensitization of human glioma U87 cells induced by upregulated expression of DUSP-2 after treatment with curcumin. Curr. Signal Transduct. Ther. 2015, 10, 119–125. [Google Scholar] [CrossRef]
- Li, M.; Zhang, Z.; Hill, D.L.; Wang, H.; Zhang, R. Curcumin, a dietary component, has anticancer, chemosensitization, and radiosensitization effects by down-regulating the MDM2 oncogene through the PI3K/mTOR/ETS2 pathway. Cancer Res. 2007, 67, 1988–1996. [Google Scholar] [CrossRef] [Green Version]
- Zanotto-Filho, A.; Braganhol, E.; Edelweiss, M.I.; Behr, G.A.; Zanin, R.; Schröder, R.; Simões-Pires, A.; Battastini, A.M.; Moreira, J.C. The curry spice curcumin selectively inhibits cancer cells growth in vitro and in preclinical model of glioblastoma. J. Nutr. Biochem. 2012, 23, 591–601. [Google Scholar] [CrossRef]
- Gersey, Z.C.; Rodriguez, G.A.; Barbarite, E.; Sanchez, A.; Walters, W.M.; Ohaeto, K.C.; Komotar, R.J.; Graham, R.M. Curcumin decreases malignant characteristics of glioblastoma stem cells via induction of reactive oxygen species. BMC Cancer 2017, 17, 99. [Google Scholar] [CrossRef] [Green Version]
- Jurenka, J.S. Anti-inflammatory properties of curcumin, a major constituent of Curcuma longa: A review of preclinical and clinical research. Altern. Med. Rev. 2009, 14, 141–153. [Google Scholar]
- Shehzad, A.; Rehman, G.; Lee, Y.S. Curcumin in inflammatory diseases. BioFactors 2013, 39, 69–77. [Google Scholar] [CrossRef]
- Levin, V.A.; Yung, W.K.; Bruner, J.; Kyritsis, A.; Leeds, N.; Gleason, M.J.; Hess, K.R.; Meyers, C.A.; Ictech, S.A.; Chang, E.; et al. Phase II study of accelerated fractionation radiation therapy with carboplatin followed by PCV chemotherapy for the treatment of anaplastic gliomas. Int. J. Radiat. Oncol. Biol. Phys. 2002, 53, 58–66. [Google Scholar] [CrossRef]
- Khafif, A.V.I.; Hurst, R.; Kyker, K.; Fliss, D.M.; Gil, Z.I.V.; Medina, J.E. Curcumin: A new radio-sensitizer of squamous cell carcinoma cells. Otolaryngol. Head Neck Surg. 2005, 132, 317–321. [Google Scholar] [CrossRef] [PubMed]
- Jagetia, G.C. Radioprotection and radiosensitization by curcumin. Adv. Exp. Med. Biol. 2007, 595, 301–320. [Google Scholar] [PubMed]
- Schmidt-Ullrich, R.K.; Contessa, J.N.; Dent, P.; Mikkelsen, R.B.; Valerie, K.; Reardon, D.B.; Bowers, G.; Lin, P.S. Molecular mechanisms of radiation-induced accelerated repopulation. Radiat. Oncol. Investig. 1999, 7, 321–330. [Google Scholar] [CrossRef]
- Coleman, C.N. Radiation oncology: Linking technology and biology in the treatment of cancer. Acta Oncol. 2002, 41, 6–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jamalzaei, P.; Valojerdi, M.R.; Montazeri, L.; Baharvand, H. Effects of alginate concentration and ovarian cells on in vitro development of mouse preantral follicles: A factorial study. Int. J. Fertil. Steril. 2020, 13, 330–338. [Google Scholar]
- Santivasi, W.L.; Xia, F. Ionizing radiation-induced DNA damage, response, and repair. Antioxid. Redox Signal. 2014, 21, 251–259. [Google Scholar] [CrossRef]
- Zeng, Y.; Du, Q.; Zhang, Z.; Ma, J.; Han, L.; Wang, Y.; Yang, L.; Tao, N.; Qin, Z. Curcumin promotes cancer-associated fibroblasts apoptosis via ROS-mediated endoplasmic reticulum stress. Arch. Biochem. Biophys. 2020, 694, 108613. [Google Scholar] [CrossRef]
