Lung Fibroblasts from Idiopathic Pulmonary Fibrosis Patients Harbor Short and Unstable Telomeres Leading to Chromosomal Instability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Lung Fibroblasts
2.2. Preparation of Cytogenetic Slides
2.3. Telomere and Centromere Staining
2.3.1. Telomere Length
2.3.2. Telomere Abnormalities
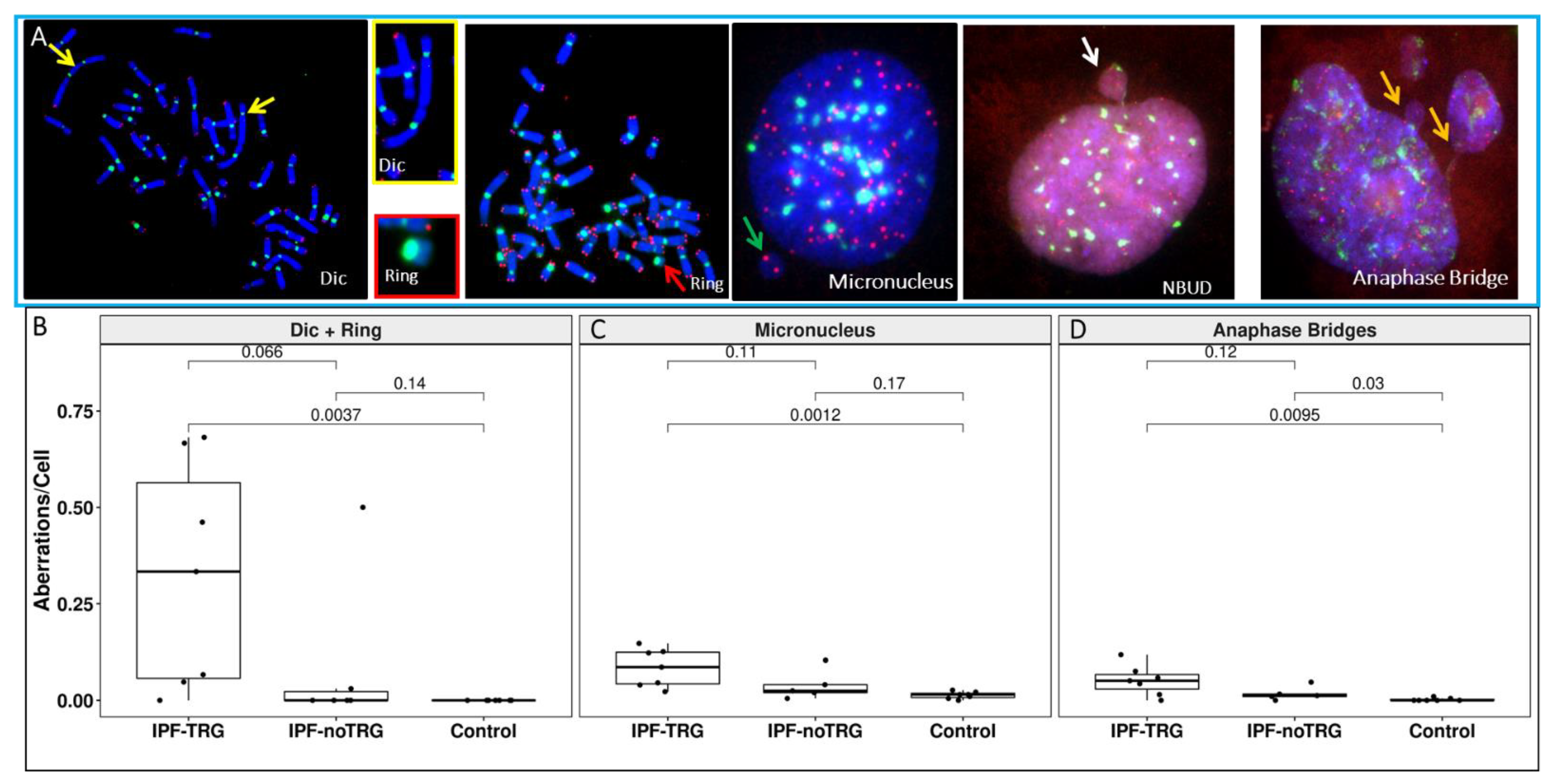
2.4. Dicentric Chromosomes, Micronuclei, and Anaphase Bridges Detection
2.5. Statistical Analysis
3. Results
3.1. Clinical Characteristics of IPF Patients
3.2. Telomere Staining Demonstrate Reduced and Heterogeneous Telomere Lengths in IPF-TRG Fibroblasts
3.3. Telomere Staining Shows Telomere Abnormalities in IPF-TRG Fibroblasts
3.4. Telomere and Centromere Staining Shows Dicentric Chromosomes and Impaired Chromosome Segregation in IPF-TRG Fibroblasts
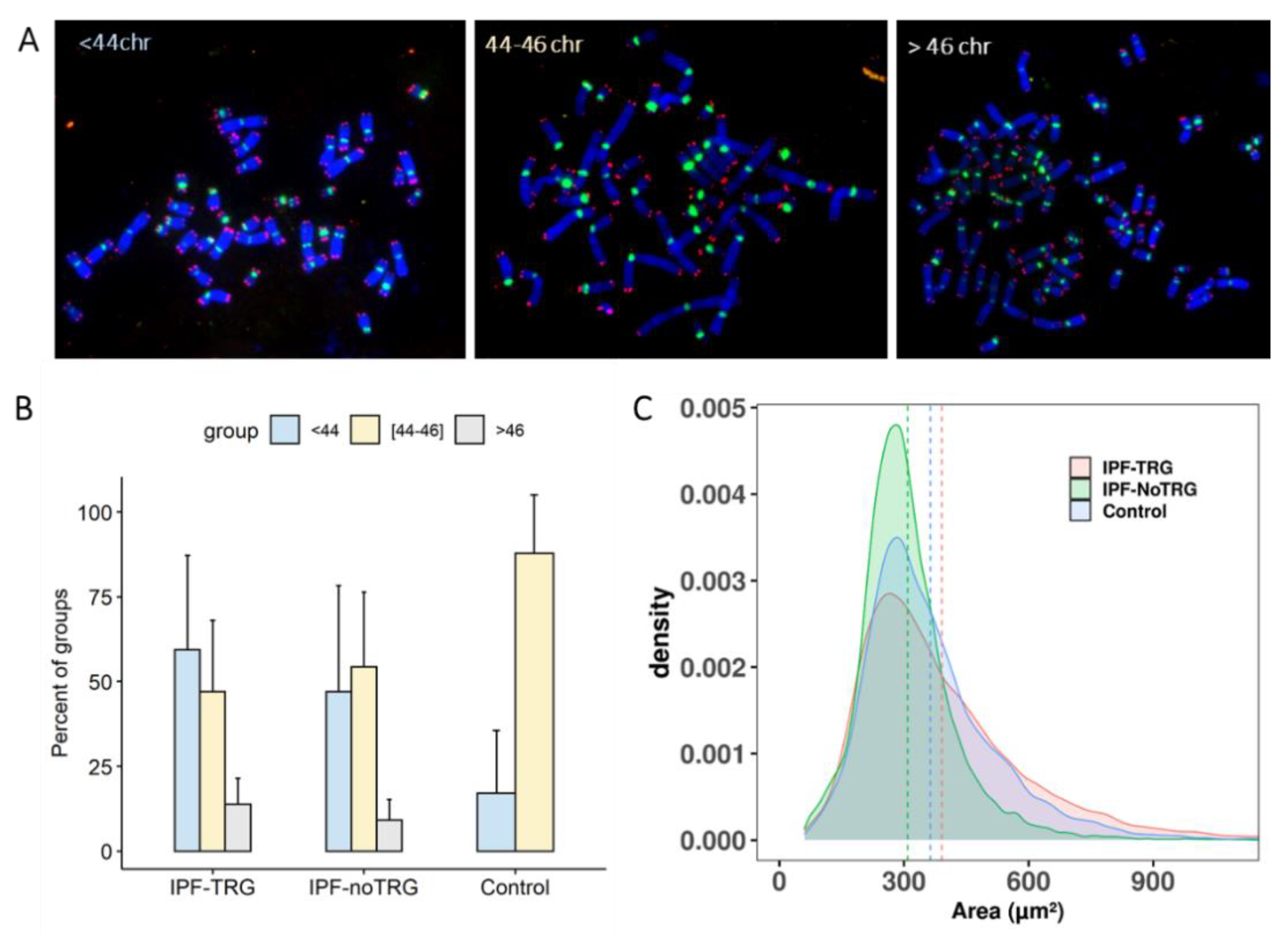
3.5. Aneuploidy in IPF-TRG Fibroblasts Identified by Telomere and Centromere Staining
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lazzerini-Denchi, E.; Sfeir, A. Stop pulling my strings—What telomeres taught us about the DNA damage response. Nat. Rev. Mol. Cell Biol. 2016, 17, 364–378. [Google Scholar] [CrossRef] [PubMed]
- Palm, W.; de Lange, T. How shelterin protects mammalian telomeres. Annu. Rev. Genet. 2008, 42, 301–334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nassour, J.; Radford, R.; Correia, A.; Fusté, J.M.; Schoell, B.; Jauch, A.; Shaw, R.J.; Karlseder, J. Autophagic cell death restricts chromosomal instability during replicative crisis. Nature 2019, 565, 659–663. [Google Scholar] [CrossRef] [PubMed]
- Murnane, J.P. Telomere dysfunction and chromosome instability. Mutat. Res. Mol. Mech. Mutagen. 2012, 730, 28–36. [Google Scholar] [CrossRef] [Green Version]
- de Lange, T. Shelterin-Mediated Telomere Protection. Annu. Rev. Genet. 2018, 52, 223–247. [Google Scholar] [CrossRef]
- Erdel, F.; Kratz, K.; Willcox, S.; Griffith, J.D.; Greene, E.C.; de Lange, T. Telomere Recognition and Assembly Mechanism of Mammalian Shelterin. Cell Rep. 2017, 18, 41–53. [Google Scholar] [CrossRef]
- Savage, S.A. Beginning at the ends: Telomeres and human disease. F1000Research 2018, 7, 524. [Google Scholar] [CrossRef] [Green Version]
- Lederer, D.J.; Martinez, F.J. Idiopathic Pulmonary Fibrosis. N. Engl. J. Med. 2018, 379, 797–798. [Google Scholar] [CrossRef]
