Reducing the Pill Burden: Immunosuppressant Adherence and Safety after Conversion from a Twice-Daily (IR-Tac) to a Novel Once-Daily (LCP-Tac) Tacrolimus Formulation in 161 Liver Transplant Patients
Abstract
:1. Introduction
2. Materials and Methods
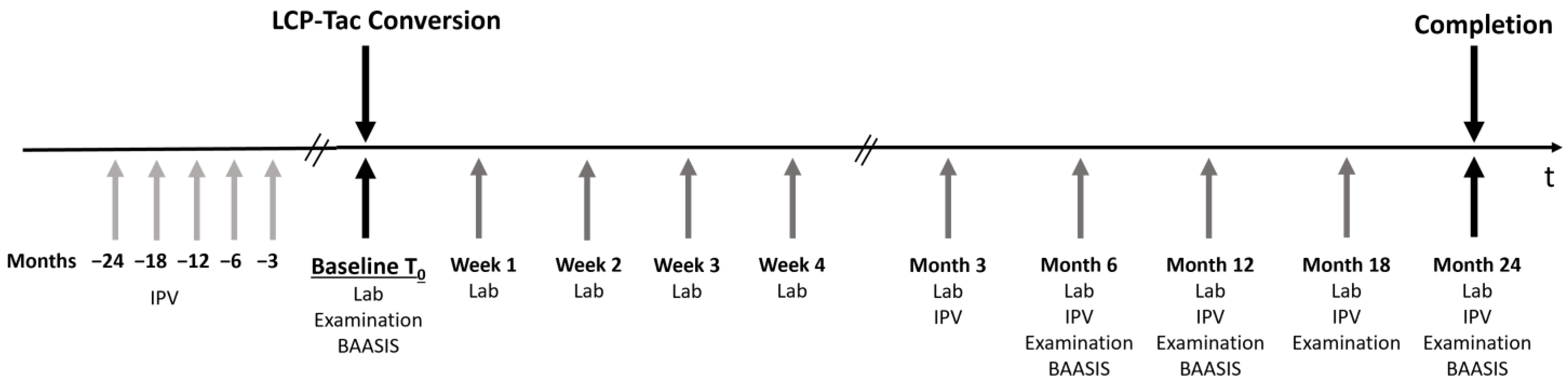
2.1. Study Design
2.2. Inclusion and Exclusion Criteria
2.3. Primary and Secondary Objectives
2.4. Statistical Analysis
3. Results
3.1. Patient Baseline Characteristics
3.2. Overall Adherence to Immunosuppressive Medication
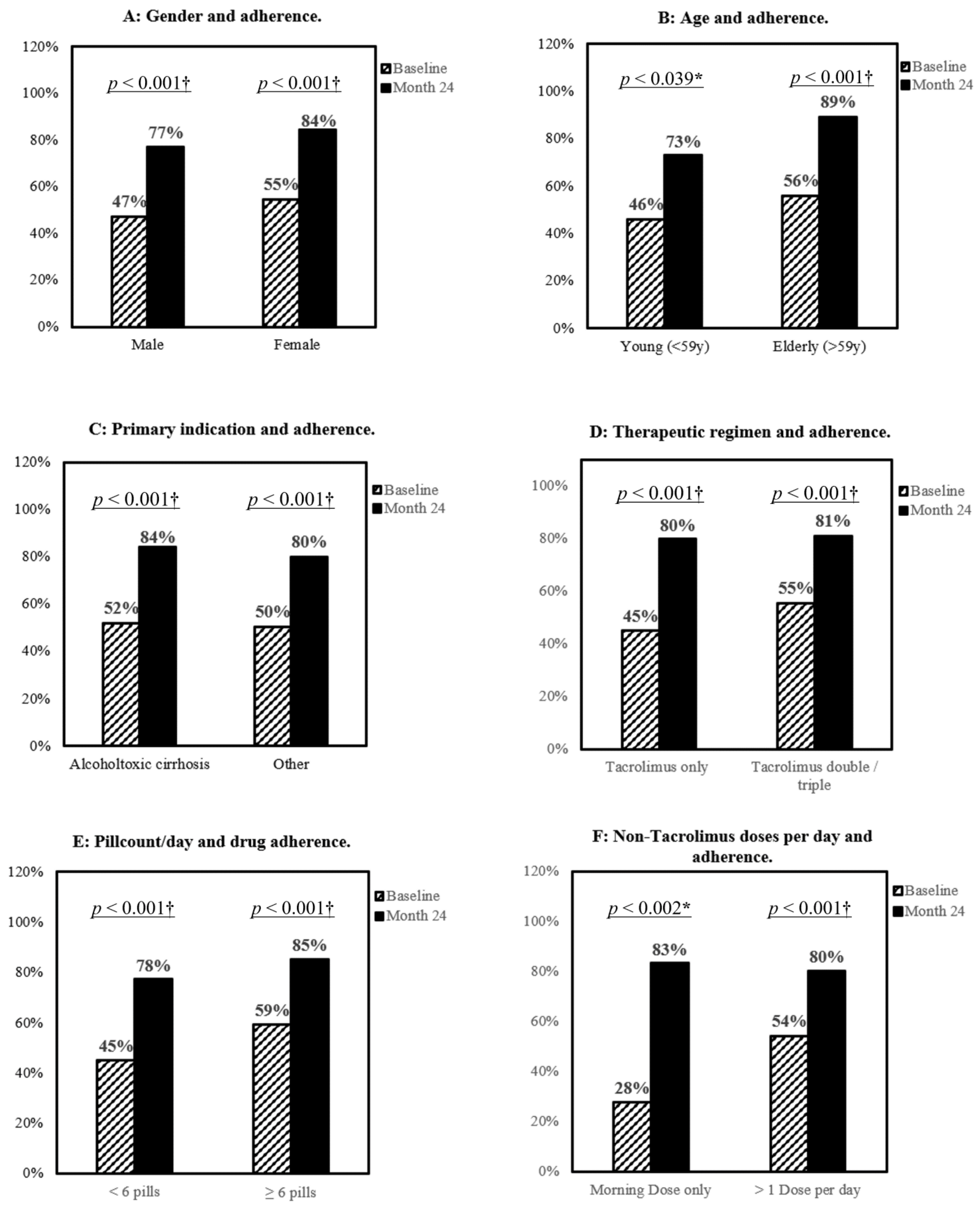
3.3. Impact of Gender, Age, and Treatment Complexity on Patients’ Adherence
3.4. Pharmacokinetic Characteristic
3.5. Efficacy and Adverse Events
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Halloran, P.F. Immunosuppressive drugs for kidney transplantation. N. Engl. J. Med. 2004, 351, 2715–2729. [Google Scholar] [CrossRef] [Green Version]
- Charlton, M.; Levitsky, J.; Aqel, B.; O’Grady, J.; Hemibach, J.; Rinella, M.; Fung, J.; Ghabril, M.; Thomason, R.; Burra, P.; et al. International Liver Transplantation Society Consensus Statement on Immunosuppression in Liver Transplant Recipients. Transplantation 2018, 102, 727–743. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Liver transplantation. J. Hepatol. 2016, 64, 433–485. [Google Scholar] [CrossRef]
- McAlister, V.C.; Haddad, E.; Renouf, E.; Malthaner, R.A.; Kjaer, M.S.; Gluud, L.L. Cyclosporin versus tacrolimus as primary immunosuppressant after liver transplantation: A meta-analysis. Am. J. Transplant. 2006, 6, 1578–1585. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.; Patel, H.; Panchal, S.; Mehta, T. Formulation strategies for drug delivery of tacrolimus: An overview. Int. J. Pharm. Investig. 2012, 2, 169–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venkataramanan, R.; Jain, A.; Warty, V.; Abu-Elmagd, K.; Alessiani, M.; Lever, J.; Krajak, A.; Flowers, J.; Mehta, S.; Zuckerman, S.; et al. Pharmacokinetics of FK 506 in transplant patients. Transplant. Proc. 1991, 23, 2736–2740. [Google Scholar]
