Relative Effect of Extracorporeal Shockwave Therapy Alone or in Combination with Noninjective Treatments on Pain and Physical Function in Knee Osteoarthritis: A Network Meta-Analysis of Randomized Controlled Trials
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Search Strategy
2.3. Selection Criteria of Studies
2.4. Outcome Measures
2.5. Data Extraction and Synthesis
2.6. Methodological Quality and Risks of Bias of Included Trials
2.7. Statistical Analysis
3. Results
3.1. Patient Demographics and Clinical Characteristics
3.2. Study Characteristics
3.3. ESWT Intervention Characteristics
3.3.1. ESWT Protocols
3.3.2. Treatment Arms of ESWT
3.4. Risk of Bias in Included Studies
3.5. Effectiveness of Treatment for Pain Reduction Assessed in NMA
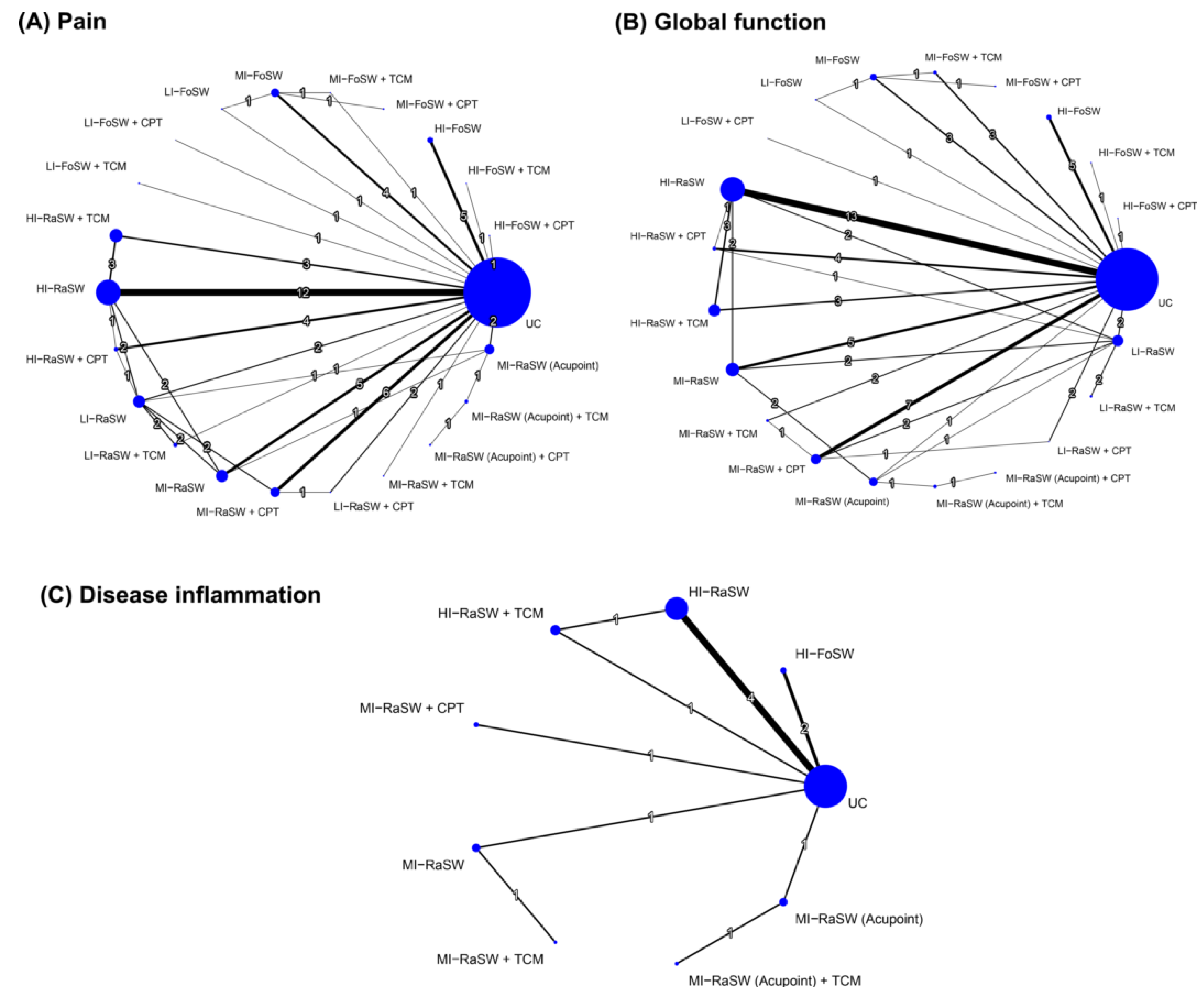
3.5.1. Pairwise Meta-Analysis
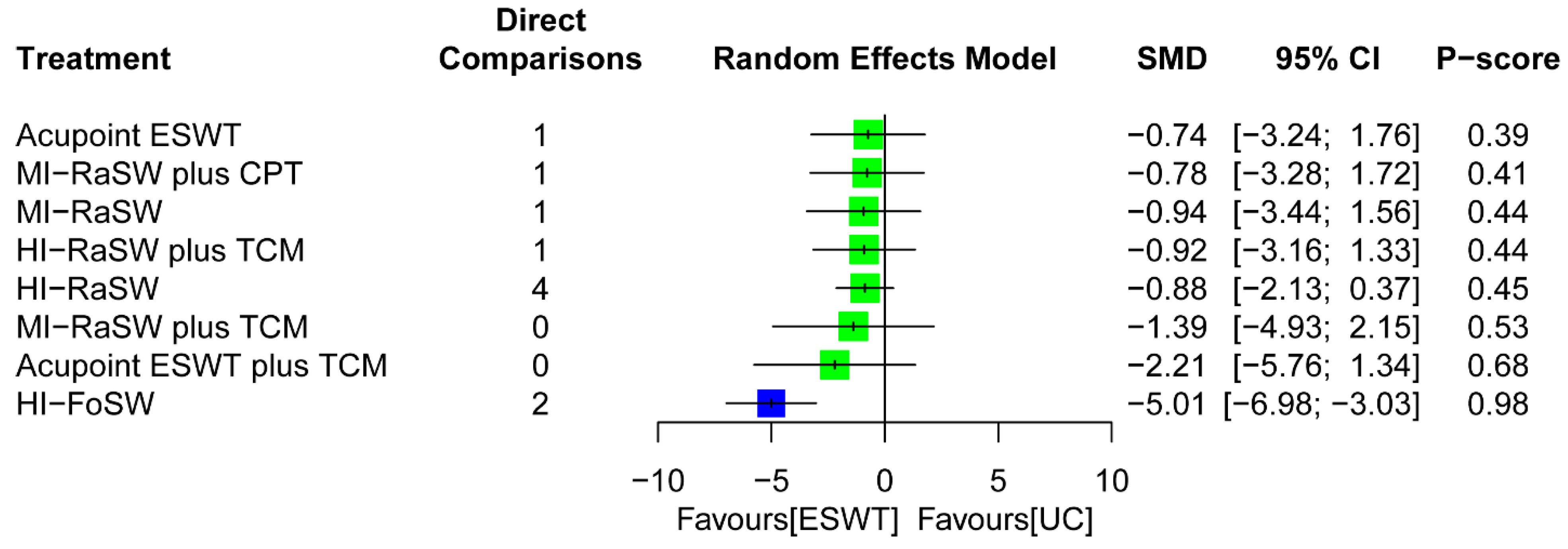
3.5.2. Global Effects in NMA
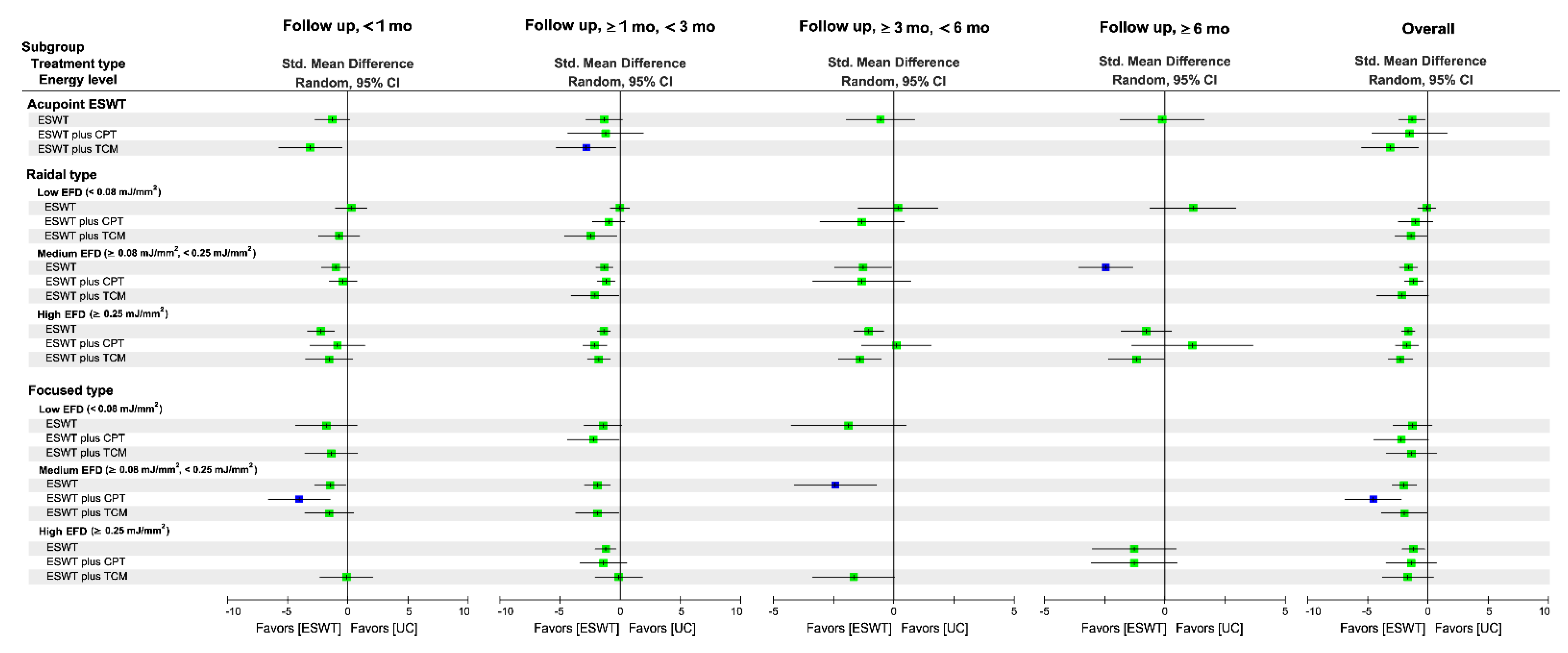
3.5.3. Subgroup Analysis of Follow-Up Duration
3.6. Effectiveness of Treatment for Global Function
3.6.1. Pairwise Meta-Analysis
3.6.2. Global Effects of NMA
3.6.3. Subgroup Analysis of Follow-Up Duration
3.7. Effectiveness of Treatment for Disease Inflammation
3.7.1. Pairwise Meta-Analysis
3.7.2. Global Effects of NMA
3.8. Network Metaregression Results for Moderators of Treatment Efficacy
3.9. Compliance and Side Effects
3.10. Publication Bias
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Di Nicola, V. Degenerative osteoarthritis a reversible chronic disease. Regen. Ther. 2020, 15, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Katz, J.N.; Arant, K.R.; Loeser, R.F. Diagnosis and Treatment of Hip and Knee Osteoarthritis: A Review. JAMA 2021, 325, 568–578. [Google Scholar] [CrossRef]
- Madry, H.; Kon, E.; Condello, V.; Peretti, G.M.; Steinwachs, M.; Seil, R.; Berruto, M.; Engebretsen, L.; Filardo, G.; Angele, P. Early osteoarthritis of the knee. Knee Surg. Sports Traumatol. Arthrosc. 2016, 24, 1753–1762. [Google Scholar] [CrossRef] [PubMed]
- Puntillo, F.; Giglio, M.; Paladini, A.; Perchiazzi, G.; Viswanath, O.; Urits, I.; Sabbà, C.; Varrassi, G.; Brienza, N. Pathophysiology of musculoskeletal pain: A narrative review. Ther. Adv. Musculoskelet. Dis. 2021, 13, 1759720X21995067. [Google Scholar] [CrossRef] [PubMed]
- Hunter, D.J.; Bierma-Zeinstra, S. Osteoarthritis. Lancet 2019, 393, 1745–1759. [Google Scholar] [CrossRef]
- Hawker, G.A. Osteoarthritis is a serious disease. Clin. Exp. Rheumatol. 2019, 37 (Suppl. 120), 3–6. [Google Scholar]
- Zitko, P.; Bilbeny, N.; Balmaceda, C.; Abbott, T.; Carcamo, C.; Espinoza, M. Prevalence, burden of disease, and lost in health state utilities attributable to chronic musculoskeletal disorders and pain in Chile. BMC Public Health 2021, 21, 937. [Google Scholar] [CrossRef]
- Kiadaliri, A.A.; Lamm, C.J.; De Verdier, M.G.; Engström, G.; Turkiewicz, A.; Lohmander, S.; Englund, M. Association of knee pain and different definitions of knee osteoarthritis with health-related quality of life: A population-based cohort study in southern Sweden. Health Qual. Life Outcomes 2016, 14, 121. [Google Scholar] [CrossRef]
- Kim, I.J.; Kim, H.A.; Seo, Y.-I.; Jung, Y.O.; Song, Y.W.; Jeong, J.Y.; Kim, D.H. Prevalence of knee pain and its influence on quality of life and physical function in the Korean elderly population: A community based cross-sectional study. J. Korean Med. Sci. 2011, 26, 1140–1146. [Google Scholar] [CrossRef]
- Usiskin, I.M.; Yang, H.Y.; Deshpande, B.R.; Collins, J.E.; Michl, G.L.; Smith, S.R.; Klara, K.M.; Selzer, F.; Katz, J.N.; Losina, E. Association between activity limitations and pain in patients scheduled for total knee arthroplasty. BMC Musculoskelet. Disord. 2016, 17, 378. [Google Scholar] [CrossRef]
