Association of Lipoproteins with Neutrophil Extracellular Traps in Patients with Abdominal Aortic Aneurysm
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Blood Sampling
2.3. Measurement of Blood Parameters via ELISA
2.4. Detection of Circulating Cell-Free Nuclear DNA by Polymerase Chain Reaction
2.5. Lipoprotein Isolation and Modification
2.6. DNA Release Assay
2.7. Statistical Analysis
3. Results
3.1. AAA and PAD Patients Have a Comparable Profile of Comorbidities and Medication While Healthy Controls Differ Significantly
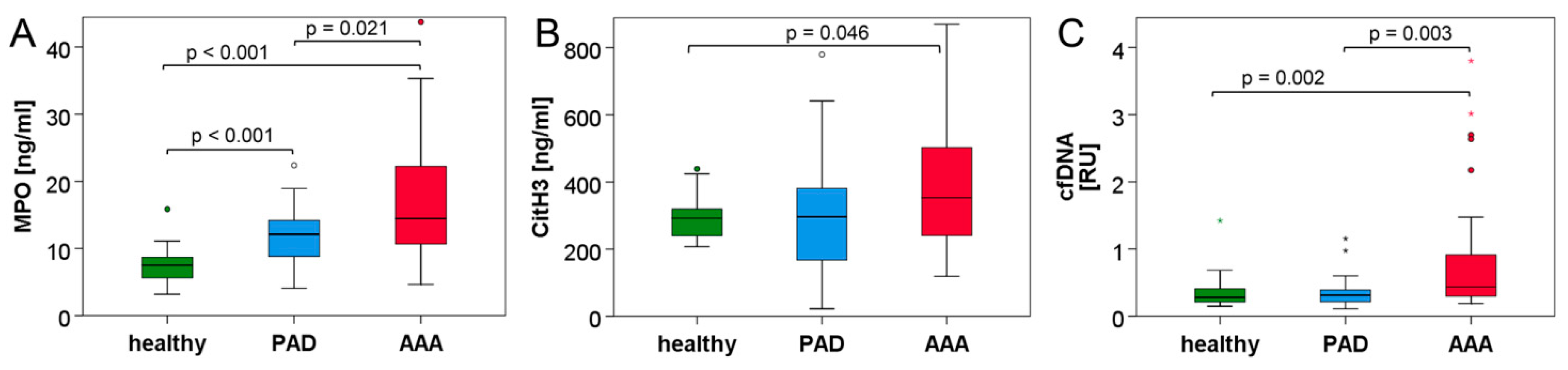
3.2. Neutrophil and NET Parameters Are Particularly Elevated in Blood of AAA Patients
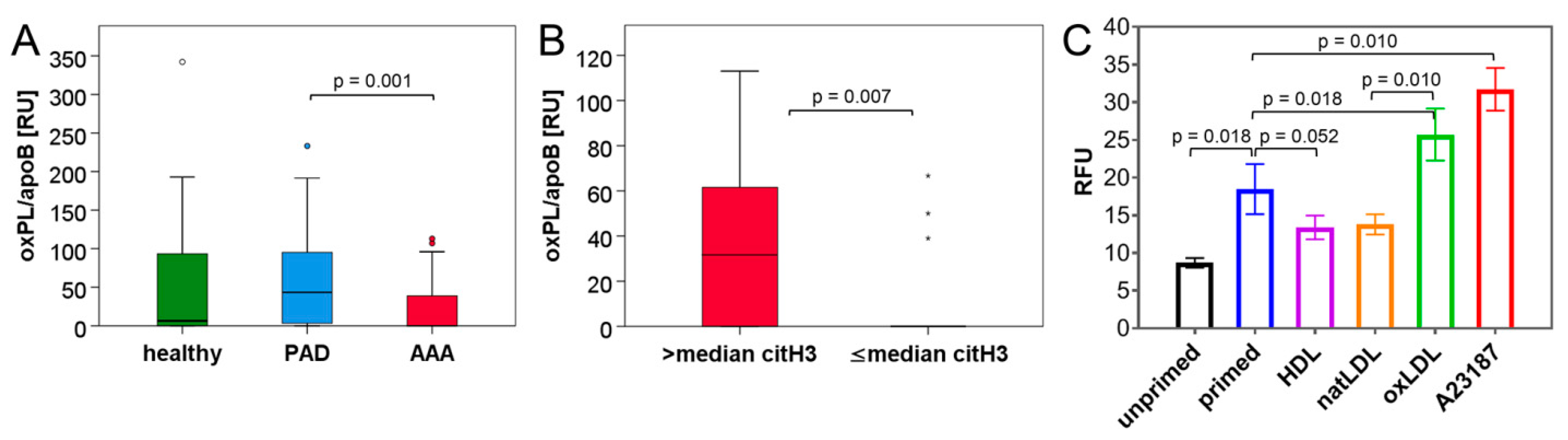
3.3. Blood Lipid Levels Show an Association with Circulating NET Markers
3.4. NET Induction Is Promoted by oxLDL but Unaffected by HDL In Vitro
4. Discussion
5. Conclusions
6. Limitations of the Study
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Wanhainen, A.; Verzini, F.; Van Herzeele, I.; Allaire, E.; Bown, M.; Cohnert, T.; Dick, F.; van Herwaarden, J.; Karkos, C.; Koelemay, M.; et al. Editor’s Choice—European Society for Vascular Surgery (ESVS) 2019 Clinical Practice Guidelines on the Management of Abdominal Aorto-iliac Artery Aneurysms. Eur. J. Vasc. Endovasc. Surg. 2019, 57, 8–93. [Google Scholar] [CrossRef] [Green Version]
- Piechota-Polanczyk, A.; Jozkowicz, A.; Nowak, W.; Eilenberg, W.; Neumayer, C.; Malinski, T.; Huk, I.; Brostjan, C. The Abdominal Aortic Aneurysm and Intraluminal Thrombus: Current Concepts of Development and Treatment. Front. Cardiovasc. Med. 2015, 2, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Golledge, J. Abdominal aortic aneurysm: Update on pathogenesis and medical treatments. Nat. Rev. Cardiol. 2019, 16, 225–242. [Google Scholar] [CrossRef]
- Kessler, V.; Klopf, J.; Eilenberg, W.; Neumayer, C.; Brostjan, C. AAA Revisited: A Comprehensive Review of Risk Factors, Management, and Hallmarks of Pathogenesis. Biomedicines 2022, 10, 94. [Google Scholar] [CrossRef]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil extracellular traps kill bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef] [PubMed]
- Brostjan, C.; Oehler, R. The role of neutrophil death in chronic inflammation and cancer. Cell Death Discov. 2020, 6, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klopf, J.; Brostjan, C.; Eilenberg, W.; Neumayer, C. Neutrophil Extracellular Traps and Their Implications in Cardiovascular and Inflammatory Disease. Int. J. Mol. Sci. 2021, 22, 559. [Google Scholar] [CrossRef]