- Stuart, B.D.; Choi, J.; Zaidi, S.; Xing, C.; Holohan, B.; Chen, R.; Choi, M.; Dharwadkar, P.; Torres, F.; Girod, C.E.; et al. Exome sequencing links mutations in PARN and RTEL1 with familial pulmonary fibrosis and telomere shortening. Nat. Genet. 2015, 47, 512–517. [Google Scholar] [CrossRef] [Green Version]
- Stanley, S.E.; Gable, D.L.; Wagner, C.L.; Carlile, T.M.; Hanumanthu, V.S.; Podlevsky, J.D.; Khalil, S.E.; DeZem, A.E.; Rojas-Duran, M.F.; Applegate, C.D.; et al. Loss-of-function mutations in the RNA biogenesis factor NAF1 predispose to pulmonary fibro-sis-emphysema. Sci. Transl. Med. 2016, 8, 351ra107. [Google Scholar] [CrossRef] [Green Version]
- Kannengiesser, C.; Borie, R.; Ménard, C.; Réocreux, M.; Nitschké, P.; Gazal, S.; Mal, H.; Taillé, C.; Cadranel, J.; Nunes, H.; et al. HeterozygousRTEL1mutations are associated with familial pulmonary fibrosis. Eur. Respir. J. 2015, 46, 474–485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsakiri, K.D.; Cronkhite, J.T.; Kuan, P.J.; Xing, C.; Raghu, G.; Weissler, J.C.; Rosenblatt, R.L.; Shay, J.W.; Garcia, C.K. Adult-onset pulmonary fibrosis caused by mutations in telomerase. Proc. Natl. Acad. Sci. USA 2007, 104, 7552–7557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armanios, M.Y.; Chen, J.; Cogan, J.D.; Alder, J.; Ingersoll, R.G.; Markin, C.; Lawson, W.E.; Xie, M.; Vulto, I.; Phillips, J.A.; et al. Telomerase Mutations in Families with Idiopathic Pulmonary Fibrosis. N. Engl. J. Med. 2007, 356, 1317–1326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stuart, B.D.; Lee, J.S.; Kozlitina, J.; Noth, I.; Devine, M.S.; Glazer, C.S.; Torres, F.; Kaza, V.; Girod, C.E.; Jones, K.D.; et al. Effect of telomere length on survival in patients with idiopathic pulmonary fibrosis: An observational cohort study with independent validation. Lancet Respir. Med. 2014, 2, 557–565. [Google Scholar] [CrossRef] [Green Version]
- Joannes, A.; Brayer, S.; Besnard, V.; Marchal-Sommé, J.; Jaille, M.; Mordant, P.; Mal, H.; Borie, R.; Crestani, B.; Mailleux, A.A. FGF9 and FGF18 in idiopathic pulmonary fibrosis promote survival and migration and inhibit myofibroblast differentiation of human lung fibroblasts in vitro. Am. J. Physiol. Lung Cell. Mol. Physiol. 2016, 310, L615–L629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borie, R.; Kannengiesser, C.; Hirschi, S.; Le Pavec, J.; Mal, H.; Bergot, E.; Jouneau, S.; Naccache, J.-M.; Revy, P.; Boutboul, D.; et al. Severe hematologic complications after lung transplantation in patients with telomerase complex mutations. J. Hear. Lung Transplant. 2015, 34, 538–546. [Google Scholar] [CrossRef]
