Structure-Based Analysis of Protein Cluster Size for Super-Resolution Microscopy in the Nervous System
Abstract
1. Introduction
2. Materials and Methods
2.1. Fluorescent Microsphere Sample Preparation
2.2. HEK 293T Cell Culture
2.3. Sample Preparation of TDP-43 Protein Inclusions
2.4. Establishment and Maintenance of Cortical Primary Neuron Culture and PSD-95 Sample Preparation
2.5. Immunofluorescence
2.6. Imaging Buffer Preparation
2.7. STORM Imaging
2.8. Numerical Fitting of SBA Analysis in STORM Images
3. Results
3.1. Structure-Based Analysis
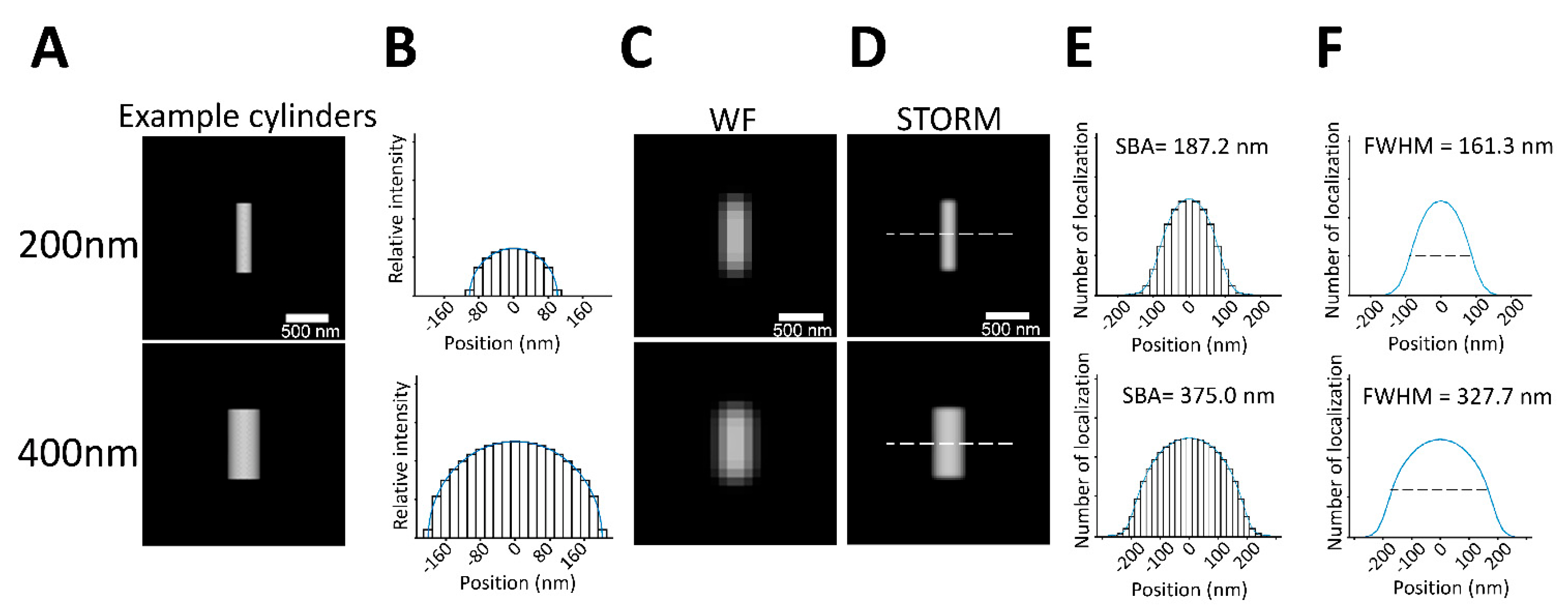
3.2. SBA of a Spherical Structural Function
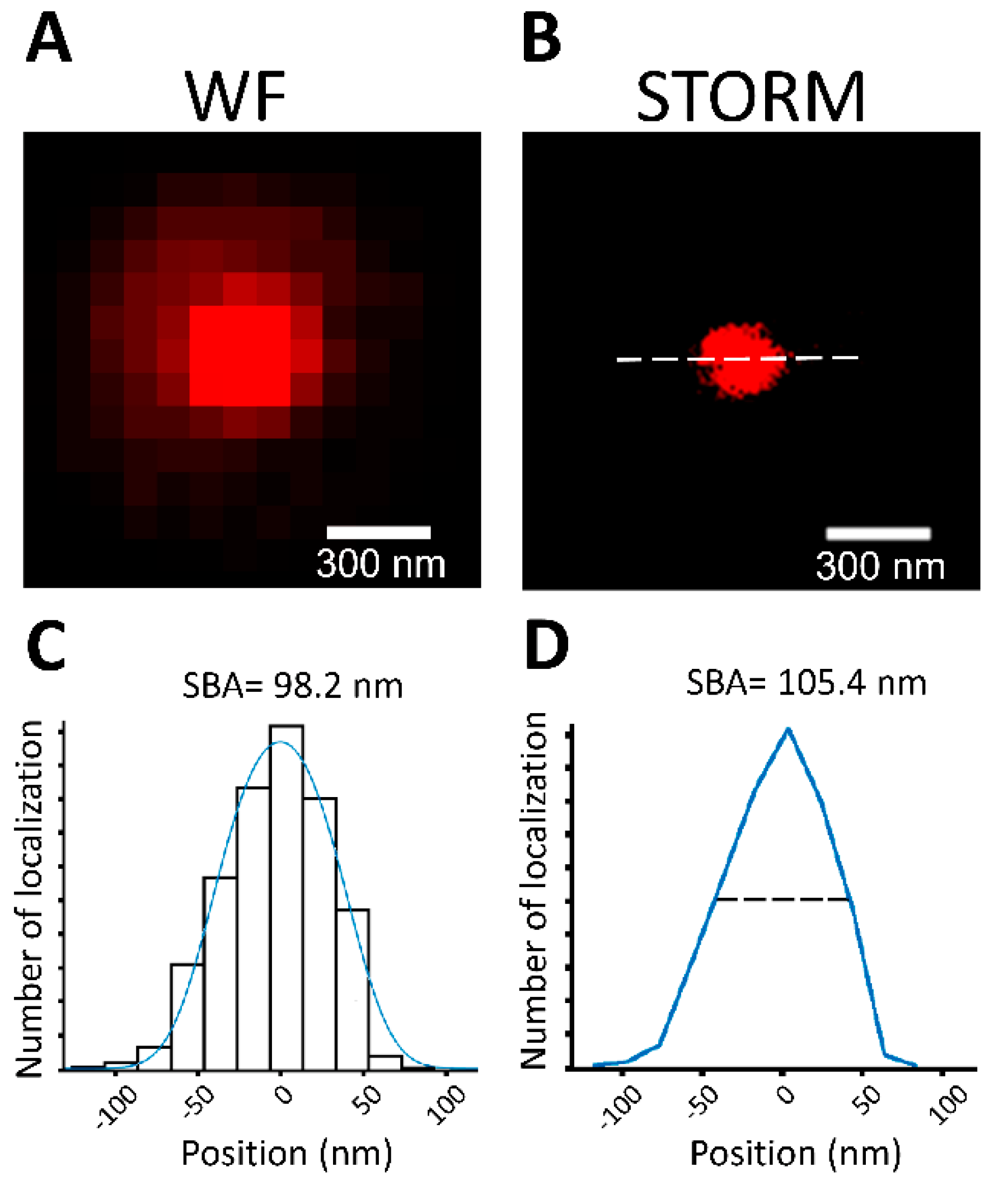
3.3. SBA Diameter Estimation Using 100 nm Fluorescent Microspheres
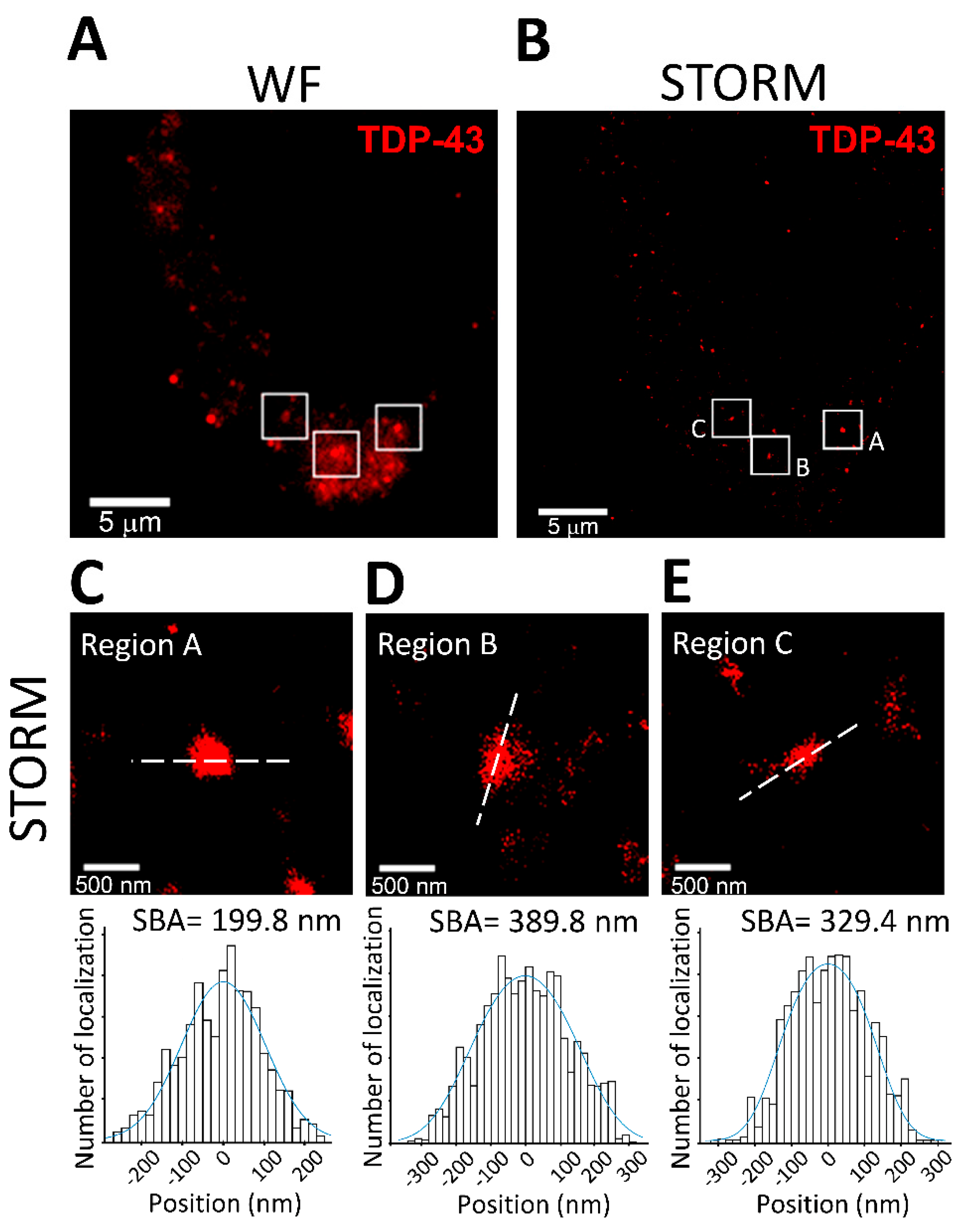
3.4. SBA in Mammalian Cells: Analysis of TDP-43 Cluster Size
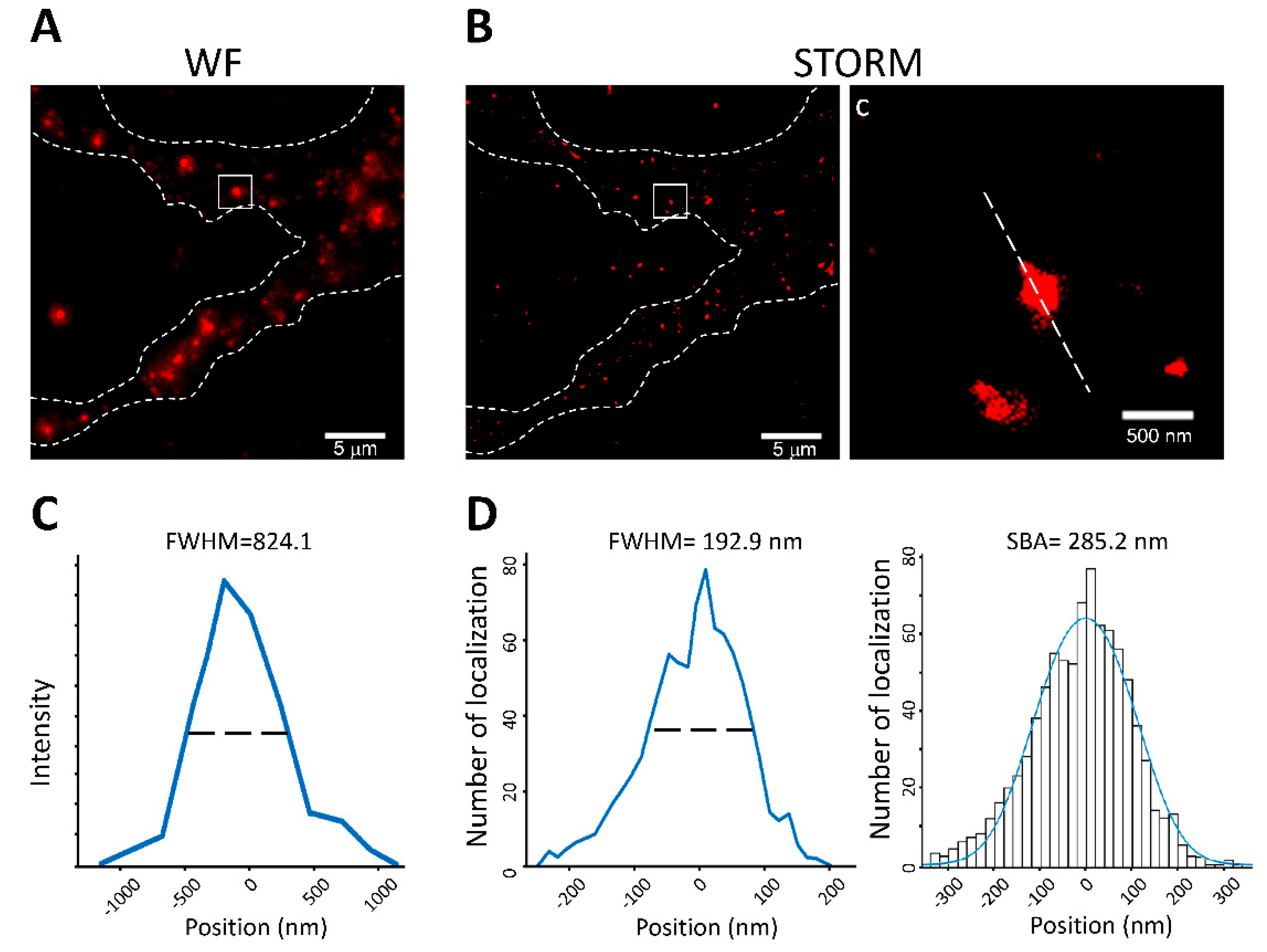
3.5. Application of SBA in the Neuronal System: Analysis of PSD Size in Neuronal Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Born, M.; Wolf, E. Principles of Optics: Electromagnetic Theory of Propagation, Interference and Diffraction of Light, 7th ed.; Cambridge University Press: Cambridge, UK, 1999. [Google Scholar]
- Hecht, E.; Zajac, A. Optics; Addison-Wesley Pub. Co., Reading, Mass.: Boston, MA, USA, 1987. [Google Scholar]
- Hell, S.W. Microscopy and its focal switch. Nat. Methods 2009, 6, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Jones, S.A.; Brandenburg, B.; Zhuang, X. Whole-cell 3D STORM reveals interactions between cellular structures with nanometer-scale resolution. Nat. Methods 2008, 5, 1047–1052. [Google Scholar] [CrossRef] [PubMed]