- Nakamae, I.; Morimoto, T.; Shima, H.; Shionyu, M.; Fujiki, H.; Yoneda-Kato, N.; Yokoyama, T.; Kanaya, S.; Kakiuchi, K.; Shirai, T.; et al. Curcumin Derivatives Verify the Essentiality of ROS Upregulation in Tumor Suppression. Molecules 2019, 24, 4067. [Google Scholar] [CrossRef] [Green Version]
- Cho, W.; Kim, M.S.; Lee, K.H.; Park, S.J.; Shin, H.J.; Lee, Y.J.; Kim, S.B.; Son, Y.; Kim, C.H. Ionizing radiation attracts tumor targeting and apoptosis by radiotropic lysyl oxidase traceable nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2020, 24, 102141. [Google Scholar] [CrossRef]
- Takasawa, R.; Nakamura, H.; Mori, T.; Tanuma, S. Differential apoptotic pathways in human keratinocyte HaCaT cells exposed to UVB and UVC. Apoptosis 2005, 10, 1121–1130. [Google Scholar] [CrossRef] [PubMed]
- Mortezaee, K.; Salehi, E.; Mirtavoos-Mahyari, H.; Motevaseli, E.; Najafi, M.; Farhood, B.; Rosengren, R.J.; Sahebkar, A. Mechanisms of apoptosis modulation by curcumin: Implications for cancer therapy. J. Cell. Physiol. 2019, 234, 12537–12550. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Sheng, Z.; Liang, S. Radiosensitization effects of curcumin plus cisplatin on non-small cell lung cancer A549 cells. Oncol. Lett. 2019, 18, 529–534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rego, R.L.; Foster, N.R.; Smyrk, T.C.; Le, M.; O’Connell, M.J.; Sargent, D.J.; Windschitl, H.; Sinicrope, F.A. Prognostic effect of activated EGFR expression in human colon carcinomas: Comparison with EGFR status. Br. J. Cancer 2010, 102, 165–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, X.; Chen, C.; Wu, F.; Qiu, L.; Ke, Q.; Sun, R.; Duan, Q.; Luo, M.; Luo, Z. Curcumin Inhibits the Migration and Invasion of Non-Small-Cell Lung Cancer Cells through Radiation-Induced Suppression of Epithelial-Mesenchymal Transition and Soluble E-Cadherin Expression. Technol. Cancer Res. Treat. 2020, 19, 1533033820947485. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Lin, Q.; Huang, P.; Liang, Y. Antiepithelial-Mesenchymal Transition of Herbal Active Substance in Tumor Cells via Different Signaling. Oxidative Med. Cell. Longev. 2020, 2020, 9253745. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, S.; Patwardhan, R.S.; Pal, D.; Sharma, D.; Sandur, S.K. Dimethoxycurcumin, a metabolically stable analogue of curcumin enhances the radiosensitivity of cancer cells: Possible involvement of ROS and thioredoxin reductase. Biochem. Biophys. Res. Commun. 2016, 478, 446–454. [Google Scholar] [CrossRef]
- Cuixia, W.; Yun, Z.; Chong, Z.; Yifan, Z.; Xiang, H.; Jun, L.; Haitao, Y. Enhanced Radiosensitization Effect of Curcumin Delivered by PVP-PCL Nanoparticle in Lung Cancer. J. Nanomater. 2017, 8, 9625909. [Google Scholar]
- Fan, H.; Shao, M.; Huang, S.; Liu, Y.; Liu, J.; Wang, Z.; Diao, J.; Liu, Y.; Tong, L.; Fan, Q.; et al. MiR-593 mediates curcumin-induced radiosensitization of nasopharyngeal carcinoma cells via MDR1. Oncol. Lett. 2016, 11, 3729–3734. [Google Scholar] [CrossRef] [Green Version]