- Davison, M.J.; Ioannidis, G.; Maly, M.R.; Adachi, J.D.; Beattie, K.A. Intermittent and constant pain and physical function or performance in men and women with knee osteoarthritis: Data from the osteoarthritis initiative. Clin. Rheumatol. 2016, 35, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Lamb, S.E.; Guralnik, J.M.; Buchner, D.M.; Ferrucci, L.M.; Hochberg, M.C.; Simonsick, E.M.; Fried, L.P. Factors that modify the association between knee pain and mobility limitation in older women: The Women’s Health and Aging Study. Ann. Rheum. Dis. 2000, 59, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Whitchelo, T.; McClelland, J.A.; Webster, K.E. Factors associated with stair climbing ability in patients with knee osteoarthritis and knee arthroplasty: A systematic review. Disabil. Rehabil. 2014, 36, 1051–1060. [Google Scholar] [CrossRef] [PubMed]
- Korakakis, V.; Whiteley, R.; Tzavara, A.; Malliaropoulos, N. The effectiveness of extracorporeal shockwave therapy in common lower limb conditions: A systematic review including quantification of patient-rated pain reduction. Br. J. Sports Med. 2018, 52, 387–407. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.D.; Tsauo, J.Y.; Chen, H.C.; Liou, T.H. Efficacy of Extracorporeal Shock Wave Therapy for Lower Extremity Tendinopathy: A Meta-analysis of Randomised Controlled Trials. Am. J. Phys. Med. Rehabil. 2018, 97, 605–619. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.D.; Xie, G.M.; Tsauo, J.Y.; Chen, H.C.; Liou, T.H. Efficacy of extracorporeal shock wave therapy for knee tendinopathies and other soft tissue disorders: A meta-analysis of randomized controlled trials. BMC Musculoskelet. Disord. 2018, 19, 278. [Google Scholar] [CrossRef]
- Mani-Babu, S.; Morrissey, D.; Waugh, C.; Screen, H.; Barton, C. The effectiveness of extracorporeal shock wave therapy in lower limb tendinopathy: A systematic review. Am. J. Sports Med. 2015, 43, 752–761. [Google Scholar] [CrossRef]
- Leal, C.; Ramon, S.; Furia, J.; Fernandez, A.; Romero, L.; Hernandez-Sierra, L. Current concepts of shockwave therapy in chronic patellar tendinopathy. Int. J. Surg. 2015, 24, 160–164. [Google Scholar] [CrossRef]
- Langer, P.R. Two emerging technologies for achilles tendinopathy and plantar fasciopathy. Clin. Podiatr. Med. Surg. 2015, 32, 183–193. [Google Scholar] [CrossRef]
- Smith, W.B.; Melton, W.; Davies, J. Midsubstance Tendinopathy, Percutaneous Techniques (Platelet-Rich Plasma, Extracorporeal Shock Wave Therapy, Prolotherapy, Radiofrequency Ablation). Clin. Podiatr. Med. Surg. 2017, 34, 161–174. [Google Scholar] [CrossRef]
- Vetrano, M.; d’Alessandro, F.; Torrisi, M.R.; Ferretti, A.; Vulpiani, M.C.; Visco, V. Extracorporeal shock wave therapy promotes cell proliferation and collagen synthesis of primary cultured human tenocytes. Knee Surg. Sports Traumatol. Arthrosc. 2011, 19, 2159–2168. [Google Scholar] [CrossRef] [PubMed]
- Leone, L.; Raffa, S.; Vetrano, M.; Ranieri, D.; Malisan, F.; Scrofani, C.; Vulpiani, M.C.; Ferretti, A.; Torrisi, M.R.; Visco, V. Extracorporeal Shock Wave Treatment (ESWT) enhances the in vitro-induced differentiation of human tendon-derived stem/progenitor cells (hTSPCs). Oncotarget 2016, 7, 6410–6423. [Google Scholar] [CrossRef] [PubMed]
- de Girolamo, L.; Stanco, D.; Galliera, E.; Viganò, M.; Lovati, A.; Marazzi, M.G.; Romeo, P.; Sansone, V. Soft-focused extracorporeal shock waves increase the expression of tendon-specific markers and the release of anti-inflammatory cytokines in an adherent culture model of primary human tendon cells. Ultrasound Med. Biol. 2014, 40, 1204–1215. [Google Scholar] [CrossRef]
