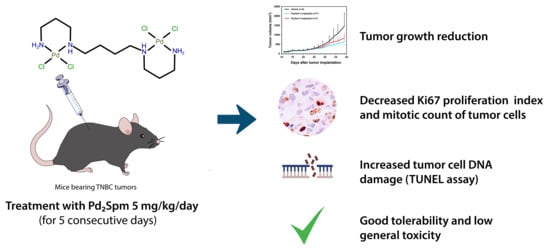
Pd2Spermine Complex Shows Cancer Selectivity and Efficacy to Inhibit Growth of Triple-Negative Breast Tumors in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Pd2Spm Synthesis and Formulation
2.3. Cell Cultures
2.4. Cell Proliferation Assay
2.5. Cell Migration—Wound Healing Assay
2.6. Quantitative Analysis of Vascular Endothelial Growth Factor (VEGF) Production
2.7. Mice
2.8. Subcutaneous In Vivo Breast Cancer Xenograft Study
2.9. Histological Analysis and Immunohistochemistry
2.10. Detection of Apoptosis with Labeling of DNA Strand Breaks (TUNEL Assay)
2.11. Biochemical and Hematological Analyses
2.12. In Vivo CAM Assay
2.13. Ethical Considerations
2.14. Statistical Data Analysis
3. Results
3.1. Pd2Spm Showed Selective Antiproliferative Activity for MDA-MB-231 Breast Cancer Cells
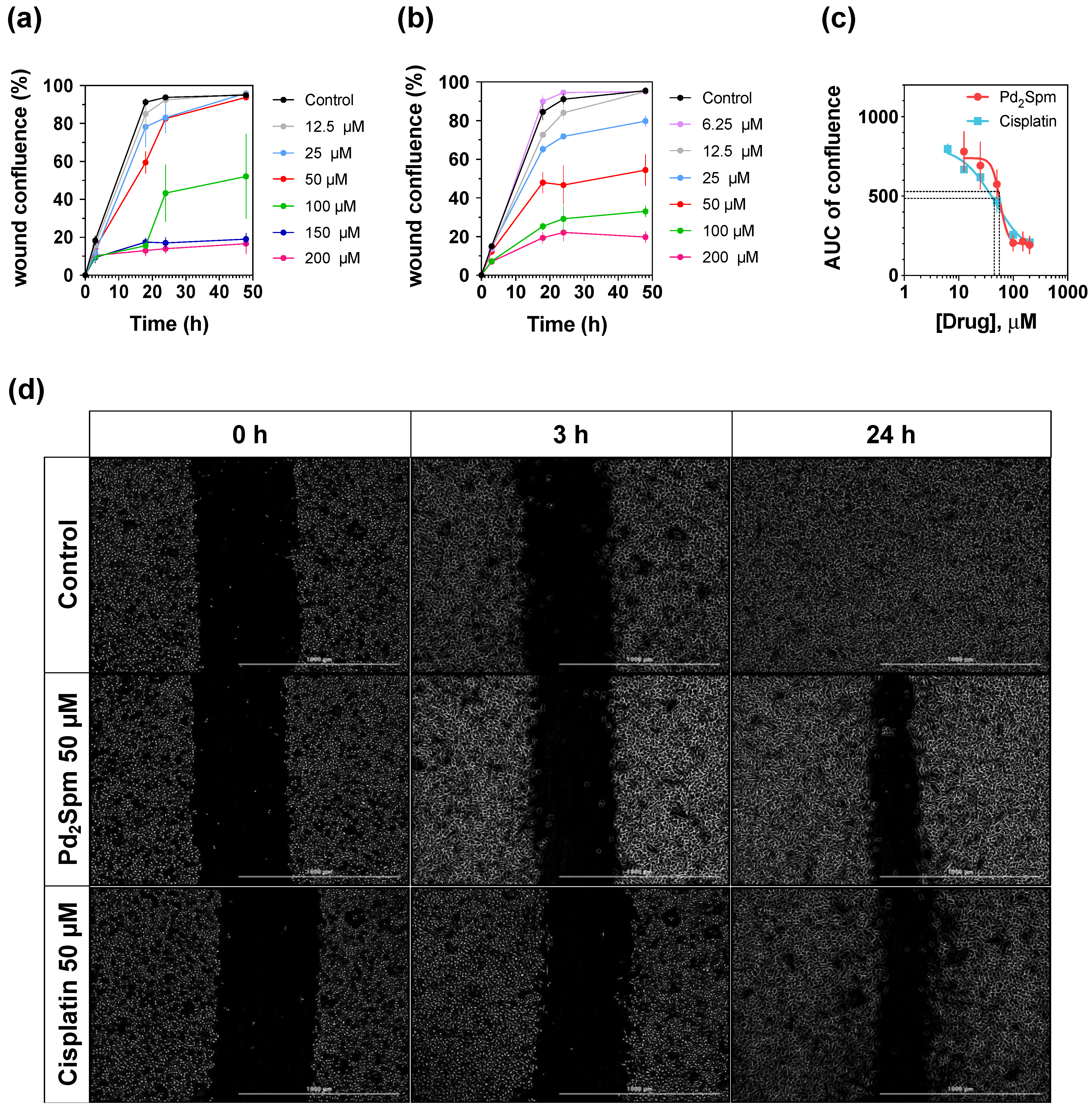
3.2. Pd2Spm Inhibited In Vitro Migration of MDA-MB-231 Cells
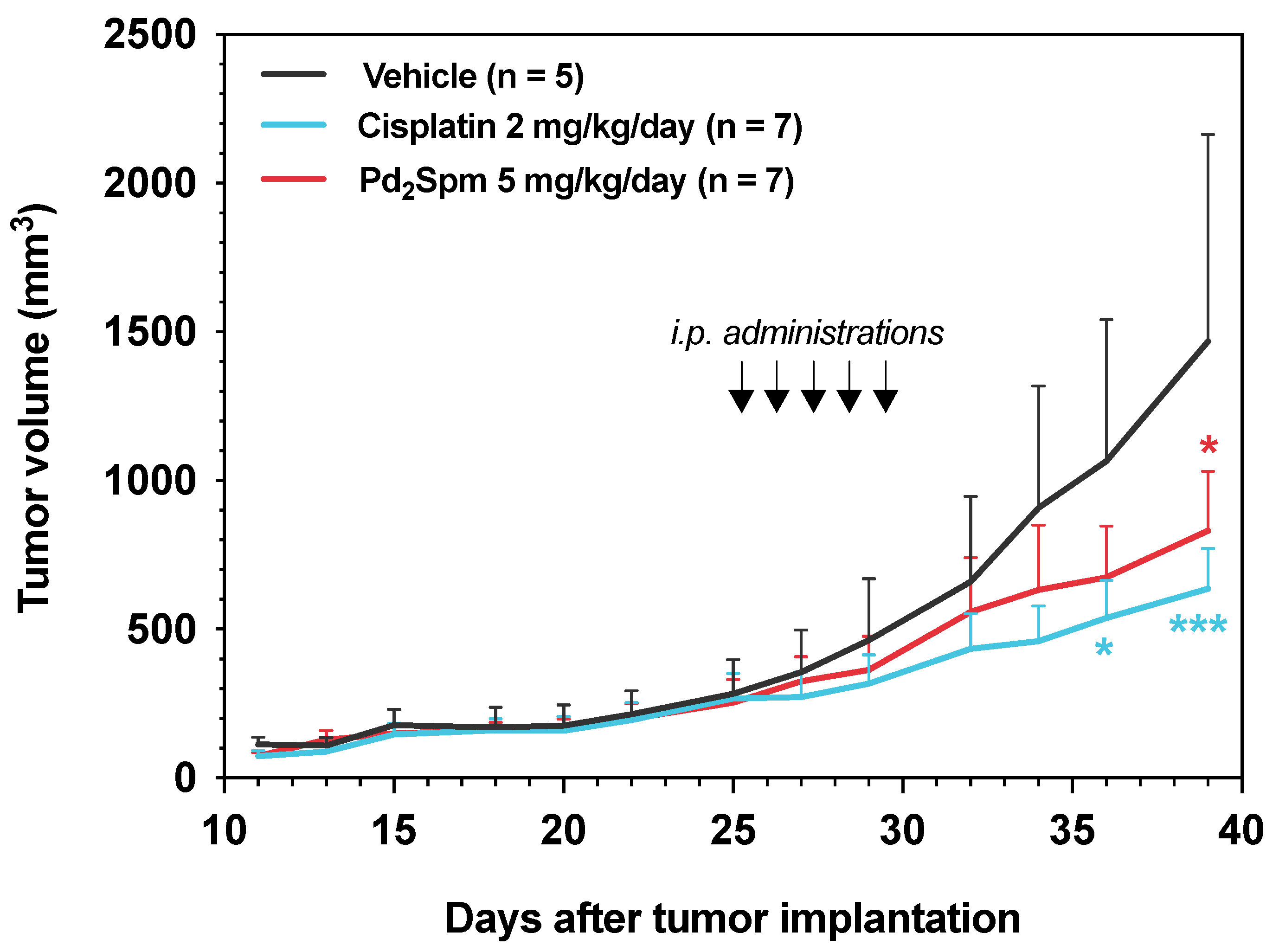
3.3. Pd2Spm Administration Inhibited the Growth of MDA-MB-231 Xenografts in Female Nude Mice
3.4. Pd2Spm Administration Suppressed Cell Proliferation and Induced DNA Damage in Tumors Collected from MDA-MB-231 Breast Tumor-Bearing Mice
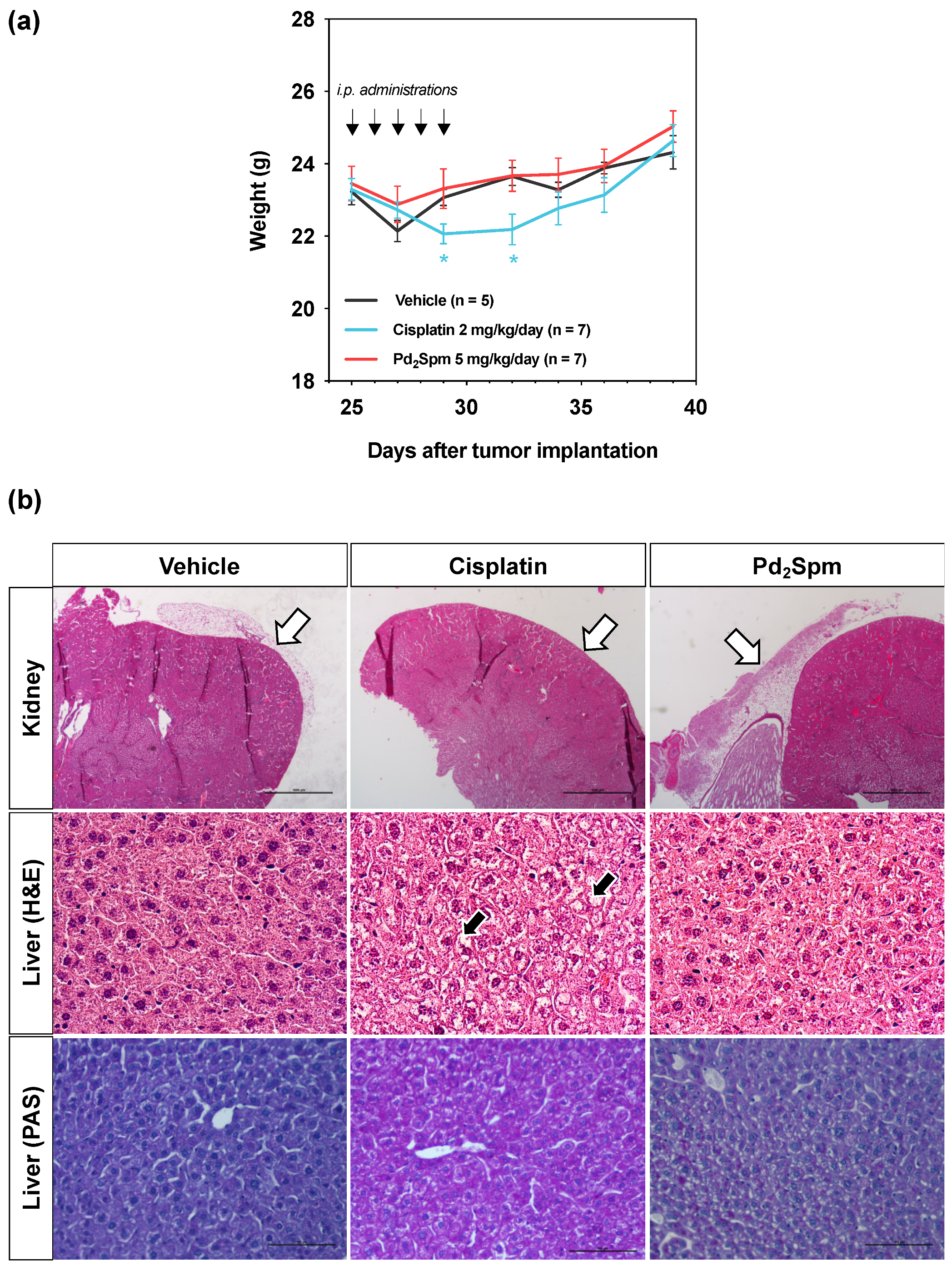
3.5. Impact of Pd2Spm on the Weight of MDA-MB-231 Breast Tumor-Bearing Mice
3.6. Impact of Pd2Spm on the Hematologic and Biochemical Parameters of MDA-MB-231 Breast Tumor-Bearing Mice
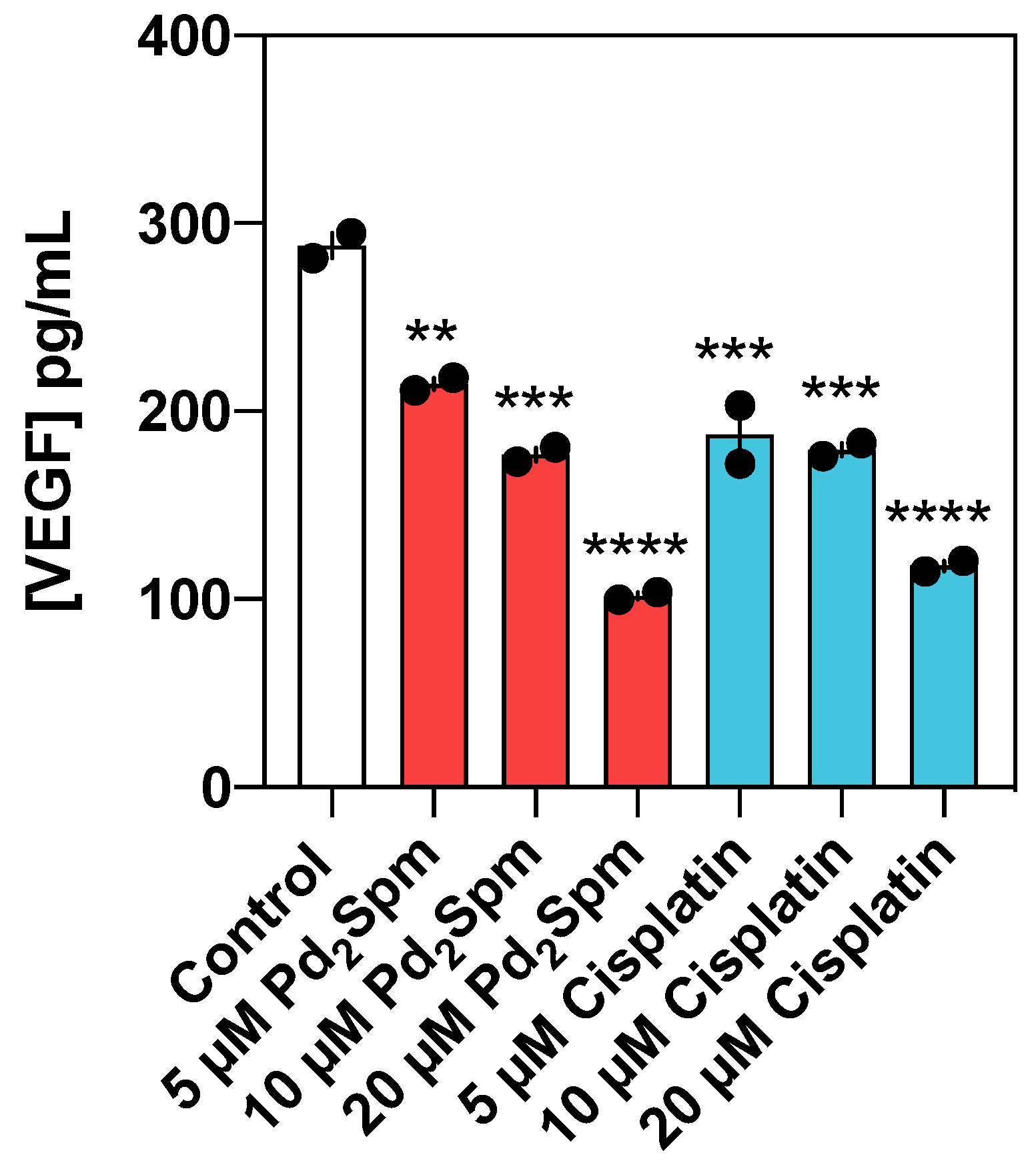