- Hess, S.T.; Girirajan, T.P.; Mason, M.D. Ultra-high resolution imaging by fluorescence photoactivation localization microscopy. Biophys, J. 2006, 91, 4258–4272. [Google Scholar] [CrossRef] [PubMed]
- Rust, M.J.; Bates, M.; Zhuang, X. Sub-diffraction-limit imaging by stochastic optical reconstruction microscopy (STORM). Nat. Methods 2006, 3, 793–795. [Google Scholar] [CrossRef]
- Rowlands, C.J.; Ströhl, F.; Ramirez, P.P.V.; Scherer, K.M.; Kaminski, C.F. Flat-Field Super-Resolution Localization Microscopy with a Low-Cost Refractive Beam-Shaping Element. Sci. Rep. 2018, 8, 5630. [Google Scholar] [CrossRef]
- Mortensen, K.I.; Churchman, L.S.; Spudich, J.A.; Flyvbjerg, H. Optimized localization analysis for single-molecule tracking and super-resolution microscopy. Nat. Methods 2010, 7, 377–381. [Google Scholar] [CrossRef]
- Wong, C.E.; Jin, L.W.; Chu, Y.P.; Wei, W.Y.; Ho, P.C.; Tsai, K.J. TDP-43 proteinopathy impairs mRNP granule mediated postsynaptic translation and mRNA metabolism. Theranostics 2021, 11, 330–345. [Google Scholar] [CrossRef]
- Small, A.; Stahlheber, S. Fluorophore localization algorithms for super-resolution microscopy. Nat. Methods 2014, 11, 267–279. [Google Scholar] [CrossRef]
- Agarwal, K.; Macháň, R. Multiple signal classification algorithm for super-resolution fluorescence microscopy. Nat. Commun. 2016, 7, 13752. [Google Scholar] [CrossRef]
- Holden, S.J.; Uphoff, S.; Kapanidis, A.N. DAOSTORM: An algorithm for high-density super-resolution microscopy. Nat. Methods 2011, 8, 279–280. [Google Scholar] [CrossRef]