- Wang, C.J.; Cheng, J.H.; Chou, W.Y.; Hsu, S.L.; Chen, J.H.; Huang, C.Y. Changes of articular cartilage and subchondral bone after extracorporeal shockwave therapy in osteoarthritis of the knee. Int. J. Med. Sci. 2017, 14, 213–223. [Google Scholar] [CrossRef]
- Chu, C.H.; Yen, Y.S.; Chen, P.L.; Wen, C.Y. Repair of articular cartilage in rabbit osteochondral defects promoted by extracorporeal shock wave therapy. Shock Waves 2015, 25, 205–214. [Google Scholar] [CrossRef]
- Simplicio, C.L.; Purita, J.; Murrell, W.; Santos, G.S.; Dos Santos, R.G.; Lana, J. Extracorporeal shock wave therapy mechanisms in musculoskeletal regenerative medicine. J. Clin. Orthop. Trauma 2020, 11, S309–S318. [Google Scholar] [CrossRef]
- Cheing, G.L.; Chang, H. Extracorporeal shock wave therapy. J. Orthop. Sports Phys. Ther. 2003, 33, 337–343. [Google Scholar] [CrossRef]
- Ogden, J.A.; Toth-Kischkat, A.; Schultheiss, R. Principles of shock wave therapy. Clin. Orthop. Relat. Res. 2001, 387, 8–17. [Google Scholar] [CrossRef]
- van der Worp, H.; van den Akker-Scheek, I.; van Schie, H.; Zwerver, J. ESWT for tendinopathy: Technology and clinical implications. Knee Surg. Sports Traumatol. Arthrosc. 2013, 21, 1451–1458. [Google Scholar] [CrossRef]
- Foldager, C.B.; Kearney, C.; Spector, M. Clinical application of extracorporeal shock wave therapy in orthopedics: Focused versus unfocused shock waves. Ultrasound Med. Biol. 2012, 38, 1673–1680. [Google Scholar] [CrossRef]
- Schmitz, C.; Császár, N.B.M.; Milz, S.; Schieker, M.; Maffulli, N.; Rompe, J.-D.; Furia, J.P. Efficacy and safety of extracorporeal shock wave therapy for orthopedic conditions: A systematic review on studies listed in the PEDro database. Br. Med. Bull. 2015, 116, 115–138. [Google Scholar] [CrossRef]
- Speed, C. A systematic review of shockwave therapies in soft tissue conditions: Focusing on the evidence. Br. J. Sports Med. 2014, 48, 1538–1542. [Google Scholar] [CrossRef]
- Storheim, K.; Gjersing, L.; Bolstad, K.; Risberg, M.A. Extracorporeal shock wave therapy (ESWT) and radial extracorporeal shock wave therapy (rESWT) in chronic musculoskeletal pain. Tidsskr. Nor. Laegeforen. 2010, 130, 2360–2364. [Google Scholar] [CrossRef]
- Rompe, J.D.; Furia, J.; Weil, L.; Maffulli, N. Shock wave therapy for chronic plantar fasciopathy. Br. Med. Bull. 2007, 81-82, 183–208. [Google Scholar] [CrossRef]
- Speed, C.A. Extracorporeal shock-wave therapy in the management of chronic soft-tissue conditions. J. Bone Jt. Surg. Br. 2004, 86, 165–171. [Google Scholar] [CrossRef]
- Notarnicola, A.; Maccagnano, G.; Tafuri, S.; Fiore, A.; Margiotta, C.; Pesce, V.; Moretti, B. Prognostic factors of extracorporeal shock wave therapy for tendinopathies. Musculoskelet. Surg. 2016, 100, 53–61. [Google Scholar] [CrossRef]
- Wang, Y.C.; Huang, H.T.; Huang, P.J.; Liu, Z.M.; Shih, C.L. Efficacy and Safety of Extracorporeal Shockwave Therapy for Treatment of Knee Osteoarthritis: A Systematic Review and Meta-analysis. Pain Med. 2020, 21, 822–835. [Google Scholar] [CrossRef]
- Ma, H.; Zhang, W.; Shi, J.; Zhou, D.; Wang, J. The efficacy and safety of extracorporeal shockwave therapy in knee osteoarthritis: A systematic review and meta-analysis. Int. J. Surg. 2020, 75, 24–34. [Google Scholar] [CrossRef]
- Hsieh, C.K.; Chang, C.J.; Liu, Z.W.; Tai, T.W. Extracorporeal shockwave therapy for the treatment of knee osteoarthritis: A meta-analysis. Int. Orthop. 2020, 44, 877–884. [Google Scholar] [CrossRef]