- Leshner, M.; Wang, S.; Lewis, C.; Zheng, H.; Chen, X.A.; Santy, L.; Wang, Y. PAD4 mediated histone hypercitrullination induces heterochromatin decondensation and chromatin unfolding to form neutrophil extracellular trap-like structures. Front. Immunol. 2012, 3, 307. [Google Scholar] [CrossRef] [Green Version]
- Lewis, H.D.; Liddle, J.; Coote, J.E.; Atkinson, S.J.; Barker, M.D.; Bax, B.D.; Bicker, K.L.; Bingham, R.P.; Campbell, M.; Chen, Y.H.; et al. Inhibition of PAD4 activity is sufficient to disrupt mouse and human NET formation. Nat. Chem. Biol. 2015, 11, 189–191. [Google Scholar] [CrossRef]
- Cassatella, M.A.; Östberg, N.K.; Tamassia, N.; Soehnlein, O. Biological Roles of Neutrophil-Derived Granule Proteins and Cytokines. Trends Immunol. 2019, 40, 648–664. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, A.; Libby, P.; Soehnlein, O.; Aramburu, I.V.; Papayannopoulos, V.; Silvestre-Roig, C. Neutrophil extracellular traps: From physiology to pathology. Cardiovasc. Res. 2021. [Google Scholar] [CrossRef] [PubMed]
- Döring, Y.; Libby, P.; Soehnlein, O. Neutrophil Extracellular Traps Participate in Cardiovascular Diseases: Recent Experimental and Clinical Insights. Circ. Res. 2020, 126, 1228–1241. [Google Scholar] [CrossRef]
- Megens, R.T.; Vijayan, S.; Lievens, D.; Döring, Y.; van Zandvoort, M.A.; Grommes, J.; Weber, C.; Soehnlein, O. Presence of luminal neutrophil extracellular traps in atherosclerosis. Thromb. Haemost. 2012, 107, 597–598. [Google Scholar] [CrossRef] [PubMed]
- Borissoff, J.I.; Joosen, I.A.; Versteylen, M.O.; Brill, A.; Fuchs, T.A.; Savchenko, A.S.; Gallant, M.; Martinod, K.; Ten Cate, H.; Hofstra, L.; et al. Elevated levels of circulating DNA and chromatin are independently associated with severe coronary atherosclerosis and a prothrombotic state. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 2032–2040. [Google Scholar] [CrossRef] [Green Version]
- Blasco, A.; Coronado, M.J.; Vela, P.; Martin, P.; Solano, J.; Ramil, E.; Mesquida, A.; Santos, A.; Cozar, B.; Royuela, A.; et al. Prognostic implications of Neutrophil Extracellular Traps in coronary thrombi of patients with ST-elevation myocardial infarction. Thromb. Haemost. 2021. [Google Scholar] [CrossRef]