- Zhu, D.; Shao, M.; Yang, J.; Fang, M.; Liu, S.; Lou, D.; Gao, R.; Liu, Y.; Li, A.; Lv, Y.; et al. Curcumin Enhances Radiosensitization of Nasopharyngeal Carcinoma via Mediating Regulation of Tumor Stem-like Cells by a CircRNA Network. J. Cancer 2020, 11, 2360–2370. [Google Scholar] [CrossRef]
- O’Leary, V.B.; Smida, J.; Matjanovski, M.; Brockhaus, C.; Winkler, K.; Moertl, S.; Ovsepian, S.V.; Atkinson, M.J. The circRNA interactome-innovative hallmarks of the intra- and extracellular radiation response. Oncotarget 2017, 8, 78397–78409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minafra, L.; Porcino, N.; Bravatà, V.; Gaglio, D.; Bonanomi, M.; Amore, E.; Cammarata, F.P.; Russo, G.; Militello, C.; Savoca, G.; et al. Radiosensitizing effect of curcumin-loaded lipid nanoparticles in breast cancer cells. Sci. Rep. 2019, 9, 11134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, K.; Liao, Z.; Wu, Y.; Li, M.; Guo, T.; Lin, J.; Li, Y.; Hu, C. Curcumin and Glu-GNPs Induce Radiosensitivity against Breast Cancer Stem-Like Cells. BioMed Res. Int. 2020, 2020, 3189217. [Google Scholar] [CrossRef] [PubMed]
- Assad, D.X.; Borges, G.A.; Avelino, S.R.; Guerra, E.N.S. Additive cytotoxic effects of radiation and mTOR inhibitors in a cervical cancer cell line. Pathol. Res. Pract. 2018, 214, 259–262. [Google Scholar] [CrossRef] [PubMed]
- Hidayat, Y.M.; Wagey, F.; Suardi, D.; Susanto, H.; Laihad, B.J.; Tobing, M.D.L. Analysis of Curcumin as a Radiosensitizer in Cancer Therapy with Serum Survivin Examination: Randomised Control Trial. Asian Pac. J. Cancer Prev. 2021, 22, 139–143. [Google Scholar] [CrossRef]
- Liu, J.; Li, M.; Wang, Y.; Luo, J. Curcumin sensitizes prostate cancer cells to radiation partly via epigenetic activation of miR-143 and miR-143 mediated autophagy inhibition. J. Drug Target. 2017, 25, 645–652. [Google Scholar] [CrossRef]
- Rutz, J.; Benchellal, A.; Kassabra, W.; Maxeiner, S.; Bernd, A.; Kippenberger, S.; Zöller, N.; Chun, F.K.; Juengel, E.; Blaheta, R.A. Growth, Proliferation and Metastasis of Prostate Cancer Cells Is Blocked by Low-Dose Curcumin in Combination with Light Irradiation. Int. J. Mol. Sci. 2021, 22, 9966. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Cai, J.; Liu, J.; Han, B.; Gao, F.; Gao, W.; Zhang, Y.; Zhang, J.; Zhao, Z.; Jiang, C. Curcumin increases efficiency of γ-irradiation in gliomas by inhibiting Hedgehog signaling pathway. Cell Cycle 2017, 16, 1181–1192. [Google Scholar] [CrossRef] [Green Version]
- Zoi, V.; Galani, V.; Vartholomatos, E.; Zacharopoulou, N.; Tsoumeleka, E.; Gkizas, G.; Bozios, G.; Tsekeris, P.; Chousidis, I.; Leonardos, I.; et al. Curcumin and Radiotherapy Exert Synergistic Anti-Glioma Effect In Vitro. Biomedicines 2021, 9, 1562. [Google Scholar] [CrossRef]
- Wang, W.H.; Shen, C.Y.; Chien, Y.C.; Chang, W.S.; Tsai, C.W.; Lin, Y.H.; Hwang, J.J. Validation of Enhancing Effects of Curcumin on Radiotherapy with F98/FGT Glioblastoma-Bearing Rat Model. Int. J. Mol. Sci. 2020, 21, 4385. [Google Scholar] [CrossRef]