- Chen, L.; Ye, L.; Liu, H.; Yang, P.; Yang, B. Extracorporeal Shock Wave Therapy for the Treatment of Osteoarthritis: A Systematic Review and Meta-Analysis. BioMed Res. Int. 2020, 2020, 1907821. [Google Scholar] [CrossRef]
- Avendaño-Coy, J.; Comino-Suárez, N.; Grande-Muñoz, J.; Avendaño-López, C.; Gómez-Soriano, J. Extracorporeal shockwave therapy improves pain and function in subjects with knee osteoarthritis: A systematic review and meta-analysis of randomized clinical trials. Int. J. Surg. 2020, 82, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.D.; Tsauo, J.Y.; Liou, T.H.; Chen, H.C.; Huang, S.W. Clinical efficacy of extracorporeal shockwave therapy for knee osteoarthritis: A systematic review and meta-regression of randomized controlled trials. Clin. Rehabil. 2019, 33, 1419–1430. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Ma, J.; Zhao, T.; Gao, F.; Sun, W. Application and efficacy of extracorporeal shockwave treatment for knee osteoarthritis: A systematic review and meta-analysis. Exp. Ther. Med. 2019, 18, 2843–2850. [Google Scholar] [CrossRef] [PubMed]
- Atisook, R.; Euasobhon, P.; Saengsanon, A.; Jensen, M.P. Validity and Utility of Four Pain Intensity Measures for Use in International Research. J. Pain Res. 2021, 14, 1129–1139. [Google Scholar] [CrossRef]
- Collins, N.J.; Misra, D.; Felson, D.T.; Crossley, K.M.; Roos, E.M. Measures of knee function: International Knee Documentation Committee (IKDC) Subjective Knee Evaluation Form, Knee Injury and Osteoarthritis Outcome Score (KOOS), Knee Injury and Osteoarthritis Outcome Score Physical Function Short Form (KOOS-PS), Knee Outcome Survey Activities of Daily Living Scale (KOS-ADL), Lysholm Knee Scoring Scale, Oxford Knee Score (OKS), Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC), Activity Rating Scale (ARS), and Tegner Activity Score (TAS). Arthritis Care Res. 2011, 63 (Suppl. 11), S208–S228. [Google Scholar]
- Bilbao, A.; Martín-Fernández, J.; García-Pérez, L.; Arenaza, J.C.; Ariza-Cardiel, G.; Ramallo-Fariña, Y.; Ansola, L. Mapping WOMAC Onto the EQ-5D-5L Utility Index in Patients With Hip or Knee Osteoarthritis. Value Health 2020, 23, 379–387. [Google Scholar] [CrossRef]
- Roos, E.M.; Roos, H.P.; Lohmander, L.S.; Ekdahl, C.; Beynnon, B.D. Knee Injury and Osteoarthritis Outcome Score (KOOS)--development of a self-administered outcome measure. J. Orthop. Sports Phys. Ther. 1998, 28, 88–96. [Google Scholar] [CrossRef]
- Lequesne, M.G.; Mery, C.; Samson, M.; Gerard, P. Indexes of severity for osteoarthritis of the hip and knee. Validation—value in comparison with other assessment tests. Scand. J. Rheumatol. Suppl. 1987, 65, 85–89. [Google Scholar] [CrossRef]
- Wang, W.; Liu, L.; Chang, X.; Jia, Z.Y.; Zhao, J.Z.; Xu, W.D. Cross-cultural translation of the Lysholm knee score in Chinese and its validation in patients with anterior cruciate ligament injury. BMC Musculoskelet. Disord. 2016, 17, 436. [Google Scholar] [CrossRef]
- Metsavaht, L.; Leporace, G.; de Mello Sposito, M.M.; Riberto, M.; Batista, L.A. What is the best questionnaire for monitoring the physical characteristics of patients with knee osteoarthritis in the Brazilian population? Rev. Bras. Ortop. 2011, 46, 256–261. [Google Scholar] [CrossRef][Green Version]
- Wang, D.; Jones, M.H.; Khair, M.M.; Miniaci, A. Patient-reported outcome measures for the knee. J. Knee Surg. 2010, 23, 137–151. [Google Scholar] [CrossRef]
- Molnar, V.; Matišić, V.; Kodvanj, I.; Bjelica, R.; Jeleč, Ž.; Hudetz, D.; Rod, E.; Čukelj, F.; Vrdoljak, T.; Vidović, D. Cytokines and Chemokines Involved in Osteoarthritis Pathogenesis. Int. J. Mol. Sci. 2021, 22, 9208. [Google Scholar] [CrossRef]
- Hancock, C.M.; Riegger-Krugh, C. Modulation of pain in osteoarthritis: The role of nitric oxide. Clin. J. Pain 2008, 24, 353–365. [Google Scholar] [CrossRef]