- Juge, P.-A.; Borie, R.; Kannengiesser, C.; Gazal, S.; Revy, P.; Wemeau-Stervinou, L.; Debray, M.-P.; Ottaviani, S.; Marchand-Adam, S.; Nathan, N.; et al. Shared genetic predisposition in rheumatoid arthritis-interstitial lung disease and familial pulmonary fibrosis. Eur. Respir. J. 2017, 49, 1602314. [Google Scholar] [CrossRef]
- De Lange, T. Telomere-related genome instability in cancer. Cold Spring Harb. Symp. Quant. Biol. 2005, 70, 197–204. [Google Scholar] [CrossRef] [Green Version]
- Artandi, S.E.; Chang, S.; Lee, S.-L.; Alson, S.; Gottlieb, G.J.; Chin, L.; DePinho, R. Telomere dysfunction promotes non-reciprocal translocations and epithelial cancers in mice. Nature 2000, 406, 641–645. [Google Scholar] [CrossRef]
- Calado, R.T.; Regal, J.A.; Hills, M.; Yewdell, W.T.; Dalmazzo, L.F.; Zago, M.A.; Lansdorp, P.M.; Hogge, D.; Chanock, S.J.; Estey, E.H.; et al. Constitutional hypomorphic telomerase mutations in patients with acute myeloid leukemia. Proc. Natl. Acad. Sci. USA 2009, 106, 1187–1192. [Google Scholar] [CrossRef] [Green Version]
- Calado, R.T.; Cooper, J.N.; Padilla-Nash, H.M.; Sloand, E.M.; Wu, C.O.; Scheinberg, P.; Ried, T.; Young, N.S. Short telomeres result in chromosomal instability in hematopoietic cells and precede malignant evolution in human aplastic anemia. Leukemia 2012, 26, 700–707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyer, D.H.; Bailis, A.M. Telomerase Deficiency Affects the Formation of Chromosomal Translocations by Homologous Recombination in Saccharomyces cerevisiae. PLoS ONE 2008, 3, e3318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- M’kacher, R.; Colicchio, B.; Borie, C.; Junker, S.; Marquet, V.; Heidingsfelder, L.; Soehnlen, K.; Najar, W.; Hempel, W.M.; Oudrhiri, N.; et al. Telomere and Centromere Staining Followed by M-FISH Improves Diagnosis of Chromosomal Instability and Its Clinical Utility. Genes 2020, 11, 475. [Google Scholar] [CrossRef] [PubMed]