- Ojo, A.O.; Held, P.J.; Port, F.K.; Wolfe, R.A.; Leichtman, A.B.; Young, E.W.; Arndorfer, J.; Christensen, L.; Merion, R.M. Chronic renal failure after transplantation of a nonrenal organ. N. Engl. J. Med. 2003, 349, 931–940. [Google Scholar] [CrossRef]
- Christians, U.; Klawitter, J.; Brunner, N.; Schmitz, V. Biomarkers of Immunosuppressant Organ Toxicity after Transplantation—Status, Concepts and Misconceptions. Expert. Opin. Drug Metab. Toxicol. 2011, 7, 175–200. [Google Scholar] [CrossRef] [Green Version]
- Burra, P.; Shalaby, S.; Zanetto, A. Long-term care of transplant recipients: De novo neoplasms after liver transplantation. Curr. Opin. Organ Transplant. 2018, 23, 187–195. [Google Scholar] [CrossRef]
- Jacobson, P.A.; Schladt, D.; Israni, A.; Oetting, W.S.; Lin, Y.C. Genetic and Clinical Determinants of Early, Acute Calcineurin Inhibitor-Related Nephrotoxicity: Results from a Kidney Transplant Consortium. Transplatation 2012, 93, 624–631. [Google Scholar] [CrossRef] [Green Version]
- Van Gelder, T.; Etsouli, O.; Moes, D.J.; Swen, J.J. Comparison of the Impact of Pharmacogenetic Variability on the PK of Slow Release and Immediate Release Tacrolimus Formulations. Genes 2020, 11, 1205. [Google Scholar] [CrossRef] [PubMed]
- Coleman, C.I.; Roberts, M.S.; Sobieraj, D.M.; Lee, S.; Alam, T.; Kaur, R. Effect of dosing frequency on chronic cardiovascular disease medication adherence. Curr. Med. Res. Opin. 2012, 28, 669–680. [Google Scholar] [CrossRef] [PubMed]