- Liu, G.; Wang, Y.; Li, M. Curcumin sensitized the antitumour effects of irradiation in promoting apoptosis of oesophageal squamous-cell carcinoma through NF-κB signalling pathway. J. Pharm. Pharmacol. 2018, 70, 1340–1348. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Qiu, J.; Wang, D.; Tao, Y.; Song, Y.; Wang, H.; Tang, J.; Wang, X.; Sun, Y.U.; Yang, Z.; et al. Traditional Chinese Medicine Curcumin Sensitizes Human Colon Cancer to Radiation by Altering the Expression of DNA Repair-related Genes. Anticancer. Res. 2018, 38, 131–136. [Google Scholar] [PubMed]
- Schwarz, K.; Dobiasch, S.; Nguyen, L.; Schilling, D.; Combs, S.E. Modification of radiosensitivity by Curcumin in human pancreatic cancer cell lines. Sci. Rep. 2020, 10, 3815. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Wang, Z.; Chong, T.; Yang, J.; Li, H.; Chen, H. Curcumin enhances the radiosensitivity of renal cancer cells by suppressing NF-κB signaling pathway. Biomed. Pharmacother. 2017, 94, 974–981. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Li, H.; Wu, S.; Qu, J.; Yuan, H.; Zhou, Y.; Lu, Q. MicroRNA-1246 regulates the radio-sensitizing effect of curcumin in bladder cancer cells via activating P53. Int. Urol. Nephrol. 2019, 51, 1771–1779. [Google Scholar] [CrossRef]
- Bryant, A.K.; Banegas, M.P.; Martinez, M.E.; Mell, L.K.; Murphy, J.D. Trends in Radiation Therapy among Cancer Survivors in the United States, 2000–2030. Cancer Epidemiol. Biomark. Prev. 2017, 26, 963–970. [Google Scholar] [CrossRef] [Green Version]
- FitzGerald, T.J.; Bishop-Jodoin, M.; Laurie, F.; Lukez, A.; O’Loughlin, L.; Sacher, A. Treatment Toxicity: Radiation. Oncol. Emerg. Med. 2019, 33, 1027–1039. [Google Scholar]
- Tawfik, S.S.; Abouelella, A.M.; Shahein, Y.E. Curcumin protection activities against γ-rays-induced molecular and biochemical lesions. BMC Res. Notes 2013, 6, 375. [Google Scholar] [CrossRef] [Green Version]
- Ryan, J.L. Ionizing radiation: The good, the bad, and the ugly. J. Investig. Dermatol. 2012, 132, 985–993. [Google Scholar] [CrossRef] [Green Version]
- Najafi, M.; Motevaseli, E.; Shirazi, A.; Geraily, G.; Rezaeyan, A.; Norouzi, F.; Rezapoor, S.; Abdollahi, H. Mechanisms of inflammatory responses to radiation and normal tissues toxicity: Clinical implications. Int. J. Radiat. Biol. 2018, 94, 335–356. [Google Scholar] [CrossRef]
- Shabeeb, D.; Musa, A.E.; Ali, H.S.A.; Najafi, M. Curcumin Protects Against Radiotherapy-Induced Oxidative Injury to the Skin. Drug Des. Dev. Ther. 2020, 14, 3159–3163. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Park, S.; Jeon, B.S.; Jang, W.-S.; Lee, S.-J.; Son, Y.; Rhim, K.-J.; Lee, S.I.; Lee, S.-S. Therapeutic effect of topical application of curcumin during treatment of radiation burns in a mini-pig model. J. Vet. Sci. 2016, 17, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Kole, A.J.; Kole, L.; Moran, M.S. Acute radiation dermatitis in breast cancer patients: Challenges and solutions. Breast Cancer Targets Ther. 2017, 9, 313–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryan Wolf, J.; Gewandter, J.S.; Bautista, J.; Heckler, C.E.; Strasser, J.; Dyk, P.; Anderson, T.; Gross, H.; Speer, T.; Dolohanty, L.; et al. Utility of topical agents for radiation dermatitis and pain: A randomized clinical trial. Support. Care Cancer 2020, 28, 3303–3311. [Google Scholar] [CrossRef]