- Vuolteenaho, K.; Moilanen, T.; Knowles, R.G.; Moilanen, E. The role of nitric oxide in osteoarthritis. Scand. J. Rheumatol. 2007, 36, 247–258. [Google Scholar] [CrossRef]
- Calvet, J.; Orellana, C.; Gratacós, J.; Berenguer-Llergo, A.; Caixàs, A.; Chillarón, J.J.; Pedro-Botet, J.; García-Manrique, M.; Navarro, N.; Larrosa, M. Synovial fluid adipokines are associated with clinical severity in knee osteoarthritis: A cross-sectional study in female patients with joint effusion. Arthritis Res. Ther. 2016, 18, 207. [Google Scholar] [CrossRef]
- Carrión, M.; Frommer, K.W.; Pérez-García, S.; Müller-Ladner, U.; Gomariz, R.P.; Neumann, E. The Adipokine Network in Rheumatic Joint Diseases. Int. J. Mol. Sci. 2019, 20, 4190. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Li, T.; Deeks, J.J. Cochrane Handbook for Systematic Reviews of Interventions. In Cochrane Handbook for Systematic Reviews of Interventions; Higgins, J.P., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., Eds.; The Cochrane Collaboration: London, UK, 2021. [Google Scholar]
- Chang, K.V.; Chen, S.Y.; Chen, W.S.; Tu, Y.K.; Chien, K.L. Comparative effectiveness of focused shock wave therapy of different intensity levels and radial shock wave therapy for treating plantar fasciitis: A systematic review and network meta-analysis. Arch. Phys. Med. Rehabil. 2012, 93, 1259–1268. [Google Scholar] [CrossRef]
- Lei, H.; Liu, J.; Li, H.; Wang, L.; Xu, Y.; Tian, W.; Lin, G.; Xin, Z. Low-intensity shock wave therapy and its application to erectile dysfunction. World J. Mens. Health 2013, 31, 208–214. [Google Scholar] [CrossRef]
- Bannuru, R.R.; Flavin, N.E.; Vaysbrot, E.; Harvey, W.; McAlindon, T. High-energy extracorporeal shock-wave therapy for treating chronic calcific tendinitis of the shoulder: A systematic review. Ann. Intern. Med. 2014, 160, 542–549. [Google Scholar] [CrossRef]
- Liu, B.Y.; Li, H.Y.; Xi, L.C.; Huang, S.C.; Wei, M.Z.; Wang, H.R. Comparison of early clinical outcomes of extracorporeal shock wave therapy in different energy flux densities for moderate knee osteoarthritis. Orthop. J. China 2020, 28, 908–912. [Google Scholar]
- Wu, C.-H.; Chen, K.-T.; Hou, M.-T.; Chang, Y.-F.; Chang, C.-S.; Liu, P.-Y.; Wu, S.-J.; Chiu, C.-J.; Jou, I.-M.; Chen, C.-Y. Prevalence and associated factors of sarcopenia and severe sarcopenia in older Taiwanese living in rural community: The Tianliao Old People study 04. Geriatr. Gerontol. Int. 2014, 14 (Suppl. 1), 69–75. [Google Scholar] [CrossRef]
- Tooth, L.; Bennett, S.; McCluskey, A.; Hoffmann, T.; McKenna, K.; Lovarini, M. Appraising the quality of randomized controlled trials: Inter-rater reliability for the OTseeker evidence database. J. Eval. Clin. Pract. 2005, 11, 547–555. [Google Scholar] [CrossRef]
- Foley, N.C.; Bhogal, S.K.; Teasell, R.W.; Bureau, Y.; Speechley, M.R. Estimates of quality and reliability with the physiotherapy evidence-based database scale to assess the methodology of randomized controlled trials of pharmacological and nonpharmacological interventions. Phys. Ther. 2006, 86, 817–824. [Google Scholar] [CrossRef]
- Briani, R.V.; Ferreira, A.S.; Pazzinatto, M.F.; Pappas, E.; De Oliveira Silva, D.; Azevedo, F.M. What interventions can improve quality of life or psychosocial factors of individuals with knee osteoarthritis? A systematic review with meta-analysis of primary outcomes from randomised controlled trials. Br. J. Sports Med. 2018, 52, 1031–1038. [Google Scholar] [CrossRef]