- De Geest, S.; Burkhalter, H.; Bogert, L.; Berben, L.; Glass, T.R.; Denhaerynck, K. Psychosocial Interest Group; Swiss Transplant Cohort Study. Describing the evolution of medication nonadherence from pretransplant until 3 years post-transplant and determining pretransplant medication nonadherence as risk factor for post-transplant nonadherence to immunosuppressives: The Swiss Transplant Cohort Study. Transpl. Int. 2014, 27, 657–666. [Google Scholar] [CrossRef]
- O’Carroll, R.E.; McGregor, L.M.M.; Swanson, V.; Masterton, G.; Hayes, P.C. Adherence to Medication after Liver Transplantation in Scotland: A Pilot Study. Liver Transpl. 2006, 12, 1862–1868. [Google Scholar] [CrossRef] [PubMed]
- Beckebaum, S.; Iacob, S.; Sweid, D.; Sotiropoulos, G.C.; Saner, F.; Kaiser, G.; Radtke, A.; Klein, C.G.; Erim, Y.; De Geest, S.; et al. Efficacy, safety, and immunosuppressant adherence in stable liver transplant patients converted from a twice-daily tacrolimus-based regimen to once-daily tacrolimus extended-release formulation. Transpl. Int. 2011, 24, 666–675. [Google Scholar] [CrossRef]
- Baraldo, M. Meltdose Tacrolimus Pharmacokinetics. Transpl. Proc. 2016, 48, 420–423. [Google Scholar] [CrossRef] [Green Version]
- Alloway, R.R.; Eckhoff, D.E.; Washburn, W.K.; Teperman, L.W. Conversion from Twice Daily Tacrolimus Capsules to Once Daily Extended-Release Tacrolimus (LCP-Tacro): Phase 2 Trial of Stable Liver Transplant Recipients. Liver Transplant. 2014, 20, 564–575. [Google Scholar] [CrossRef]
- Dobbels, F.; Berben, L.; De Geest, S.; Drent, G.; Lennerling, A.; Whittaker, C.; Kugler, C. The psychometric properties and practicability of self-report instruments to identify medication nonadherence in adult transplant patients: A systematic review. Transplantation 2010, 90, 205–219. [Google Scholar] [CrossRef] [PubMed]
- Summary of Product Characteristics for LCP-Tac 0.75 mg/1 mg/4 mg Prolonged-Release Tablets. Available online: https://www.ema.europa.eu/en/documents/assessment-report/envarsus-epar-public-assessment-report_en.pdf (accessed on 1 January 2022).