- Wolf, J.R.; Heckler, C.E.; Guido, J.J.; Peoples, A.R.; Gewandter, J.S.; Ling, M.; Vinciguerra, V.P.; Anderson, T.; Evans, L.; Wade, J.; et al. Oral curcumin for radiation dermatitis: A URCC NCORP study of 686 breast cancer patients. Support Care Cancer 2018, 26, 1543–1552. [Google Scholar] [CrossRef]
- Proklou, A.; Diamantaki, E.; Pediaditis, E.; Kondili, E. Radiation Therapy: Impact on Lung Function and Acute Respiratory Failure. In Mechanical Ventilation in Critically Ill Cancer Patients; Springer: Berlin/Heidelberg, Germany, 2018; pp. 33–39. [Google Scholar]
- Amini, P.; Saffar, H.; Nourani, M.R.; Motevaseli, E.; Najafi, M.; Taheri, R.A.; Qazvini, A. Curcumin Mitigates Radiation-induced Lung Pneumonitis and Fibrosis in Rats. Int. J. Mol. Cell. Med. 2018, 7, 212–219. [Google Scholar]
- Groves, A.M.; Johnston, C.J.; Misra, R.S.; Williams, J.P.; Finkelstein, J.N. Effects of IL-4 on pulmonary fibrosis and the accumulation and phenotype of macrophage subpopulations following thoracic irradiation. Int. J. Radiat. Biol. 2016, 92, 754–765. [Google Scholar] [CrossRef] [Green Version]
- Ameziane-El-Hassani, R.; Talbot, M.; de Souza Dos Santos, M.C.; Al Ghuzlan, A.; Hartl, D.; Bidart, J.M.; De Deken, X.; Miot, F.; Diallo, I.; de Vathaire, F. NADPH oxidase DUOX1 promotes long-term persistence of oxidative stress after an exposure to irradiation. Proc. Natl. Acad. Sci. USA 2015, 112, 5051–5056. [Google Scholar] [CrossRef] [Green Version]
- Chen, T.; Zhuang, B.; Huang, Y.; Liu, Y.; Yuan, B.; Wang, W.; Yuan, T.; Du, L.; Jin, Y. Inhaled curcumin mesoporous polydopamine nanoparticles against radiation pneumonitis. Acta Pharm. Sin. B 2021, 11, 11. [Google Scholar] [CrossRef]
- Chaveli-López, B. Oral toxicity produced by chemotherapy: A systematic review. J. Clin. Exp. Dent. 2014, 6, 81–90. [Google Scholar] [CrossRef] [Green Version]
- Kia, S.J.; Basirat, M.; Saedi, H.S.; Arab, S.A. Effects of nanomicelle curcumin capsules on prevention and treatment of oral mucosits in patients under chemotherapy with or without head and neck radiotherapy: A randomized clinical trial. BMC Complement Med. Ther. 2021, 21, 232. [Google Scholar] [CrossRef] [PubMed]
- Charantimath, S. Use of curcumin in Radiochemotherapy induced Oral mucositis patients: A control trial study. Int. J. Med. Health Sci. 2016, 10, 147–152. [Google Scholar]
- Shah, S.; Rath, H.; Sharma, G.; Senapati, S.N.; Mishra, E. Effectiveness of curcumin mouthwash on radiation-induced oral mucositis among head and neck cancer patients: A triple-blind, pilot randomised controlled trial. Indian, J. Dent. Res. 2020, 31, 718–727. [Google Scholar]
- Farhadi, M.; Bakhshandeh, M.; Shafiei, B.; Mahmoudzadeh, A.; Hosseinimehr, S.J. The Radioprotective Effects of Nano-Curcumin Against Genotoxicity Induced by Iodine-131 in Patients with Differentiated Thyroid Carcinoma (DTC) by Micronucleus Assay, Int. J. Cancer Manag. 2018, 11, e14193. [Google Scholar]
- Jagetia, G.C. Antioxidant activity of curcumin protects against the radiation-induced micronuclei formation in cultured human peripheral blood lymphocytes exposed to various doses of γ-Radiation. Int. J. Radiat. Biol. 2021, 97, 485–493. [Google Scholar] [CrossRef]