3.7. Pd2Spm Suppressed Neovascularization in Chorioallantoic Membrane and Reduced VEGF Production in MDA-MB-231 Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Wu, Q.; Siddharth, S.; Sharma, D. Triple Negative Breast Cancer: A Mountain Yet to Be Scaled Despite the Triumphs. Cancers 2021, 13, 3697. [Google Scholar] [CrossRef]
- Bianchini, G.; De Angelis, C.; Licata, L.; Gianni, L. Treatment Landscape of Triple-Negative Breast Cancer—Expanded Options, Evolving Needs. Nat. Rev. Clin. Oncol. 2021, 274. [Google Scholar] [CrossRef]
- Manjunath, M.; Choudhary, B. Triple-negative Breast Cancer: A Run-through of Features, Classification and Current Therapies (Review). Oncol. Lett. 2021, 22, 512. [Google Scholar] [CrossRef]
- Yang, R.; Shi, Y.-Y.; Han, X.-H.; Liu, S. The Impact of Platinum-Containing Chemotherapies in Advanced Triple-Negative Breast Cancer: Meta-Analytical Approach to Evaluating Its Efficacy and Safety. Oncol. Res. Treat. 2021, 44, 333–343. [Google Scholar] [CrossRef]
- Crona, D.J.; Faso, A.; Nishijima, T.F.; McGraw, K.A.; Galsky, M.D.; Milowsky, M.I. A Systematic Review of Strategies to Prevent Cisplatin-Induced Nephrotoxicity. Oncologist 2017, 22, 609. [Google Scholar] [CrossRef] [Green Version]
- Vojtek, M.; Marques, M.P.M.; Ferreira, I.M.P.L.V.O.; Mota-Filipe, H.; Diniz, C. Anticancer Activity of Palladium-Based Complexes against Triple-Negative Breast Cancer. Drug Discov. Today 2019, 24, 1044–1058. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.S.; Batista de Carvalho, A.L.M.; Lamego, I.; Marques, M.P.M.; Gil, A.M. Cytotoxicity of Platinum and Palladium Chelates against Osteosarcoma. ChemistrySelect 2020, 5, 5993–6000. [Google Scholar] [CrossRef]