- Germani, G.; Lazzaro, S.; Gnoato, F.; Senzolo, M.; Borella, V.; Rupolo, G.; Cillo, U.; Rigotti, P.; Feltrin, G.; Loy, M.; et al. Nonadherent behaviors after solid organ transplantation. Transplant. Proc. 2011, 43, 318–323. [Google Scholar] [CrossRef]
- Lieber, S.R.; Volk, M.L. Non-Adherence and Graft Failure in Adult Liver Transplant Recipients. Dig. Dis. Sci. 2013, 58, 824–834. [Google Scholar] [CrossRef]
- Kuypers, D.R.; Peeters, P.C.; Sennesael, J.J.; Kianda, M.N.; Vrijens, B.; Kristanto, P.; Dobbels, F.; Vanrenterghem, Y.; Kanaan, N. Improved adherence to tacrolimus once-daily formulation in renal recipients: A randomized controlled trial using electronic monitoring. Transplantation 2013, 95, 333–340. [Google Scholar] [CrossRef] [Green Version]
- Myaskovsky, L.; Jesse, M.T.; Kuntz, K.; Leino, A.; Peipert, J.D.; Russell, C.L.; Spivey, C.A.; Sulejmani, N.; Dew, M.A. Report from the American Society of Transplantation Psychosocial Community of Practice Adherence Task Force: Real-world options for promoting adherence in adult recipients. Clin. Transpl. 2018, 32, e13353. [Google Scholar] [CrossRef]
- Kuypers, D.R.J. Intrapatient Variability of Tacrolimus Exposure in Solid Organ Transplantation: A Novel Marker for Clinical Outcome. Clin. Pharmacol. Ther. 2020, 107, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Thölking, G.; Siats, L.; Fortmann, C.; Koch, R.; Hüsing, A.; Cicinnati, V.R.; Gerth, H.U.; Wolters, H.H.; Anthoni, C.; Pavenstädt, H.; et al. Tacrolimus Concentration/Dose Ratio is Associated with Renal Function After Liver Transplantation. Ann. Transplant. 2016, 21, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Von Einsiedel, J.; Thölking, G.; Wilms, C.; Vorona, E.; Bokemeyer, A.; Schmidt, H.H.; Kabar, Y.; Hüsing-Kabar, A. Conversion from Standard-Release Tacrolimus to MeltDose® Tacrolimus (LCPT) Improves Renal Function after Liver Transplantation. J. Clin. Med. 2020, 9, 1654. [Google Scholar] [CrossRef]
- Langone, A.; Steinberg, S.M.; Gedaly, R.; Chan, L.K.; Shah, T.; Sethi, K.D.; Nigro, V.; Morgan, J.C.; Formica, R.N.; Barr, Y.M.; et al. Switching STudy of Kidney Transplant PAtients with Tremor to LCP-TacrO (STRATO): An open-label, multicenter, prospective phase 3b study. Clin. Transplant. 2015, 29, 796–805. [Google Scholar] [CrossRef] [PubMed]

| Characteristic | Completers | Non-Completers |
|---|---|---|
| N | 134 | 31 |
| Female | 64 (48%) | 18 (58%) |
| Age at study entry, years | 55 ± 13 | 56 ± 13 |
| Time since LT, months | 91 ± 85 | 101 ± 87 |
| Median (Range) | 58 (4–336) | 62 (5–312) |
| <1 year | 14 (10%) | 2 (7%) |
| 2–5 years | 57 (43%) | 12 (39%) |
| 5–10 years | 19 (15%) | 6 (19%) |
| >10 years | 43 (32%) | 9 (30%) |
| BMI | 26 ± 5 | 25 ± 5 |
| <18,5 | 5 (4%) | 2 (6%) |
| 18.5–25 | 53 (40%) | 14 (45%) |
| 25–30 | 47 (35%) | 9 (29%) |
| >30 | 30 (22%) | 6 (19%) |
| Race | ||