- Minh-Hiep, N.; Ngoc-Duy, P.; Bingxue, D.; Thi-Huynh-Nga, N.; Chi-Bao, B.; Hadinoto, K. Radioprotective activity of curcumin-encapsulated liposomes against genotoxicity caused by Gamma Cobalt-60 irradiation in human blood cells. Int. J. Radiat. Biol. 2017, 93, 1267–1273. [Google Scholar]
- Kolivand, S.; Amini, P.; Saffar, H.; Rezapoor, S.; Motevaseli, E.; Najafi, M.; Nouruzi, F.; Shabeeb, D.; Musa, A.E. Evaluating the Radioprotective Effect of Curcumin on Rat’s Heart Tissues. Curr. Radiopharm. 2019, 12, 23–28. [Google Scholar] [CrossRef]
- Eassawy, M.M.T.; Salem, A.A.; Ismail, A.F.M. Biochemical study on the protective effect of curcumin on acetaminophen and gammairradiation induced hepatic toxicity in rats. Environ. Toxicol. 2021, 36, 748–763. [Google Scholar] [CrossRef]
- Li, W.; Jiang, L.; Lu, X.; Liu, X.; Ling, M. Curcumin protects radiation-induced liver damage in rats through the NF-κBsignaling pathway. BMC Complementary Med. Ther. 2021, 21, 10. [Google Scholar]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef]
- Shi, H.S.; Gao, X.; Li, D.; Zhang, Q.W.; Wang, Y.S.; Zheng, Y.; Cai, L.L.; Zhong, R.M.; Rui, A.; Li, Z.; et al. A systemic administration of liposomal curcumin inhibits radiation pneumonitis and sensitizes lung carcinoma to radiation. Int. J. Nanomed. 2012, 7, 2601–2611. [Google Scholar]
- Jäger, R.; Lowery, R.P.; Calvanese, A.V.; Joy, J.M.; Purpura, M.; Wilson, J.M. Comparative absorption of curcumin formulations. Nutr. J. 2014, 13, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, S.P.; Sharma, M.; Gupta, P.K. Cytotoxicity of curcumin silica nanoparticle complexes conjugated with hyaluronic acid on colon cancer cells. Int. J. Biol. Macromol. 2015, 74, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Jyoti, K.; Bhatia, R.K.; Martis, E.A.F.; Coutinho, E.C.; Jain, U.K.; Chandra, R.; Madan, J. Soluble curcumin amalgamated chitosan microspheres augmented drug delivery and cytotoxicity in colon cancer cells: In vitro, and in vivo, study. Colloids Surf. B Biointerfaces 2016, 148, 674–683. [Google Scholar] [CrossRef]
- Shinde, R.L.; Devarajan, P.V. Docosahexaenoic acid-mediated, targeted and sustained brain delivery of curcumin microemulsion. Drug Deliv. 2017, 24, 152–161. [Google Scholar] [CrossRef] [Green Version]
- Choi, Y.H.; Han, D.H.; Kim, S.W.; Kim, M.J.; Sung, H.H.; Jeon, H.G.; Jeong, B.C.; Seo, S.I.; Jeon, S.S.; Lee, H.M.; et al. A randomized, double-blind, placebo-controlled trial to evaluate the role of curcumin in prostate cancer patients with intermittent androgen deprivation. Prostate 2019, 79, 614–621. [Google Scholar] [CrossRef]
- Lanjekar, A.B.; Bhowate, R.R.; Bakhle, S.; Narayane, A.; Pawar, V.; Gandagule, R. Comparison of efficacy of topical curcumin gel with triamcinolone-hyaluronidase gel individually and in combination in the treatment of oral submucous fibrosis. J. Contemp. Dent. Pract. 2020, 21, 83–90. [Google Scholar]
- Delavarian, Z.; Pakfetrat, A.; Ghazi, A.; Jaafari, M.R.; Homaei, S.F.; Dalirsani, Z.; Mohammadpour, A.H.; Rahimi, H.R. Oral administration of nanomicelle curcumin in the prevention of radiotherapy-induced mucositis in head and neck cancers. Spec. Care Dent. 2019, 39, 166–172. [Google Scholar] [CrossRef]