- Mangold, A.; Alias, S.; Scherz, T.; Hofbauer, M.; Jakowitsch, J.; Panzenbock, A.; Simon, D.; Laimer, D.; Bangert, C.; Kammerlander, A.; et al. Coronary neutrophil extracellular trap burden and deoxyribonuclease activity in ST-elevation acute coronary syndrome are predictors of ST-segment resolution and infarct size. Circ. Res. 2015, 116, 1182–1192. [Google Scholar] [CrossRef] [Green Version]
- Meher, A.K.; Spinosa, M.; Davis, J.P.; Pope, N.; Laubach, V.E.; Su, G.; Serbulea, V.; Leitinger, N.; Ailawadi, G.; Upchurch, G.R., Jr. Novel Role of IL (Interleukin)-1beta in Neutrophil Extracellular Trap Formation and Abdominal Aortic Aneurysms. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 843–853. [Google Scholar] [CrossRef] [Green Version]
- Yan, H.; Zhou, H.F.; Akk, A.; Hu, Y.; Springer, L.E.; Ennis, T.L.; Pham, C.T.N. Neutrophil Proteases Promote Experimental Abdominal Aortic Aneurysm via Extracellular Trap Release and Plasmacytoid Dendritic Cell Activation. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 1660–1669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delbosc, S.; Alsac, J.M.; Journe, C.; Louedec, L.; Castier, Y.; Bonnaure-Mallet, M.; Ruimy, R.; Rossignol, P.; Bouchard, P.; Michel, J.B.; et al. Porphyromonas gingivalis participates in pathogenesis of human abdominal aortic aneurysm by neutrophil activation. Proof of concept in rats. PLoS ONE 2011, 6, e18679. [Google Scholar] [CrossRef] [Green Version]
- Eilenberg, W.; Zagrapan, B.; Bleichert, S.; Ibrahim, N.; Knobl, V.; Brandau, A.; Martelanz, L.; Grasl, M.T.; Hayden, H.; Nawrozi, P.; et al. Histone citrullination as a novel biomarker and target to inhibit progression of abdominal aortic aneurysms. Transl. Res. 2021, 233, 32–46. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Tan, J.; Diamond, S.L. Hemodynamic force triggers rapid NETosis within sterile thrombotic occlusions. J. Thromb. Haemost. 2018, 16, 316–329. [Google Scholar] [CrossRef]
- Warnatsch, A.; Ioannou, M.; Wang, Q.; Papayannopoulos, V. Inflammation. Neutrophil extracellular traps license macrophages for cytokine production in atherosclerosis. Science 2015, 349, 316–320. [Google Scholar] [CrossRef] [Green Version]