| Caucasian | 129 (96%) | 31 (100%) |
| Other | 5 (4%) | 0 (0%) |
| Primary indication | ||
| Autoimmune hepatitis | 27 (20%) | 3 (10%) |
| Hepatocellular carcinoma | 26 (19%) | 6 (19%) |
| Alcoholic liver disease | 25 (19%) | 7 (23%) |
| Acute liver failure | 14 (10%) | 2 (6%) |
| Chronic viral hepatitis C | 9 (7%) | 3 (10%) |
| Cryptogenic cirrhosis | 8 (6%) | 2 (6%) |
| Viral hepatitis B a | 8 (6%) | 1 (3%) |
| Liver cysts | 4 (3%) | 0 (0%) |
| Nonalcoholic steatohepatitis | 2 (1%) | 3 (10%) |
| Wilson’s disease | 2 (1%) | 0 (0%) |
| Other | 9 (7%) | 4 (13%) |
| Arterial hypertension | 73 (54%) | 17 (55%) |
| Number of antihypertensive drugs | ||
| 1 | 43 (32%) | 10 (32%) |
| 2 | 22 (16%) | 6 (20%) |
| 3 | 6 (4%) | 1 (3%) |
| 4 | 1 (1%) | 0 (0%) |
| Dyslipidemia | 29 (22%) | 10 (32%) |
| Statins/fibrates | 17 (13%) | 5 (16%) |
| Diabetes | 25 (19%) | 9 (29%) |
| Insulin/oral antidiabetics | 20 (15%) | 7 (23%) |
| Tacrolimus-based immunosuppression | ||
| Plus mycophenolate mofetil | 52 (39%) | 7 (23%) |
| Plus prednisone | 11 (8%) | 2 (6%) |
| Plus everolimus | 17 (13%) | 2 (6%) |
| Tac-based monotherapy | 60 (45%) | 20 (65%) |
| Tac-based dual therapy regime | 68 (51%) | 11 (35%) |
| Tac-based triple therapy regime | 6 (4%) | 0 (0%) |
| Item | Baseline | Month 6 | Month 12 | Month 24 | ||||
|---|---|---|---|---|---|---|---|---|
| Questionnaire Dose not taken | 20 | (15%) | 8 | (6%) | 5 | (4%) | 3 | (2%) |
| Consecutive doses not taken | 1 | (1%) | 0 | - | 1 | (1%) | 0 | - |
| Dose taken with >2 h delay | 57 | (43%) | 41 | (32%) | 33 | (25%) | 26 | (20%) |
| Dose reduced | 0 | - | 0 | - | 1 | (1%) | 0 | - |
| Overall non-adherence | 66 | (49%) | 43 | (33%) | 35 | (26%) | 26 | (20%) |
| Visual Analog Scale | ||||||||
| (0–100) | 93 ± 11 | 97 ± 9 | 98 ± 6 | 98 ± 4 | ||||
| Frequency optimal adherence a | 59 | (44%) | 85 | (67%) | 98 | (72%) | 102 | (78%) |
| IR-Tac | LCP-Tac | ||||
|---|---|---|---|---|---|
| n = 85 | n = 85 | ||||
| Median | Range | Median | Range | p | |
| Coefficient of variation C0/Dose | 21% | 4–85% | 22% | 4–70% | 0.85 |
| Standard deviation C0 | 1.1 | 0.2–5.0 | 1.2 | 0.2–3.6 | 0.68 |
| t = 0 | Week 1 | Month 1 | Month 3 | Month 6 | Month 12 | Month 18 | Month 24 | |
|---|---|---|---|---|---|---|---|---|
| n = 162 | n = 143 | n = 135 | n = 141 | n = 143 | n = 140 | n = 138 | n = 134 | |
| Tacrolimus dose (mg/day) (min−max) | 3.5 ± 1.6 (1.0–8.0) | 2.4 ± 1.2 (0.8–6.0) | 2.2 ± 1.1 (0.8–6.0) | 2.1 ± 1.1 (0.8–5.0) | 2.0 ± 1.0 (0.8–6.0) | 1.8 ± 1.0 (0.8–6.0) | 1.7 ± 1.0 (0.8–6.0) | 1.6 ± 0.9 (0.8–6.0) |
| Tacrolimus blood concentration (ng/mL) (min-max) | 5.4 ± 2.1 (2.4–12.7) | 5.5 ± 2,4 (1.0–12.0) | 4.9 ± 2.0 (1.6–10.6) | 5.0 ± 2.1 (1.3–11.3) | 4.7 ± 2.0 (1.6–10−0) | 4.6 ± 2.1 (1–0−11.8) | 4.3 ± 1.7 (1.0–9.3) | 4.1 ± 1.9 (1.0–11.8) |