- M’kacher, R.; Colicchio, B.; Marquet, V.; Borie, C.; Najar, W.; Hempel, W.M.; Heidingsfelder, L.; Oudrhiri, N.; Al Jawhari, M.; Wilhelm-Murer, N.; et al. Telomere aberrations, including telomere loss, doublets, and extreme shortening, are increased in patients with infertility. Fertil. Steril. 2021, 115, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Raghu, G.; Remy-Jardin, M.; Myers, J.L.; Richeldi, L.; Ryerson, C.J.; Lederer, D.J.; Behr, J.; Cottin, V.; Danoff, S.K.; Morell, F.; et al. Diagnosis of Idiopathic Pulmonary Fibrosis. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2018, 198, e44–e68. [Google Scholar] [CrossRef]
- Borie, R.; Tabèze, L.; Thabut, G.; Nunes, H.; Cottin, V.; Marchand-Adam, S.; Prevot, G.; Tazi, A.; Cadranel, J.; Mal, H.; et al. Prevalence and characteristics of TERT and TERC mutations in suspected genetic pulmonary fibrosis. Eur. Respir. J. 2016, 48, 1721–1731. [Google Scholar] [CrossRef] [Green Version]
- M’kacher, R.; Maalouf, E.E.; Ricoul, M.; Heidingsfelder, L.; Laplagne, E.; Cuceu, C.; Hempel, W.M.; Colicchio, B.; Dieterlen, A.; Sabatier, L. New tool for biological dosimetry: Reevaluation and automation of the gold standard method following telomere and centromere staining. Mutat. Res. 2014, 770, 45–53. [Google Scholar] [CrossRef]
- Vodicka, P.; Andera, L.; Opattova, A.; Vodickova, L. The Interactions of DNA Repair, Telomere Homeostasis, and p53 Mutational Status in Solid Cancers: Risk, Prognosis, and Prediction. Cancers 2021, 13, 479. [Google Scholar] [CrossRef]
- Finot, F.; Kaddour, A.; Morat, L.; Mouche, I.; Zaguia, N.; Cuceu, C.; Souverville, D.; Négrault, S.; Cariou, O.; Essahli, A.; et al. Genotoxic risk of ethyl-paraben could be related to telomere shortening. J. Appl. Toxicol. 2017, 37, 758–771. [Google Scholar] [CrossRef]
- Armanios, M.; Chen, J.-L.; Christy Chang, Y.-P.; Brodsky, R.A.; Hawkins, A.; Griffin, C.A.; Eshleman, J.R.; Cohen, A.R.; Chakravarti, A.; Hamosh, A.; et al. Haploinsufficiency of telomerase reverse transcriptase leads to anticipation in autosomal dominant dys-keratosis congenita. Proc. Natl. Acad. Sci. USA 2005, 102, 15960–15964. [Google Scholar] [CrossRef] [Green Version]
- Cronkhite, J.T.; Xing, C.; Raghu, G.; Chin, K.; Torres, F.; Rosenblatt, R.L.; Garcia, C.K. Telomere Shortening in Familial and Sporadic Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 2008, 178, 729–737. [Google Scholar] [CrossRef] [PubMed]