- Beaudreuil, J.; Coudreuse, J.M.; Guyen, C.N.; Deat, P.; Chabaud, A.; Pereira, B.; Lorenzo, A.; Sailhan, F.; Rannou, F.; Coudeyre, E. An algorithm to improve knee orthosis prescription for osteoarthritis patients. Ann. Phys. Rehabil. Med. 2016, 59, e156. [Google Scholar] [CrossRef]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Rosenthal, R. (Ed.) Meta-Analytic Procedures for Social Research; Sage Publications: Newbury Park, CA, USA, 1993. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Harrer, M.; Cuijpers, P.; Furukawa, T.A.; Ebert, D.D. Doing Meta-Analysis in R: A Hands-on Guide; PROTECT Lab Erlangen: Erlangen, Germany, 2019. [Google Scholar]
- Shim, S.R.; Kim, S.-J.; Lee, J.; Rücker, G. Network meta-analysis: Application and practice using R software. Epidemiol. Health 2019, 41, e2019013. [Google Scholar] [CrossRef]
- Higgins, J.P.; Jackson, D.; Barrett, J.K.; Lu, G.; Ades, A.E.; White, I.R. Consistency and inconsistency in network meta-analysis: Concepts and models for multi-arm studies. Res. Synth. Methods. 2012, 3, 98–110. [Google Scholar] [CrossRef]
- Jackson, D.; Barrett, J.K.; Rice, S.; White, I.R.; Higgins, J.P. A design-by-treatment interaction model for network meta-analysis with random inconsistency effects. Stat. Med. 2014, 33, 3639–3654. [Google Scholar] [CrossRef]
- Rücker, G.; Schwarzer, G. Ranking treatments in frequentist network meta-analysis works without resampling methods. BMC Med. Res. Methodol. 2015, 15, 58. [Google Scholar] [CrossRef]
- Mbuagbaw, L.; Rochwerg, B.; Jaeschke, R.; Heels-Andsell, D.; Alhazzani, W.; Thabane, L.; Guyatt, G.H. Approaches to interpreting and choosing the best treatments in network meta-analyses. Syst. Rev. 2017, 6, 79. [Google Scholar] [CrossRef]
- Sedgwick, P.; Marston, L. How to read a funnel plot in a meta-analysis. BMJ. 2015, 351, h4718. [Google Scholar] [CrossRef]
- Caldwell, D.M.; Welton, N.J.; Ades, A.E. Mixed treatment comparison analysis provides internally coherent treatment effect estimates based on overviews of reviews and can reveal inconsistency. J. Clin. Epidemiol. 2010, 63, 875–882. [Google Scholar] [CrossRef]
- Reken, S.; Sturtz, S.; Kiefer, C.; Böhler, Y.B.; Wieseler, B. Assumptions of Mixed Treatment Comparisons in Health Technology Assessments—Challenges and Possible Steps for Practical Application. PLoS ONE 2016, 11, e0160712. [Google Scholar] [CrossRef]
- Król, P.; Franek, A.; Durmała, J.; Błaszczak, E.; Ficek, K.; Król, B.; Detko, E.; Wnuk, B.; Białek, L.; Taradaj, J. Focused and Radial Shock Wave Therapy in the Treatment of Tennis Elbow: A Pilot Randomised Controlled Study. J. Hum. Kinet. 2015, 47, 127–135. [Google Scholar] [CrossRef]
- Lohrer, H.; Nauck, T.; Dorn-Lange, N.V.; Scholl, J.; Vester, J.C. Comparison of radial versus focused extracorporeal shock waves in plantar fasciitis using functional measures. Foot Ankle Int. 2010, 31, 1–9. [Google Scholar] [CrossRef]
- Sun, J.; Gao, F.; Wang, Y.; Sun, W.; Jiang, B.; Li, Z. Extracorporeal shock wave therapy is effective in treating chronic plantar fasciitis: A meta-analysis of RCTs. Medicine 2017, 96, e6621. [Google Scholar] [CrossRef]
- van der Worp, H.; Zwerver, J.; Hamstra, M.; van den Akker-Scheek, I.; Diercks, R.L. No difference in effectiveness between focused and radial shockwave therapy for treating patellar tendinopathy: A randomized controlled trial. Knee Surg. Sports Traumatol. Arthrosc. 2014, 22, 2026–2032. [Google Scholar] [CrossRef]
- Zhao, Z.; Ji, H.; Jing, R.; Liu, C.; Wang, M.; Zhai, L.; Bai, X.; Xing, G. Extracorporeal shock-wave therapy reduces progression of knee osteoarthritis in rabbits by reducing nitric oxide level and chondrocyte apoptosis. Arch. Orthop. Trauma. Surg. 2012, 132, 1547–1553. [Google Scholar] [CrossRef]