- Pinheiro, S.L.; Bonadiman, A.C.; Borges-Lemos, A.L.; Annicchino, B.M.; Segatti, B.; Pucca, D.S.; Dutra, P.T.; de Carvalho, E.; Silva, R.M.; Leal, F. Photobiomodulation Therapy in Cancer Patients with Mucositis: A Clinical Evaluation. Photobiomodulation Photomed. Laser Surg. 2019, 37, 142–150. [Google Scholar] [CrossRef]
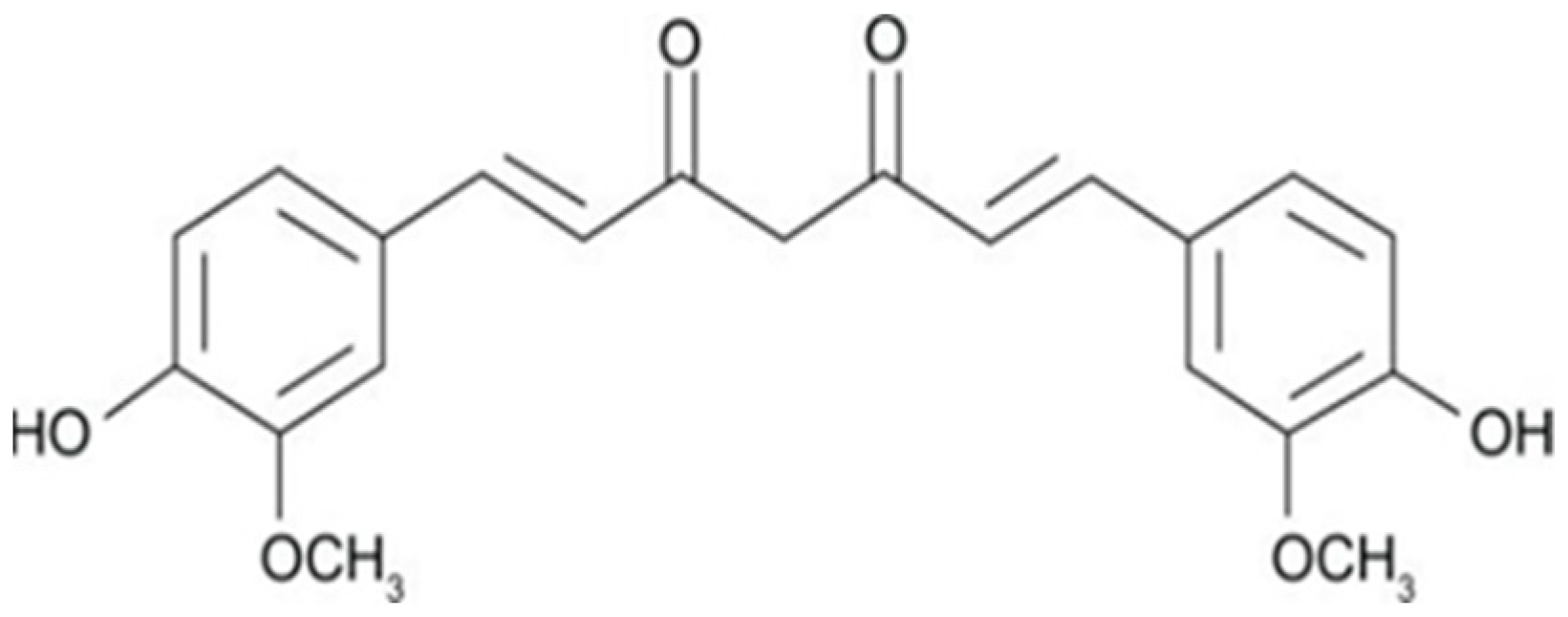
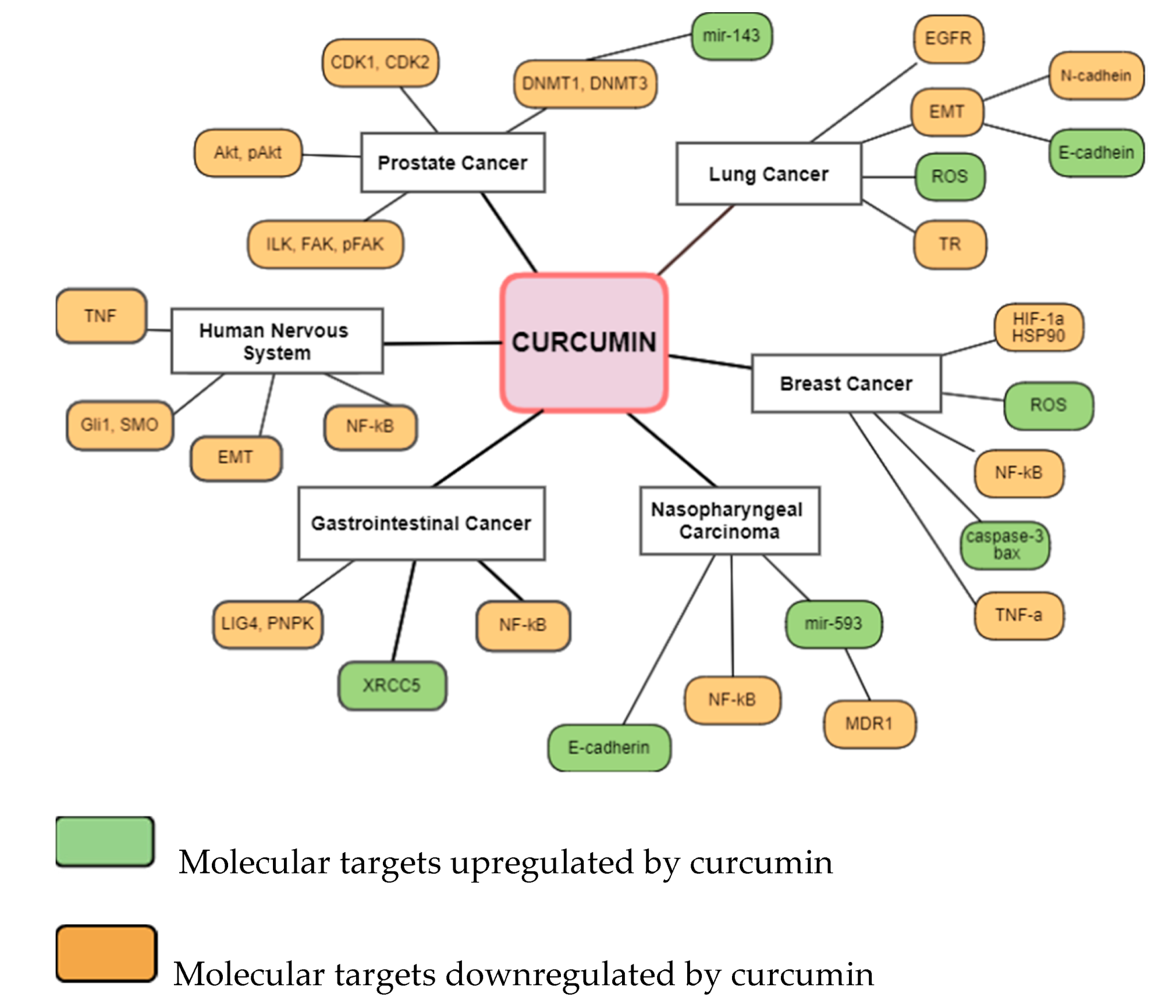
| Cancer Site | Subject | Curcumin Dosage | Effect | Ref. |
|---|---|---|---|---|
| Lung | Human A549 cells | 10 μΜ | Ιncrease in IR-induced reduction of cell viability via inhibition of EGFR protein | [33] |
| Human A549 cells | 5–20 μΜ | Inhibition of migration, invasion through suppression of radiation-induced EMT | [35] | |
| Xenograft model of A549 cells | 20 uM | Induction of apoptosis | [38] | |
| Nasopharynx | Human nasopharyngeal carcinoma (NPC) cells | 10 μΜ | Increase in radiosensitivity through depression of MDR1 expression | [39] |
| Human NPC cell line CNE-2 | 20 μΜ | Increase in IR-induced cell death through modulation of circRNAs | [40] | |
| Breast | Human MCF10A, MCF7 and MDA-MB-231 BC cells | 2.5–10 μΜ | Deregulation of molecules involved in the induction of apoptosis, in the inflammatory process, in the cell cycle, and tyrosine metabolism | [42] |
| Breast cancer stem cells | 30 μΜ | Reduction of RT resistance through inhibition of HIF-1a, HSP90 | [43] | |
| Prostate | Human PC-3, DU145, and LNCaP cells | 30 μΜ | Increase in IR-induced apoptosis/reduction in the expression of DNMT1 and DNMT3B | [46] |
| DU145 and PC-3 cells | 0.1–0.4 µg/mL | Tumor growth suppression, decreased invasion, and migration | [47] | |
| Cervix | Human HeLa cells | 40 μΜ | Increased cell cytotoxicity of the combination | [44] |
| Cervical carcinoma stage IIB–IIIB patients | 4 g/day | Decreased levels of surviving | [45] | |
| CNS | LN229 and U251 glioma cells | 20 μΜ | Reduction in cell migration and invasion/inhibition of the Hedgehog signaling pathway | [48] |
| U87 and T98 human glioma cells | 10–20 μΜ | Increased cytotoxicity and G2/M arrest | [49] | |
| Orthotopic F98/FGT glioma-bearing rat model. | 5-20 μΜ | Suppression of the growth of in situ brain tumors | [50] | |
| Esophagus | Human ESCC-07, ESCC-12, ESCC-19, ESCC-27 and ESCC-31 cell lines | 10 μΜ | Increase in IR-induced apoptosis/Inhibition of Nf-Kb signaling | [51] |
| ESCC-07 xenograft mice | 10 μΜ | Decrease in tumor volume and weight | [51] | |
| Colon | Human colon cancer HT-29 cells | 2.5 μΜ | Inhibition of cell proliferation/modulation of expression of DNA repair-related genes | [52] |
| HT-29 bearing mice | 2.5 μΜ | Intratumoral apoptosis and suppression of neoplastic growth | [52] | |
| Pancreas | Human Panc-1 and MiaPaCa-2 cells | 6 or 12 μΜ | Increased cytotoxicity and G2/M arrest | [53] |
| Kidney | Renal ACHN cancer cells | 5–80 μΜ | Increased cell death, suppression of the NF-κB signaling pathway | [54] |
| ACHN tumor-bearing nude mice | 5–80 μΜ | Decrease in tumor volume increased apoptosis | [54] | |
| Urinary Bladder | Urinary bladder cancer T24 cells | 10 μΜ | Inhibition of p53 nuclear transcription factor | [55] |
| Adverse Reaction | Subject | Curcumin Dosage | Mechanism/Conclusion | Ref |