- Alder, J.K.; Chen, J.J.-L.; Lancaster, L.; Danoff, S.; Su, S.-C.; Cogan, J.D.; Vulto, I.; Xie, M.; Qi, X.; Tuder, R.M.; et al. Short telomeres are a risk factor for idiopathic pulmonary fibrosis. Proc. Natl. Acad. Sci. USA 2008, 105, 13051–13056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newton, C.A.; Kozlitina, J.; Lines, J.R.; Kaza, V.; Torres, F.; Garcia, C.K. Telomere length in patients with pulmonary fibrosis associated with chronic lung allograft dysfunction and post–lung transplantation survival. J. Hear. Lung Transplant. 2017, 36, 845–853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Batenburg, A.A.; Kazemier, K.M.; van Oosterhout, M.F.; van der Vis, J.J.; Grutters, J.C.; Goldschmeding, R.; van Moorsel, C.H. Telomere shortening and DNA damage in culprit cells of different types of progressive fibrosing interstitial lung disease. ERJ Open Res. 2021, 7, 691–2020. [Google Scholar] [CrossRef] [PubMed]
- Alder, J.K.; Hanumanthu, V.S.; Strong, M.A.; De Zern, A.E.; Stanley, S.E.; Takemoto, C.M.; Danilova, L.; Applegate, C.D.; Bolton, S.G.; Mohr, D.W.; et al. Diagnostic utility of telomere length testing in a hospital-based setting. Proc. Natl. Acad. Sci. USA 2018, 115, E2358–E2365. [Google Scholar] [CrossRef] [Green Version]
- van Batenburg, A.A.; Kazemier, K.M.; van Oosterhout, M.F.M.; van der Vis, J.J.; van Es, H.W.; Grutters, J.C.; Goldschmeding, R.; van Moorsel, C.H.M. From organ to cell: Multi-level telomere length assessment in patients with idiopathic pulmonary fibrosis. PLoS ONE 2020, 15, e0226785. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Zhang, J.; Liu, Y.; Zhang, S.; Wang, Y.; Liu, B.; Liu, H.; Li, R.; Lv, C.; Song, X. Regulation of TERRA on telomeric and mitochondrial functions in IPF pathogenesis. BMC Pulm. Med. 2017, 17, 163. [Google Scholar] [CrossRef] [Green Version]
- Townsley, D.M.; Dumitriu, B.; Young, N.S. Bone marrow failure and the telomeropathies. Blood 2014, 124, 2775–2783. [Google Scholar] [CrossRef]
- Yanai, H.; Shteinberg, A.; Porat, Z.; Budovsky, A.; Braiman, A.; Zeische, R.; Fraifeld, V.E. Cellular senescence-like features of lung fibroblasts derived from idiopathic pulmonary fibrosis patients. Aging 2015, 7, 664–672. [Google Scholar] [CrossRef] [Green Version]
- Parimon, T.; Hohmann, M.; Yao, C. Cellular Senescence: Pathogenic Mechanisms in Lung Fibrosis. Int. J. Mol. Sci. 2021, 22, 6214. [Google Scholar] [CrossRef]