- Cheng, J.H.; Jhan, S.W.; Hsu, C.C.; Chiu, H.W.; Hsu, S.L. Extracorporeal Shockwave Therapy Modulates the Expressions of Proinflammatory Cytokines IL33 and IL17A, and Their Receptors ST2 and IL17RA, within the Articular Cartilage in Early Avascular Necrosis of the Femoral Head in a Rat Model. Mediat. Inflamm. 2021, 2021, 9915877. [Google Scholar] [CrossRef]
- Moretti, B.; Iannone, F.; Notarnicola, A.; Lapadula, G.; Moretti, L.; Patella, V.; Garofalo, R. Extracorporeal shock waves down-regulate the expression of interleukin-10 and tumor necrosis factor-alpha in osteoarthritic chondrocytes. BMC Musculoskelet. Disord. 2008, 9, 16. [Google Scholar] [CrossRef]
- Liu, B.Y.; Li, H.Y.; Jin, X.Y.; Orthopedics, D. Effect of extracorporeal shock wave therapy on expression of IL-1β,TNF-α and MMP-13 in joint fluid for early and middle stage knee osteoarthritis. Orthop. J. Chin. 2016, 24, 1807–1810. [Google Scholar]


| Trial design | Randomized controlled trial; quasirandomized controlled trial |
| Participant | Symptomatic or radiographic diagnosis of knee osteoarthritis |
| Treatment group | Received ESWT alone, ESWT plus CPT, or ESWT plus TCM |
| Control group | Received a placebo ESWT, relatively low-dosage ESWT, or non-ESWT intervention (i.e., CPT or TCM) |
| Outcome | Pain, global function, disease inflammation |
| Experimental Group | Control Group | Total | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Trials (n) a | Study Arm (n) | Patients (n) | Mean (Range) b | Trials (n) a | Study Arm (n) | Patients (n) | Mean (Range) b | Trials (n) a | Study Arm (n) | Patients (n) | Mean (Range) b | |
| Age, years | 68 | 93 | 3701 | 60.1 (40.1–80.3) | 53 | 60 | 2209 | 59.3 (43.6–72.7) | 68 | 153 | 5910 | 59.8 (40.1–80.3) |
| BMI, kg/m2 | 29 | 38 | 1275 | 25.8 (22.3–35.3) | 23 | 25 | 784 | 25.7 (22.3–36.4) | 29 | 63 | 2059 | 25.7 (22.3–36.4) |
| Sex, n | ||||||||||||
| Male | 50 | 72 | 1242 | 37 | 38 | 618 | 57 | 128 | 2048 | |||
| Female | 58 | 82 | 2113 | 43 | 45 | 1155 | 65 | 145 | 3706 | |||
| Disease duration, mo | 48 | 69 | 2982 | 51.4 (6–200) | 33 | 34 | 1443 | 47.6 (6–138) | 53 | 116 | 4850 | 52.4 (6–200) |
| K-L grade | ||||||||||||
| ≤II | 26 | 37 | 1062 | 20 | 20 | 571 | 46 | 57 | 1633 | |||
| II–III | 31 | 44 | 1597 | 24 | 29 | 974 | 55 | 73 | 2571 | |||
| I–III | 47 | 66 | 2697 | 36 | 42 | 1592 | 83 | 108 | 4289 | |||
| ≥III | 3 | 3 | 98 | 3 | 4 | 113 | 6 | 7 | 211 | |||
| Involved knee, n | ||||||||||||
| Unilateral | 28 | 36 | 1225 | 24 | 25 | 810 | 33 | 74 | 2280 | |||
| Bilateral | 13 | 19 | 329 | 10 | 13 | 254 | 18 | 45 | 769 | |||
| Population (area) | ||||||||||||
| Europe | 4 | 4 | 132 | 4 | 4 | 133 | 4 | 8 | 265 | |||
| Africa | 6 | 7 | 110 | 5 | 8 | 130 | 6 | 15 | 240 | |||
| Asia | 59 | 83 | 3494 | 45 | 49 | 1981 | 59 | 132 | 5475 | |||
| Intervention design (compliance, %) | ||||||||||||
| ESWT alone | 46 | 57 | 2234 | 98.8 (88.1–100) | 46 | 57 | 2234 | 98.8 (88.1–100) | ||||
| ESWT + CPT | 19 | 22 | 710 | 95.0 (44.4–100) | 19 | 22 | 710 | 95.0 (44.4–100) | ||||
| ESWT + TCM | 15 | 15 | 792 | 100 (100–100) | 15 | 15 | 792 | 100 (100–100) | ||||
| Comparator type (compliance, %) | ||||||||||||
| None | 4 | 4 | 187 | 98.0 (90.0–100) | 4 | 4 | 187 | 98.0 (90.0–100) | ||||
| Placebo | 13 | 13 | 466 | 95.6 (83.3–100) | 13 | 13 | 466 | 95.6 (83.3–100) | ||||
| PM | 11 | 11 | 546 | 97.8 (80.8–100) | 11 | 11 | 546 | 97.8 (80.8–100) | ||||
| CPT | 21 | 24 | 652 | 97.4 (86.7–100) | 21 | 24 | 652 | 97.4 (86.7–100) | ||||
| TCM | 9 | 9 | 393 | 93.0 (98.6–100) | 9 | 9 | 393 | 93.0 (98.6–100) | ||||
| Pain (10-point VAS) | 49 | 62 | 2542 | 8.8 (4.5–8.1) | 42 | 45 | 1688 | 6.6 (4.0–8.7) | 49 | 107 | 4230 | 7.9 (4.0–8.7) |