|---|---|---|---|---|
| Acute skin reactions (prevention) | 40 rats | 150 mg/kg 1 day before to 3 days post-radiation | ↑ antioxidant enzymes (CAT, SOD, MDA) | [61] |
| Acute skin reactions (management) | mini-pig model | 200 mg/cm² twice a day for 35 days after RT | ↓ NF-κB and COX-2 expression, ↑ wound healing | [62] |
| Radiation dermatitis (prevention) | 191 breast cancer patients | Curcumin gel 3 times daily for 1 week after RT | ↓ RDS and Pain scores in patients with high breast separation (≥25 cm) | [64] |
| Radiation dermatitis (prevention) | 686 breast cancer patients | 500 mg three times daily for 1 week after RT | No sig. difference between curcumin and placebo in RDS | [65] |
| Radiation pneumonitis/fibrosis | 20 rats | 150 mg/kg for 4 days before and 6 consecutive days after RT | ↓ IL-4, IL4Ra1, DUOX1 and 2 expression | [67] |
| Radiation pneumonitis | 20 rats | 2 mg i.t. 5 h pre-irradiation | ↓ proinflammatory cytokines, MDA, and ↑ SOD expression | [69] |
| Oral mucositis (treatment) | 50 head and neck cancer patients | 80 mg of curcumin nanomicelle capsules twice a day for 7 weeks | ↓ OM severity and pain | [72] |
| Oral mucositis (treatment) | 40 patients with OM | Gel containing 10 mg of curcumin, 3 times a day for 2 weeks | ↓ OM severity | [73] |
| Lymphocytes genotoxicity (treatment) | 21 patients with differentiated thyroid carcinoma (DTC) | 160 mg/day for 10 days post-RT | ↓ frequency of micronuclei in peripheral blood lymphocytes | [75] |
| Lymphocytes genotoxicity (prevention) | Human peripheral blood lymphocytes (HPBLs) | 0.125–50 µg/mL prior to RT | ↓ formation of OH, NO, DPPH, micronuclei | [76] |
| Heart tissue toxicity (prevention) | 20 rats | 150 mg/kg curcumin for 7days after RT | ↓ Duox1 and Duox2, IL-4 protein and its receptor ↑ infiltration lymphocytes, macrophages | [78] |
| Hepatic toxicity (prevention) | 20 rats | 100 mg/kg orally for 21 days before RT | Regulation of Nrf2, mir-122, Ca2+ level, NF-κB | [79] |
| Liver toxicity (treatment) | 30 rats | 30 mg/kg for 2 weeks once a day post-RT | ↑ SOD, CAD, GSH, Bcl-2 ↓ TNF-α, IL-1β, IL-6, ↓ p-NF-κB/NF-κB | [80] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zoi, V.; Galani, V.; Tsekeris, P.; Kyritsis, A.P.; Alexiou, G.A. Radiosensitization and Radioprotection by Curcumin in Glioblastoma and Other Cancers. Biomedicines 2022, 10, 312. https://doi.org/10.3390/biomedicines10020312
Zoi V, Galani V, Tsekeris P, Kyritsis AP, Alexiou GA. Radiosensitization and Radioprotection by Curcumin in Glioblastoma and Other Cancers. Biomedicines. 2022; 10(2):312. https://doi.org/10.3390/biomedicines10020312
Chicago/Turabian StyleZoi, Vasiliki, Vasiliki Galani, Pericles Tsekeris, Athanasios P. Kyritsis, and George A. Alexiou. 2022. "Radiosensitization and Radioprotection by Curcumin in Glioblastoma and Other Cancers" Biomedicines 10, no. 2: 312. https://doi.org/10.3390/biomedicines10020312
APA StyleZoi, V., Galani, V., Tsekeris, P., Kyritsis, A. P., & Alexiou, G. A. (2022). Radiosensitization and Radioprotection by Curcumin in Glioblastoma and Other Cancers. Biomedicines, 10(2), 312. https://doi.org/10.3390/biomedicines10020312