- Fuchs, T.A.; Brill, A.; Wagner, D.D. Neutrophil extracellular trap (NET) impact on deep vein thrombosis. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 1777–1783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maugeri, N.; Campana, L.; Gavina, M.; Covino, C.; De Metrio, M.; Panciroli, C.; Maiuri, L.; Maseri, A.; D’Angelo, A.; Bianchi, M.E.; et al. Activated platelets present high mobility group box 1 to neutrophils, inducing autophagy and promoting the extrusion of neutrophil extracellular traps. J. Thromb. Haemost. 2014, 12, 2074–2088. [Google Scholar] [CrossRef]
- Douda, D.N.; Khan, M.A.; Grasemann, H.; Palaniyar, N. SK3 channel and mitochondrial ROS mediate NADPH oxidase-independent NETosis induced by calcium influx. Proc. Natl. Acad. Sci. USA 2015, 112, 2817–2822. [Google Scholar] [CrossRef] [Green Version]
- Parker, H.; Dragunow, M.; Hampton, M.B.; Kettle, A.J.; Winterbourn, C.C. Requirements for NADPH oxidase and myeloperoxidase in neutrophil extracellular trap formation differ depending on the stimulus. J. Leukoc. Biol. 2012, 92, 841–849. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, D.; Nagarkoti, S.; Kumar, A.; Dubey, M.; Singh, A.K.; Pathak, P.; Chandra, T.; Barthwal, M.K.; Dikshit, M. Oxidized LDL induced extracellular trap formation in human neutrophils via TLR-PKC-IRAK-MAPK and NADPH-oxidase activation. Free Radic. Biol. Med. 2016, 93, 190–203. [Google Scholar] [CrossRef]
- Obama, T.; Ohinata, H.; Takaki, T.; Iwamoto, S.; Sawada, N.; Aiuchi, T.; Kato, R.; Itabe, H. Cooperative Action of Oxidized Low-Density Lipoproteins and Neutrophils on Endothelial Inflammatory Responses Through Neutrophil Extracellular Trap Formation. Front. Immunol. 2019, 10, 1899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thålin, C.; Daleskog, M.; Göransson, S.P.; Schatzberg, D.; Lasselin, J.; Laska, A.C.; Kallner, A.; Helleday, T.; Wallén, H.; Demers, M. Validation of an enzyme-linked immunosorbent assay for the quantification of citrullinated histone H3 as a marker for neutrophil extracellular traps in human plasma. Immunol. Res. 2017, 65, 706–712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsimikas, S.; Brilakis, E.S.; Miller, E.R.; McConnell, J.P.; Lennon, R.J.; Kornman, K.S.; Witztum, J.L.; Berger, P.B. Oxidized phospholipids, Lp(a) lipoprotein, and coronary artery disease. N. Engl. J. Med. 2005, 353, 46–57. [Google Scholar] [CrossRef]