- Fiuza, S.M.; Holy, J.; Batista de Carvalho, L.A.E.; Marques, M.P.M. Biologic Activity of a Dinuclear Pd(II)-Spermine Complex Toward Human Breast Cancer. Chem. Biol. Drug Des. 2011, 77, 477–488. [Google Scholar] [CrossRef] [Green Version]
- Tummala, R.; Diegelman, P.; Fiuza, S.M.; Batista de Carvalho, L.A.E.; Marques, M.P.M.; Kramer, D.L.; Clark, K.; Vujcic, S.; Porter, C.W.; Pendyala, L. Characterization of Pt-, Pd-Spermine Complexes for Their Effect on Polyamine Pathway and Cisplatin Resistance in A2780 Ovarian Carcinoma Cells. Oncol. Rep. 2010, 24, 15–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Batista de Carvalho, A.L.M.; Medeiros, P.S.C.; Costa, F.M.; Ribeiro, V.P.; Sousa, J.B.; Diniz, C.; Marques, M.P.M. Anti-Invasive and Anti-Proliferative Synergism between Docetaxel and a Polynuclear Pd-Spermine Agent. PLoS ONE 2016, 11, e0167218. [Google Scholar] [CrossRef] [Green Version]
- Farrell, N.P. Multi-Platinum Anti-Cancer Agents. Substitution-Inert Compounds for Tumor Selectivity and New Targets. Chem. Soc. Rev. 2015, 44, 8773–8785. [Google Scholar] [CrossRef]
- Allardyce, C.S.; Dyson, P.J. Metal-Based Drugs That Break the Rules. Dalt. Trans. 2016, 45, 3201–3209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marques, M.P.M. Platinum and Palladium Polyamine Complexes as Anticancer Agents: The Structural Factor. ISRN Spectrosc. 2013, 2013, 1–29. [Google Scholar] [CrossRef]
- Vojtek, M.; Gonçalves-Monteiro, S.; Pinto, E.; Kalivodová, S.; Almeida, A.; Marques, M.P.M.; de Carvalho, A.L.M.B.; Martins, C.B.; Mota-Filipe, H.; Ferreira, I.M.P.L.V.O.; et al. Preclinical Pharmacokinetics and Biodistribution of Anticancer Dinuclear Palladium(II)-Spermine Complex (Pd2Spm) in Mice. Pharmaceuticals 2021, 14, 173. [Google Scholar] [CrossRef] [PubMed]