| Concentration/dose ratio (ng/mL per mg/day) (min-max) | 1.7 ± 1.0 (0.4–5.8) | 2.8 ± 1.7 (0.3–10.1) | 2.7 ± 1.6 (0.5–10.6) | 3.0 ± 1.8 (0.5–12.4) | 3.0 ± 1.9 (0.6–13.2) | 3.1 ± 2.0 (0.2–10.7) | 3.1 ± 1.7 (0.5–8.3) | 3.1 ± 1.7 (0.3–11.1) |
| % change in dosage to previous visit | - | −32% | −8% | −5% | −4% | −9% | −4% | −6% |
| % change in blood concentrations to previous visit | - | +3% | −11% | +2% | −6% | −2% | −7% | −5% |
| % change in concentration/dosage to previous visit | - | +65% | −4% | +11% | 0% | +3% | +0% | +0% |
| Nr. patients with dose decrease (%) | - | 136 (95%) | 43 (26%) | 30 (22%) | 49 (36%) | 34 (24%) | 30 (22%) | 16 (12%) |
| Nr. patients with dose increase (%) | - | 4 (3%) | 6 (4%) | 4 (3%) | 6 (4%) | 6 (4%) | 7 (5%) | 4 (3%) |
| Nr. patients with no dose change (%) | - | 3 (2%) | 86 (52%) | 101 (75%) | 82 (60%) | 99 (71%) | 101 (73%) | 109 (84%) |
| Adverse Events | Frequency | |
|---|---|---|
| Respiratory, thoracic, and mediastinal disorders | ||
| Viral upper respiratory tract infection | 28 | (17%) |
| Cough | 5 | (3%) |
| Nervous system and psychiatric disorders | ||
| Headache | 25 | (16%) |
| Dizziness | 6 | (4%) |
| Paresthesia | 3 | (2%) |
| Tremor | 4 | (2%) |
| Restlessness/Agitation | 4 | (2%) |
| Insomnia | 2 | (1%) |
| Gastrointestinal disorders | ||
| Diarrhea | 18 | (11%) |
| Abdominal pain | 13 | (8%) |
| Nausea and vomiting symptoms | 11 | (7%) |
| Gastroenteritis | 4 | (2%) |
| Acid reflux (esophageal) | 3 | (2%) |
| General disorders | ||
| Fatigue | 18 | (11%) |
| Asthenia | 4 | (2%) |
| Dry mouth | 3 | (2%) |
| Pyrexia | 3 | (2%) |
| Hyperhidrosis | 2 | (1%) |
| Skin and subcutaneous disorders | ||
| Pruritus | 15 | (9%) |
| Eczema | 8 | (5%) |
| Basal cell carcinoma | 3 | (2%) |
| Metabolism and nutrition disorders | ||
| Weight gain | 14 | (9%) |
| Edema, peripheral | 3 | (2%) |
| Weight loss | 3 | (2%) |
| Renal and urinary disorders | ||
| Urinary tract infection | 8 | (5%) |
| Infections and infestations | ||
| Herpes zoster | 2 | (1%) |
| Vascular disorders | ||
| Hypertension worsened | 10 | (6%) |
| Musculoskeletal and connective tissue disorders | ||
| Bone pain/Arthralgia | 12 | (7%) |
| Muscle cramps | 3 | (2%) |
| Investigations | ||
| Hepatic enzyme increased | 5 | (3%) |
| Proteinuria | 2 | (1%) |
| Cardiac disorders | ||
| Palpitations | 2 | (1%) |
| Ear and labyrinth disorders | ||
| Tinnitus | 2 | (1%) |
| Serious Adverse Events | Frequency | |
|---|---|---|
| Death | ||
| Cardiac Arrest | 2 | (1%) |
| SIRS (Systemic inflammatory response syndrome) | 2 | (1%) |
| Hepatic failure–recurrent hepatocellular carcinoma | 1 | (1%) |
| Non-fatal serious adverse events | ||
| Infections and infestations | ||
| Abscess | 3 | (2%) |
| Pneumonia | 3 | (2%) |
| Urosepsis | 2 | (1%) |
| Hepatitis B/Epstein–Barr reactivation | 2 | (1%) |
| Herpes zoster | 1 | (1%) |
| Hepatobiliary disorders | ||
| Bile duct stenosis | 3 | (2%) |