- Min, J.; Vonesch, C.; Kirshner, H.; Carlini, L.; Olivier, N.; Holden, S.; Manley, S.; Ye, J.C.; Unser, M. FALCON: Fast and unbiased reconstruction of high-density super-resolution microscopy data. Sci. Rep. 2014, 4, 4577. [Google Scholar] [CrossRef] [PubMed]
- Mukamel, E.A.; Babcock, H.; Zhuang, X. Statistical deconvolution for superresolution fluorescence microscopy. Biophys, J. 2012, 102, 2391–2400. [Google Scholar] [CrossRef] [PubMed]
- Fullerton, S.; Bennett, K.; Toda, E.; Takahashi, T. Camera Simulation Engine Enables Efficient System Optimization for Super-Resolution Imaging. Proc. SPIE-Int. Soc. Opt. Eng. 2012, 8228, 21. [Google Scholar] [CrossRef]
- Lee, S.F.; Thompson, M.A.; Schwartz, M.A.; Shapiro, L.; Moerner, W.E. Super-resolution imaging of the nucleoid-associated protein HU in Caulobacter crescentus. Biophys, J. 2011, 100, L31–L33. [Google Scholar] [CrossRef]
- Samuylov, D.K.; Purwar, P.; Szekely, G.; Paul, G. Modeling Point Spread Function in Fluorescence Microscopy With a Sparse Gaussian Mixture: Tradeoff Between Accuracy and Efficiency. IEEE Trans. Image Process. 2019, 28, 3688–3702. [Google Scholar] [CrossRef]
- Carlin, R.K.; Grab, D.J.; Cohen, R.S.; Siekevitz, P. Isolation and characterization of postsynaptic densities from various brain regions: Enrichment of different types of postsynaptic densities. J. Cell Biol. 1980, 86, 831–845. [Google Scholar] [CrossRef]
- Ziff, E.B. Enlightening the postsynaptic density. Neuron 1997, 19, 1163–1174. [Google Scholar] [CrossRef]