- Fenech, M.; Knasmueller, S.; Bolognesi, C.; Holland, N.; Bonassi, S.; Kirsch-Volders, M. Micronuclei as biomarkers of DNA damage, aneuploidy, inducers of chromosomal hypermutation and as sources of pro-inflammatory DNA in humans. Mutat. Res. Rev. Mutat. Res. 2020, 786, 108342. [Google Scholar] [CrossRef] [PubMed]
- Kirsch-Volders, M.; Fenech, M. Inflammatory cytokine storms severity may be fueled by interactions of micronuclei and RNA viruses such as COVID-19 virus SARS-CoV-2. A hypothesis. Mutat. Res. Rev. Mutat. Res. 2021, 788, 108395. [Google Scholar] [CrossRef] [PubMed]
- Gascoigne, K.E.; Cheeseman, I.M. Induced dicentric chromosome formation promotes genomic rearrangements and tu-morigenesis. Chromosome Res. 2013, 21, 407–418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barra, V.; Fachinetti, D. The dark side of centromeres: Types, causes and consequences of structural abnormalities impli-cating centromeric DNA. Nat. Commun. 2018, 9, 4340. [Google Scholar] [CrossRef] [Green Version]
- Kaddour, A.; Colicchio, B.; Buron, D.; El Maalouf, E.; Laplagne, E.; Borie, C.; Ricoul, M.; Lenain, A.; Hempel, W.M.; Morat, L.; et al. Transmission of Induced Chromosomal Aberrations through Successive Mitotic Divisions in Human Lymphocytes after In Vitro and In Vivo Radiation. Sci. Rep. 2017, 7, 3291. [Google Scholar] [CrossRef]
- Fenech, M. Cytokinesis-block micronucleus cytome assay. Nat. Protoc. 2007, 2, 1084–1104. [Google Scholar] [CrossRef] [Green Version]
- Fenech, M. Cytokinesis-Block Micronucleus Cytome Assay Evolution into a More Comprehensive Method to Measure Chromosomal Instability. Genes 2020, 11, 1203. [Google Scholar] [CrossRef]
- Chen, M.; Chen, R.; Jin, Y.; Li, J.; Hu, X.; Zhang, J.; Fujimoto, J.; Hubert, S.M.; Gay, C.M.; Zhu, B.; et al. Cold and heterogeneous T cell repertoire is associated with copy number aberrations and loss of immune genes in small-cell lung cancer. Nat. Commun. 2021, 12, 6655. [Google Scholar] [CrossRef]
- Dejima, H.; Hu, X.; Chen, R.; Zhang, J.; Fujimoto, J.; Parra, E.R.; Haymaker, C.; Hubert, S.M.; Duose, D.; Solis, L.M.; et al. Immune evolution from preneoplasia to invasive lung adenocarcinomas and underlying molecular features. Nat. Commun. 2021, 12, 2722. [Google Scholar] [CrossRef]
- El-Zein, R.A.; Fenech, M.; Lopez, M.S.; Spitz, M.R.; Etzel, C.J. Cytokinesis-Blocked Micronucleus Cytome Assay Biomarkers Identify Lung Cancer Cases Amongst Smokers. Cancer Epidemiol. Biomark. Prev. 2008, 17, 1111–1119. [Google Scholar] [CrossRef] [Green Version]