- Tsimikas, S.; Kiechl, S.; Willeit, J.; Mayr, M.; Miller, E.R.; Kronenberg, F.; Xu, Q.; Bergmark, C.; Weger, S.; Oberhollenzer, F.; et al. Oxidized phospholipids predict the presence and progression of carotid and femoral atherosclerosis and symptomatic cardiovascular disease: Five-year prospective results from the Bruneck study. J. Am. Coll. Cardiol. 2006, 47, 2219–2228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schumaker, V.N.; Puppione, D.L. Sequential flotation ultracentrifugation. Methods Enzymol. 1986, 128, 155–170. [Google Scholar] [CrossRef]
- Yla-Herttuala, S.; Palinski, W.; Rosenfeld, M.E.; Parthasarathy, S.; Carew, T.E.; Butler, S.; Witztum, J.L.; Steinberg, D. Evidence for the presence of oxidatively modified low density lipoprotein in atherosclerotic lesions of rabbit and man. J. Clin. Investig. 1989, 84, 1086–1095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freitas, M.; Porto, G.; Lima, J.L.; Fernandes, E. Isolation and activation of human neutrophils in vitro. The importance of the anticoagulant used during blood collection. Clin. Biochem. 2008, 41, 570–575. [Google Scholar] [CrossRef] [PubMed]
- Zagrapan, B.; Eilenberg, W.; Prausmueller, S.; Nawrozi, P.; Muench, K.; Hetzer, S.; Elleder, V.; Rajic, R.; Juster, F.; Martelanz, L.; et al. A Novel Diagnostic and Prognostic Score for Abdominal Aortic Aneurysms Based on D-Dimer and a Comprehensive Analysis of Myeloid Cell Parameters. Thromb. Haemost. 2019, 119, 807–820. [Google Scholar] [CrossRef] [Green Version]
| Parameter | Healthy (n = 29) | PAD (n = 40) | AAA (n = 40) | p-Value (Groups) |
|---|---|---|---|---|
| n (%) | ||||
| Sex (male) | 26 (89.7%) | 32 (80.0%) | 32 (80.0%) | 0.336 (H/AAA) 0.336 (H/PAD) 1.000 (PAD/AAA) |
| Smoker | ||||
| never | 9 (31.0%) | 3 (7.5%) | 3 (7.5%) | 0.021 (H/AAA) |
| past | 14 (48.3%) | 24 (60.0%) | 20 (50.0%) | 0.036 (H/PAD) |
| current | 6 (20.7%) | 13 (32.5%) | 17 (42.5%) | 0.639 (PAD/AAA) |
| median (interquartile range) | ||||
| Age (years) | 66.6 (12.0) | 72.7 (13.0) | 71.8 (13.0) | 0.123 (H/AAA) 0.243 (H/PAD) 0.630 (PAD/AAA) |
| Body mass index | 26.5 (5.3) | 25.9 (4.1) | 27.3 (5.6) | 0.585 (H/AAA) 0.375 (H/PAD) 0.131 (PAD/AAA) |
| Pack years | 25.0 (31.0) | 37.0 (49.5) | 40.0 (30.0) | 0.066 (H/AAA) 0.073 (H/PAD) 0.942 (PAD/AAA) |
| Parameter | Healthy (n = 29) | PAD (n = 40) | AAA (n = 40) | p-Value (Groups) |
|---|---|---|---|---|
| Hypertension | 15 (51.7%) | 36 (90.0%) | 37 (92.5%) | <0.001 (H/AAA) <0.001 (H/PAD) 1.000 (PAD/AAA) |
| Hyperlipidemia | 8 (27.6%) | 37 (92.5%) | 35 (87.5%) | <0.001 (H/AAA) <0.001 (H/PAD) 0.712 (PAD/AAA) |