- Marques, M.P.M.; Batista De Carvalho, A.L.M.; Sakai, V.G.; Hatter, L.; Batista De Carvalho, L.A.E. Intracellular Water-an Overlooked Drug Target? Cisplatin Impact in Cancer Cells Probed by Neutrons. Phys. Chem. Chem. Phys. 2017, 19, 2702–2713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marques, M.P.M.; Batista de Carvalho, A.L.M.; Mamede, A.P.; Dopplapudi, A.; Rudić, S.; Tyagi, M.; Garcia Sakai, V.; Batista de Carvalho, L.A.E. A New Look into the Mode of Action of Metal-Based Anticancer Drugs. Molecules 2020, 25, 246. [Google Scholar] [CrossRef] [Green Version]
- Marques, M.P.M.; Batista de Carvalho, A.L.M.; Mamede, A.P.; Rudić, S.; Dopplapudi, A.; García Sakai, V.; Batista de Carvalho, L.A.E. Intracellular Water as a Mediator of Anticancer Drug Action. Int. Rev. Phys. Chem. 2020, 39, 67–81. [Google Scholar] [CrossRef]
- Carneiro, T.J.; Araújo, R.; Vojtek, M.; Gonçalves-Monteiro, S.; Diniz, C.; de Carvalho, A.L.M.B.; Marques, M.P.M.; Gil, A.M. Novel Insights into Mice Multi-Organ Metabolism upon Exposure to a Potential Anticancer Pd(II)-Agent. Metabolites 2021, 11, 114. [Google Scholar] [CrossRef] [PubMed]
- Codina, G.; Caubet, A.; López, C.; Moreno, V.; Molins, E. Palladium(II) and Platinum(II) Polyamine Complexes: X-Ray Crystal Structures of (SP-4-2)-Chloro{N-[(3-Amino-ΚN) Propyl]Propane-1,3-Diamine- ΚN,ΚN’}palladium(1+) Tetrachloropalladate (2-) (2:1) and (R,S)- Tetrachloro[μ-(Spermine)]Dipalladium(II) (={μ-{N,N’. Helv. Chim. Acta 1999, 82, 1025–1037. [Google Scholar] [CrossRef]