| IPF with TRG Mutation | IPF without TRG Mutation | Controls | p * | |
|---|---|---|---|---|
| n | 7 | 6 | 7 | |
| Familial IPF | 3 | 0 | 0 | 0.5 |
| Male (%) | 4 (57%) | 4 (66%) | 5 (71%) | 1 |
| Ex- or current Smoker | 4 (57%) | 3 (50%) | 7 (100%) | 0.05 |
| Age at diagnosis | 55.7 (41.7–63.0) | 62.7 (56.2–74.2) | 64.5 (56.1–75.9) | 0.10 |
| Age at lung surgery | 58.3 (44.6–65.6) | 65.1 (58.1–74.2) | 64.5 (56.1–75.9) | 0.33 |
| Lung transplantation | 6 (88%) | 2 (33%) | NA | NA |
| FVC | 56.6 (39.0–75.0) | 73.2 (52.0–90.0) | 96.1 (80.0–116.0) | 0.0007 |
| DLCO | 30.6 (15.0–52.0) | 47.5 (23.0–59.0) | 63.8 (48.0–78.0) | 0.002 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
M’Kacher, R.; Jaillet, M.; Colicchio, B.; Vasarmidi, E.; Mailleux, A.; Dieterlen, A.; Kannengiesser, C.; Borie, C.; Oudrhiri, N.; Junker, S.; et al. Lung Fibroblasts from Idiopathic Pulmonary Fibrosis Patients Harbor Short and Unstable Telomeres Leading to Chromosomal Instability. Biomedicines 2022, 10, 310. https://doi.org/10.3390/biomedicines10020310
M’Kacher R, Jaillet M, Colicchio B, Vasarmidi E, Mailleux A, Dieterlen A, Kannengiesser C, Borie C, Oudrhiri N, Junker S, et al. Lung Fibroblasts from Idiopathic Pulmonary Fibrosis Patients Harbor Short and Unstable Telomeres Leading to Chromosomal Instability. Biomedicines. 2022; 10(2):310. https://doi.org/10.3390/biomedicines10020310
Chicago/Turabian StyleM’Kacher, Radhia, Madeleine Jaillet, Bruno Colicchio, Eirini Vasarmidi, Arnaud Mailleux, Alain Dieterlen, Caroline Kannengiesser, Claire Borie, Noufissa Oudrhiri, Steffen Junker, and et al. 2022. "Lung Fibroblasts from Idiopathic Pulmonary Fibrosis Patients Harbor Short and Unstable Telomeres Leading to Chromosomal Instability" Biomedicines 10, no. 2: 310. https://doi.org/10.3390/biomedicines10020310
APA StyleM’Kacher, R., Jaillet, M., Colicchio, B., Vasarmidi, E., Mailleux, A., Dieterlen, A., Kannengiesser, C., Borie, C., Oudrhiri, N., Junker, S., Voisin, P., Jeandidier, E., Carde, P., Fenech, M., Bennaceur-Griscelli, A., Crestani, B., & Borie, R. (2022). Lung Fibroblasts from Idiopathic Pulmonary Fibrosis Patients Harbor Short and Unstable Telomeres Leading to Chromosomal Instability. Biomedicines, 10(2), 310. https://doi.org/10.3390/biomedicines10020310