| PAD | 0 (0%) | 40 (100%) | 0 (0%) | 1.000 (H/AAA) <0.001 (H/PAD) <0.001 (PAD/AAA) |
| Coronary heart disease | 0 (0%) | 17 (42.5%) | 20 (50.0%) | <0.001 (H/AAA) <0.001 (H/PAD) 0.501 (PAD/AAA) |
| Myocardial infarction | 0 (0%) | 9 (22.5%) | 13 (32.5%) * | 0.002 (H/AAA) 0.008 (H/PAD) 0.339 (PAD/AAA) |
| Stent | 0 (0%) | 24 (60.0%) | 9 (22.5%) * | 0.014 (H/AAA) <0.001 (H/PAD) 0.002 (PAD/AAA) |
| Coronary artery bypass graft | 0 (0%) | 3 (7.5%) | 2 (5.0%) | 0.506 (H/AAA) 0.258 (H/PAD) 1.000 (PAD/AAA) |
| Diabetes mellitus | 3 (10.3%) | 11 (27.5%) | 12 (30.0%) | 0.051 (H/AAA) 0.080 (H/PAD) 0.805 (PAD/AAA) |
| Nephropathy | 3 (10.3%) | 10 (25.0%) | 16 (40.0%) | 0.006 (H/AAA) 0.124 (H/PAD) 0.152 (PAD/AAA) |
| Chronic obstructive pulmonary disease | 1 (3.4%) | 13 (32.5%) | 8 (20.0%) | 0.069 (H/AAA) 0.003 (H/PAD) 0.204 (PAD/AAA) |
| Tumor (past >1 year) | 5 (17.2%) | 5 (12.5%) | 6 (15.0%) | 1.000 (H/AAA) 0.732 (H/PAD) 0.745 (PAD/AAA) |
| Medication | Healthy (n = 29) | PAD (n = 40) | AAA (n= 40) | p-Value (Groups) |
|---|---|---|---|---|
| Acetylsalicylic acid (Thrombo ASS) | 5 (17.2%) | 33 (82.5%) | 35 (87.5%) | <0.001 (H/AAA) <0.001 (H/PAD) 0.531 (PAD/AAA) |
| P2Y12 antagonist (Clopidogrel) | 1 (3.4%) | 8 (20.0%) | 5 (12.5%) | 0.389 (H/AAA) 0.069 (H/PAD) 0.363 (PAD/AAA) |
| Vitamin K antagonist | 1 (3.4%) | 3 (7.5%) | 3 (7.5%) | 0.634 (H/AAA) 0.634 (H/PAD) 1.000 (PAD/AAA) |
| Xa inhibitor | 1 (3.4%) | 2 (5.0%) | 0 (0.0%) | 0.420 (H/AAA) 1.000 (H/PAD) 0.494 (PAD/AAA) |
| Antihypertensive therapy | 15 (51.7%) | 33 (82.5%) | 37 (92.5%) | <0.001 (H/AAA) 0.006 (H/PAD) 0.176 (PAD/AAA) |
| ACE inhibitor | 4 (13.8%) | 12 (30.0%) | 17 (42.5%) | 0.011 (H/AAA) 0.115 (H/PAD) 0.245 (PAD/AAA) |
| Angiotensin receptor inhibitor | 6 (20.7%) | 11 (27.5%) | 14 (35.0%) | 0.196 (H/AAA) 0.517 (H/PAD) 0.469 (PAD/AAA) |
| Beta blocker | 7 (24.1%) | 20 (50.0%) | 25 (62.5%) | 0.002 (H/AAA) 0.030 (H/PAD) 0.260 (PAD/AAA) |
| Ca2+ channel blocker | 4 (13.8%) | 9 (22.5%) | 6 (15.0%) | 1.000 (H/AAA) 0.361 (H/PAD) 0.390 (PAD/AAA) |
| Diuretic | 4 (13.8%) | 11 (27.5%) | 14 (35.0%) | 0.048 (H/AAA) 0.173 (H/PAD) 0.469 (PAD/AAA) |
| Lipid-lowering agents(type) | 5 (17.2%) (80% statins; 20% drug mix) | 38 (95.0%) (100% statins) | 36 (90.0%) (91.7% statins; 8.3% drug mix) | <0.001 (H/AAA) <0.001 (H/PAD) 0.675 (PAD/AAA) |