- Fiuza, S.M.; Amado, A.M.; Parker, S.F.; Marques, M.P.M.; Batista De Carvalho, L.A.E. Conformational Insights and Vibrational Study of a Promising Anticancer Agent: The Role of the Ligand in Pd(II)-Amine Complexes. New J. Chem. 2015, 39, 6274–6283. [Google Scholar] [CrossRef] [Green Version]
- Batista de Carvalho, L.A.E.; Marques, M.P.M.; Martin, C.; Parker, S.F.; Tomkinson, J. Inelastic Neutron Scattering Study of Pt II Complexes Displaying Anticancer Properties. ChemPhysChem 2011, 12, 1334–1341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azar, H.A.; Hansen, C.T.; Costa, J. N:NIH(S)II-Nu/Nu Mice With Combined Immunodeficiency: A New Model for Human Tumor Heterotransplantation. J. Natl. Cancer Inst. 1980, 65, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, G.; Gullberg, B.; Hafström, L. Estimation of Liver Tumor Volume Using Different Formulas-An Experimental Study in Rats. J. Cancer Res. Clin. Oncol. 1983, 105, 20–23. [Google Scholar] [CrossRef]
- Elston, C.W.; Ellis, I.O. Pathological Prognostic Factors in Breast Cancer. I. The Value of Histological Grade in Breast Cancer: Experience from a Large Study with Long-Term Follow-Up. Histopathology 1991, 19, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Law, A.M.K.; Yin, J.X.M.; Castillo, L.; Young, A.I.J.; Piggin, C.; Rogers, S.; Caldon, C.E.; Burgess, A.; Millar, E.K.A.; O’Toole, S.A.; et al. Andy’s Algorithms: New Automated Digital Image Analysis Pipelines for FIJI. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef]