- Fang, Y.S.; Tsai, K.J.; Chang, Y.J.; Kao, P.; Woods, R.; Kuo, P.H.; Wu, C.C.; Liao, J.Y.; Chou, S.C.; Lin, V.; et al. Full-length TDP-43 forms toxic amyloid oligomers that are present in frontotemporal lobar dementia—TDP patients. Nat. Commun. 2014, 5, 4824. [Google Scholar] [CrossRef]
- Thorpe, J.R.; Tang, H.; Atherton, J.; Cairns, N.J. Fine structural analysis of the neuronal inclusions of frontotemporal lobar degeneration with TDP-43 proteinopathy. J. Neural. Transm. 2008, 115, 1661–1671. [Google Scholar] [CrossRef][Green Version]
- STORM. Protocol-Sample Preparation; Technical Report; Nikon Corp.: Hong Kong, China, 2013. [Google Scholar]
- Rego, E.H.; Shao, L.; Macklin, J.J.; Winoto, L.; Johansson, G.A.; Kamps-Hughes, N.; Davidson, M.W.; Gustafsson, M.G. Nonlinear structured-illumination microscopy with a photoswitchable protein reveals cellular structures at 50-nm resolution. Proc. Natl. Acad. Sci. USA 2012, 109, E135–E143. [Google Scholar] [CrossRef]
- Nair, D.; Hosy, E.; Petersen, J.D.; Constals, A.; Giannone, G.; Choquet, D.; Sibarita, J.B. Super-resolution imaging reveals that AMPA receptors inside synapses are dynamically organized in nanodomains regulated by PSD95. J. Neurosci. 2013, 33, 13204–13224. [Google Scholar] [CrossRef] [PubMed]
- Paul, H. Introduction to Quantum Optics: From Light Quanta to Quantum Teleportation; Cambridge University Press: Cambridge, UK, 2004. [Google Scholar]
- Barlow, R.J. Statistics: A Guide to the Use of Statistical Methods in the Physical Sciences; Wiley: Chichester, UK, 1989. [Google Scholar]