| Antidiabetic medication | 3 (10.3%) | 8 (20.0%) | 7 (17.5%) | 0.502 (H/AAA) 0.336 (H/PAD) 0.775 (PAD/AAA) |
| Insulin | 0 (0.0%) | 5 (12.5%) | 1 (2.5%) | 1.000 (H/AAA) 0.069 (H/PAD) 0.201 (PAD/AAA) |
| Metformin | 3 (10.3%) | 5 (12.5%) | 6 (15.0%) | 0.724 (H/AAA) 1.000 (H/PAD) 0.745 (PAD/AAA) |
| Bronchodilatators | 2 (6.9%) | 9 (22.5%) | 11 (27.5%) | 0.031 (H/AAA) 0.104 (H/PAD) 0.606 (PAD/AAA) |
| Parameter | Healthy (n = 29) | PAD (n = 40) | AAA (n= 40) | p-Value (Groups) |
|---|---|---|---|---|
| Leukocytes (103/µL) | 5.60 (1.80) | 7.16 (2.70) | 6.35 (2.70) | 0.034 (H/AAA) <0.001 (H/PAD) 0.029 (PAD/AAA) |
| Neutrophils (103/µL) | 3.00 (1.15) | 4.52 (1.96) | 4.00 (1.97) | 0.016 (H/AAA) <0.001 (H/PAD) 0.023 (PAD/AAA) |
| D-dimer (µg/mL) | 0.38 (0.50) * | 0.75 (0.68) § | 1.26 (1.13) $ | <0.001 (H/AAA) 0.008 (H/PAD) 0.007 (PAD/AAA) |
| C-reactive protein (mg/dL) | 0.24 (0.42) | 0.21 (0.32) | 0.27 (0.42) # | 0.678 (H/AAA) 0.515 (H/PAD) 0.229 (PAD/AAA) |
| Parameter | Healthy (n = 29) | PAD (n = 40) | AAA (n= 40) | p-Value (Groups) |
|---|---|---|---|---|
| citH3 (ng/mL) | 292.3 (83.6) | 296.4 (221.7) | 353.2 (263.3) * | 0.046 (H/AAA) 0.734 (H/PAD) 0.070 (PAD/AAA) |
| MPO (ng/mL) | 7.51 (3.20) | 12.11 (5.60) | 14.47 (11.74) | <0.001 (H/AAA) <0.001 (H/PAD) 0.021 (PAD/AAA) |
| cfDNA (RU) | 0.277 (0.217) | 0.311 (0.190) § | 0.437 (0.737) § | 0.002 (H/AAA) 1.000 (H/PAD) 0.003 (PAD/AAA) |
| oxPL/apoB (RU) | 6.33 (104.08) | 43.42 (92.50) | 0.00 (39.00) # | 0.197 (H/AAA) 0.181 (H/PAD) 0.001 (PAD/AAA) |
| Parameter 1 | Parameter 2 | r | p | n |
|---|---|---|---|---|
| citH3 (ng/mL) | Neutrophils (103/µL) | 0.211 | 0.029 | 107 |
| MPO (ng/mL) | 0.387 | <0.001 | 107 | |
| cfDNA (RU) | 0.599 | <0.001 | 89 | |
| Triglycerides (mg/dL) | 0.291 | 0.002 | 106 | |
| Total cholesterol/HDL ratio | 0.215 | 0.027 | 106 | |
| MPO (ng/mL) | Neutrophils (103/µL) | 0.333 | <0.001 | 109 |
| cfDNA (RU) | 0.507 | <0.001 | 89 | |
| D-dimer (µg/mL) | 0.475 | <0.001 | 98 | |
| cfDNA (RU) | Triglycerides (mg/dL) | 0.257 | 0.016 | 88 |
| oxPL/apoB (RU) | Lipoprotein (a) (nmol/L) | 0.314 | 0.001 | 106 |
| Parameter | Healthy (n = 29) | PAD (n = 40) | AAA (n= 39) | p-Value (Groups) |
|---|---|---|---|---|
| Triglycerides (mg/dL) | 123.0 (82.0) | 85.0 (63.0) | 136.0 (124.0) * | 0.097 (H/AAA) 0.256 (H/PAD) 0.001 (PAD/AAA) |
| Total cholesterol (mg/dL) | 200.0 (36.0) | 141.5 (43.0) | 162.0 (79.0) | 0.003 (H/AAA) <0.001 (H/PAD) 0.031 (PAD/AAA) |