| Global function | ||||||||||||
| WOMAC (0–100) | 42 | 54 | 2217 | 50.4 (2.7–98.2) | 34 | 35 | 1328 | 50.4 (2.7–98.2) | 42 | 89 | 3545 | 49.1 (2.7–98.2) |
| Lequesne index (0–24) | 18 | 22 | 829 | 11.9 (7.8–17.4) | 16 | 16 | 631 | 11.4 (7.9–17.2) | 18 | 38 | 1460 | 11.7 (7.8–17.4) |
| Lysholm index (0–100) | 9 | 13 | 758 | 47.7 (38.1–68.1) | 7 | 7 | 382 | 51.6 (39.7–67.5) | 9 | 20 | 1140 | 49.1 (38.1–68.1) |
| Disease inflammation | ||||||||||||
| IL-1 (pg/mL) | 9 | 11 | 571 | 75.6 (17.3–220.9) | 7 | 7 | 402 | 49.6 (16.9–113.4) | 9 | 18 | 973 | 65.5 (16.9–220.9) |
| TNF-α (pg/mL) | 10 | 12 | 625 | 37.7 (9.1–48.3) | 8 | 8 | 456 | 32.8 (9.1–45.3) | 10 | 20 | 1081 | 35.7 (9.1–48.3) |
| Nitric oxide (μmol/mL) | 5 | 7 | 489 | 80.3 (65.7–96.4) | 4 | 4 | 280 | 73.1 (64.2–76.4) | 5 | 11 | 769 | 77.7 (64.2–96.4) |
| Treatment Arm | Abbreviation |
|---|---|
| Acupoint therapy using ESWT | Acupoint ESWT |
| Acupoint ESWT plus CPT | Acupoint ESWT + CPT |
| Acupoint ESWT plus TCM | Acupoint ESWT + TCM |
| Radial shockwave | RaSW |
| High-energy radial shockwave | HI-RaSW |
| Medium-energy radial shockwave | MI-RaSW |
| Low-energy radial shockwave | LI-RaSW |
| High-energy radial shockwave plus CPT | HI-RaSW + CPT |
| Medium-energy radial shockwave plus CPT | MI-RaSW + CPT |
| Low-energy radial shockwave plus CPT | LI-RaSW + CPT |
| High-energy radial shockwave plus TCM | HI-RaSW + TCM |
| Medium-energy radial shockwave plus TCM | MI-RaSW + TCM |
| Low-energy radial shockwave plus TCM | LI-RaSW + TCM |
| Focused shockwave | FoSW |
| High-energy focused shockwave | HI-FoSW |
| Medium-energy focused shockwave | MI-FoSW |
| Low-energy focused shockwave | LI-FoSW |
| High-energy focused shockwave plus CPT | HI-FoSW + CPT |
| Medium-energy focused shockwave plus CPT | MI-FoSW + CPT |
| Low-energy focused shockwave plus CPT | LI-FoSW + CPT |
| High-energy focused shockwave plus TCM | HI-FoSW + TCM |
| Medium-energy focused shockwave plus TCM | MI-FoSW + TCM |
| Low-energy focused shockwave plus TCM | LI-FoSW + TCM |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, C.-D.; Huang, Y.-Y.; Chen, H.-C.; Liou, T.-H.; Lin, C.-L.; Huang, S.-W. Relative Effect of Extracorporeal Shockwave Therapy Alone or in Combination with Noninjective Treatments on Pain and Physical Function in Knee Osteoarthritis: A Network Meta-Analysis of Randomized Controlled Trials. Biomedicines 2022, 10, 306. https://doi.org/10.3390/biomedicines10020306
Liao C-D, Huang Y-Y, Chen H-C, Liou T-H, Lin C-L, Huang S-W. Relative Effect of Extracorporeal Shockwave Therapy Alone or in Combination with Noninjective Treatments on Pain and Physical Function in Knee Osteoarthritis: A Network Meta-Analysis of Randomized Controlled Trials. Biomedicines. 2022; 10(2):306. https://doi.org/10.3390/biomedicines10020306
Chicago/Turabian StyleLiao, Chun-De, Yu-Yun Huang, Hung-Chou Chen, Tsan-Hon Liou, Che-Li Lin, and Shih-Wei Huang. 2022. "Relative Effect of Extracorporeal Shockwave Therapy Alone or in Combination with Noninjective Treatments on Pain and Physical Function in Knee Osteoarthritis: A Network Meta-Analysis of Randomized Controlled Trials" Biomedicines 10, no. 2: 306. https://doi.org/10.3390/biomedicines10020306
APA StyleLiao, C.-D., Huang, Y.-Y., Chen, H.-C., Liou, T.-H., Lin, C.-L., & Huang, S.-W. (2022). Relative Effect of Extracorporeal Shockwave Therapy Alone or in Combination with Noninjective Treatments on Pain and Physical Function in Knee Osteoarthritis: A Network Meta-Analysis of Randomized Controlled Trials. Biomedicines, 10(2), 306. https://doi.org/10.3390/biomedicines10020306