- Ribatti, D.; Vacca, A.; Roncali, L.; Dammacco, F. The Chick Embryo Chorioallantoic Membrane as a Model for in Vivo Research on Anti-Angiogenesis. Curr. Pharm. Biotechnol. 2000, 1, 73–82. [Google Scholar] [CrossRef]
- Carpentier, G.; Martinelli, M.; Courty, J.; Cascone, I. Angiogenesis Analyzer for ImageJ. In 4th ImageJ User and Developer Conference Proceedings; Mondorf-les-Bains: Luxembourg, 2012; pp. 198–201. [Google Scholar]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE Guidelines 2.0: Updated Guidelines for Reporting Animal Research. PLoS Biol. 2020, 18, e3000410. [Google Scholar] [CrossRef]
- Sun, X.; Kaufman, P.D. Ki-67: More than a Proliferation Marker. Chromosoma 2018, 127, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D.; Nico, B.; Ruggieri, S.; Tamma, R.; Simone, G.; Mangia, A. Angiogenesis and Antiangiogenesis in Triple-Negative Breast Cancer. Transl. Oncol. 2016, 9, 453–457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tabrizi, L.; Olasunkanmi, L.O.; Fadare, O.A. Experimental and Theoretical Investigations of Cyclometalated Ruthenium(II) Complex Containing CCC-Pincer and Anti-Inflammatory Drugs as Ligands: Synthesis, Characterization, Inhibition of Cyclooxygenase and in Vitro Cytotoxicity Activities in Various Can. Dalt. Trans. 2019, 48, 728–740. [Google Scholar] [CrossRef]
- Navarro-Ranninger, C.; Zamora, F.; Masaguer, J.R.; Pérez, J.M.; González, V.M.; Alonso, C. Palladium(II) Compounds of Putrescine and Spermine. Synthesis, Characterization, and DNA-Binding and Antitumor Properties. J. Inorg. Biochem. 1993, 52, 37–49. [Google Scholar] [CrossRef]