| HDL cholesterol (mg/dL) | 55.0 (22.0) | 54.0 (28.0) | 49.0 (16.0) | 0.024 (H/AAA) 0.559 (H/PAD) 0.051 (PAD/AAA) |
| LDL cholesterol (mg/dL)—calculated | 122.2 (42.1) | 60.5 (32.5) | 85.6 (72.8) | 0.007 (H/AAA) <0.001 (H/PAD) 0.056 (PAD/AAA) |
| Total cholesterol/HDL ratio | 3.9 (2.2) | 2.7 (1.4) | 3.8 (1.8) | 0.742 (H/AAA) 0.001 (H/PAD) 0.001 (PAD/AAA) |
| LDL/HDL ratio | 2.4 (1.7) | 1.3 (0.8) | 1.7 (1.6) | 0.210 (H/AAA) <0.001 (H/PAD) 0.013 (PAD/AAA) |
| Lipoprotein (a) (nmol/L) | 13.0 (33.0) | 46.5 (123.0) | 18.0 (65.0) # | 0.394 (H/AAA) 0.051 (H/PAD) 0.177 (PAD/AAA) |
| Parameter 1 | Parameter 2 | r | p | n |
|---|---|---|---|---|
| oxPL/apoB (RU) | citH3 (ng/mL) | 0.470 | 0.003 | 37 |
| citH3 (ng/mL) | Neutrophils (103/µL) | 0.363 | 0.025 | 38 |
| cfDNA (RU) | 0.680 | <0.001 | 30 | |
| MPO (ng/mL) | 0.560 | <0.001 | 38 | |
| MPO (ng/mL) | cfDNA (RU) | 0.566 | 0.001 | 30 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brandau, A.; Ibrahim, N.; Klopf, J.; Hayden, H.; Ozsvar-Kozma, M.; Afonyushkin, T.; Bleichert, S.; Fuchs, L.; Watzinger, V.; Nairz, V.; et al. Association of Lipoproteins with Neutrophil Extracellular Traps in Patients with Abdominal Aortic Aneurysm. Biomedicines 2022, 10, 217. https://doi.org/10.3390/biomedicines10020217
Brandau A, Ibrahim N, Klopf J, Hayden H, Ozsvar-Kozma M, Afonyushkin T, Bleichert S, Fuchs L, Watzinger V, Nairz V, et al. Association of Lipoproteins with Neutrophil Extracellular Traps in Patients with Abdominal Aortic Aneurysm. Biomedicines. 2022; 10(2):217. https://doi.org/10.3390/biomedicines10020217
Chicago/Turabian StyleBrandau, Annika, Nahla Ibrahim, Johannes Klopf, Hubert Hayden, Maria Ozsvar-Kozma, Taras Afonyushkin, Sonja Bleichert, Lukas Fuchs, Viktoria Watzinger, Verena Nairz, and et al. 2022. "Association of Lipoproteins with Neutrophil Extracellular Traps in Patients with Abdominal Aortic Aneurysm" Biomedicines 10, no. 2: 217. https://doi.org/10.3390/biomedicines10020217
APA StyleBrandau, A., Ibrahim, N., Klopf, J., Hayden, H., Ozsvar-Kozma, M., Afonyushkin, T., Bleichert, S., Fuchs, L., Watzinger, V., Nairz, V., Manville, E., Kessler, V., Stangl, H., Eilenberg, W., Neumayer, C., & Brostjan, C. (2022). Association of Lipoproteins with Neutrophil Extracellular Traps in Patients with Abdominal Aortic Aneurysm. Biomedicines, 10(2), 217. https://doi.org/10.3390/biomedicines10020217