- Mackenzie, I.R.; Rademakers, R.; Neumann, M. TDP-43 and FUS in amyotrophic lateral sclerosis and frontotemporal dementia. Lancet Neurol. 2010, 9, 995–1007. [Google Scholar] [CrossRef]
- Gao, J.; Wang, L.; Huntley, M.L.; Perry, G.; Wang, X. Pathomechanisms of TDP-43 in neurodegeneration. J. Neurochem. 2018, 146, 7–20. [Google Scholar] [CrossRef] [PubMed]
- Scotter, E.L.; Chen, H.J.; Shaw, C.E. TDP-43 Proteinopathy and ALS: Insights into Disease Mechanisms and Therapeutic Targets. Neurotherapeutics 2015, 12, 352–363. [Google Scholar] [CrossRef]
- Johnson, B.S.; Snead, D.; Lee, J.J.; McCaffery, J.M.; Shorter, J.; Gitler, A.D. TDP-43 is intrinsically aggregation-prone, and amyotrophic lateral sclerosis-linked mutations accelerate aggregation and increase toxicity. J. Biol. Chem. 2009, 284, 20329–20339. [Google Scholar] [CrossRef]
- Kao, P.F.; Chen, Y.R.; Liu, X.B.; DeCarli, C.; Seeley, W.W.; Jin, L.W. Detection of TDP-43 oligomers in frontotemporal lobar degeneration-TDP. Ann. Neurol. 2015, 78, 211–221. [Google Scholar] [CrossRef]
- Kennedy, M.B. Signal-processing machines at the postsynaptic density. Science 2000, 290, 750–754. [Google Scholar] [CrossRef]
- Chen, X.; Nelson, C.D.; Li, X.; Winters, C.A.; Azzam, R.; Sousa, A.A.; Leapman, R.D.; Gainer, H.; Sheng, M.; Reese, T.S. PSD-95 is required to sustain the molecular organization of the postsynaptic density. J. Neurosci. 2011, 31, 6329–6338. [Google Scholar] [CrossRef]