| Recurrent Hepatocellular carcinoma | 2 | (1%) |
| Cholangitis | 2 | (1%) |
| Hepatic failure | 1 | (1%) |
| Graft dysfunction | 1 | (1%) |
| Hepatomegaly | 1 | (1%) |
| Vascular disorders | ||
| Thrombosis | 3 | (2%) |
| Pulmonary embolism | 2 | (1%) |
| Renal and urinary disorders | ||
| Acute renal failure | 2 | (1%) |
| Urinary calculi | 2 | (1%) |
| Respiratory, thoracic, and mediastinal disorders | ||
| Lung adenocarcinoma | 2 | (1%) |
| Pleural effusion | 2 | (1%) |
| Gastrointestinal disorders | ||
| Gastrointestinal hemorrhage (gastric, small intestine) | 2 | (1%) |
| Diarrhea | 2 | (1%) |
| Nervous system disorders | ||
| Stroke | 1 | (1%) |
| Transient ischemic attack | 1 | (1%) |
| Psychiatric disorders | ||
| Hallucinations | 1 | (1%) |
| Paranoid schizophrenia | 1 | (1%) |
| Other | ||
| Amyloidosis | 1 | (1%) |
| Anemia requiring transfusion | 1 | (1%) |
| Coronary artery disease | 1 | (1%) |
| Hyponatremia | 1 | (1%) |
| Post-transplant lymphoproliferative disorder (PTLD) | 1 | (1%) |
| Retinal detachment | 1 | (1%) |
| Rheumatoid arthritis | 1 | (1%) |
| Toxic epidermal necrolysis | 1 | (1%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maurer, M.M.; Ibach, M.; Plewe, J.; Winter, A.; Ritschl, P.; Globke, B.; Öllinger, R.; Lurje, G.; Schöning, W.; Pratschke, J.; et al. Reducing the Pill Burden: Immunosuppressant Adherence and Safety after Conversion from a Twice-Daily (IR-Tac) to a Novel Once-Daily (LCP-Tac) Tacrolimus Formulation in 161 Liver Transplant Patients. Biomedicines 2022, 10, 272. https://doi.org/10.3390/biomedicines10020272
Maurer MM, Ibach M, Plewe J, Winter A, Ritschl P, Globke B, Öllinger R, Lurje G, Schöning W, Pratschke J, et al. Reducing the Pill Burden: Immunosuppressant Adherence and Safety after Conversion from a Twice-Daily (IR-Tac) to a Novel Once-Daily (LCP-Tac) Tacrolimus Formulation in 161 Liver Transplant Patients. Biomedicines. 2022; 10(2):272. https://doi.org/10.3390/biomedicines10020272
Chicago/Turabian StyleMaurer, Max M., Marius Ibach, Julius Plewe, Axel Winter, Paul Ritschl, Brigitta Globke, Robert Öllinger, Georg Lurje, Wenzel Schöning, Johann Pratschke, and et al. 2022. "Reducing the Pill Burden: Immunosuppressant Adherence and Safety after Conversion from a Twice-Daily (IR-Tac) to a Novel Once-Daily (LCP-Tac) Tacrolimus Formulation in 161 Liver Transplant Patients" Biomedicines 10, no. 2: 272. https://doi.org/10.3390/biomedicines10020272
APA StyleMaurer, M. M., Ibach, M., Plewe, J., Winter, A., Ritschl, P., Globke, B., Öllinger, R., Lurje, G., Schöning, W., Pratschke, J., & Eurich, D. (2022). Reducing the Pill Burden: Immunosuppressant Adherence and Safety after Conversion from a Twice-Daily (IR-Tac) to a Novel Once-Daily (LCP-Tac) Tacrolimus Formulation in 161 Liver Transplant Patients. Biomedicines, 10(2), 272. https://doi.org/10.3390/biomedicines10020272