- Petersen, J.D.; Chen, X.; Vinade, L.; Dosemeci, A.; Lisman, J.E.; Reese, T.S. Distribution of postsynaptic density (PSD)-95 and Ca2+/calmodulin-dependent protein kinase II at the PSD. J. Neurosci. 2003, 23, 11270–11278. [Google Scholar] [CrossRef]
- Ovesný, M.; Křížek, P.; Borkovec, J.; Svindrych, Z.; Hagen, G.M. ThunderSTORM: A comprehensive ImageJ plug-in for PALM and STORM data analysis and super-resolution imaging. Bioinformatics 2014, 30, 2389–2390. [Google Scholar] [CrossRef]
- Bormashenko, E.; Frenkel, M.; Vilk, A.; Legchenkova, I.; Fedorets, A.A.; Aktaev, N.E.; Dombrovsky, L.A.; Nosonovsky, M. Characterization of Self-Assembled 2D Patterns with Voronoi Entropy. Entropy 2018, 20, 956. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wong, C.-E.; Chu, Y.-P.; Tsai, K.-J. Structure-Based Analysis of Protein Cluster Size for Super-Resolution Microscopy in the Nervous System. Biomedicines 2022, 10, 295. https://doi.org/10.3390/biomedicines10020295
Wong C-E, Chu Y-P, Tsai K-J. Structure-Based Analysis of Protein Cluster Size for Super-Resolution Microscopy in the Nervous System. Biomedicines. 2022; 10(2):295. https://doi.org/10.3390/biomedicines10020295
Chicago/Turabian StyleWong, Chia-En, Yuan-Ping Chu, and Kuen-Jer Tsai. 2022. "Structure-Based Analysis of Protein Cluster Size for Super-Resolution Microscopy in the Nervous System" Biomedicines 10, no. 2: 295. https://doi.org/10.3390/biomedicines10020295
APA StyleWong, C.-E., Chu, Y.-P., & Tsai, K.-J. (2022). Structure-Based Analysis of Protein Cluster Size for Super-Resolution Microscopy in the Nervous System. Biomedicines, 10(2), 295. https://doi.org/10.3390/biomedicines10020295