- Perše, M. Cisplatin Mouse Models: Treatment, Toxicity and Translatability. Biomedicines 2021, 9, 1406. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, N.; Murata, K.; Fuke, H.; Inoue, T.; Yamanaka, Y.; Saitou, Y.; Ito, K.; Sugimoto, K.; Koyama, M.; Shiraki, K.; et al. Macrocytic Anemia during Low-Dose Cisplatin and 5-Fluorouracil through Implanted Infusion Port for Unresectable Hepatobilliary Malignancies. Anticancer Res. 2005, 25, 246. [Google Scholar]
- Fei, L.; Huimei, H.; Dongmin, C. Pivalopril Improves Anti-Cancer Efficiency of CDDP in Breast Cancer through Inhibiting Proliferation, Angiogenesis and Metastasis. Biochem. Biophys. Res. Commun. 2020, 533, 853–860. [Google Scholar] [CrossRef] [PubMed]
- Duyndam, M.C.A.; van Berkel, M.P.A.; Dorsman, J.C.; Rockx, D.A.P.; Pinedo, H.M.; Boven, E. Cisplatin and Doxorubicin Repress Vascular Endothelial Growth Factor Expression and Differentially Down-Regulate Hypoxia-Inducible Factor I Activity in Human Ovarian Cancer Cells. Biochem. Pharmacol. 2007, 74, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Aydinlik, S.; Uvez, A.; Kiyan, H.T.; Gurel-Gurevin, E.; Yilmaz, V.T.; Ulukaya, E.; Armutak, E.I. Palladium (II) Complex and Thalidomide Intercept Angiogenic Signaling via Targeting FAK/Src and Erk/Akt/PLCγ Dependent Autophagy Pathways in Human Umbilical Vein Endothelial Cells. Microvasc. Res. 2021, 138, 104229. [Google Scholar] [CrossRef] [PubMed]
- Ulukaya, E.; Ari, F.; Dimas, K.; Ikitimur, E.I.; Guney, E.; Yilmaz, V.T. Anti-Cancer Activity of a Novel Palladium(II) Complex on Human Breast Cancer Cells in Vitro and in Vivo. Eur. J. Med. Chem. 2011, 46, 4957–4963. [Google Scholar] [CrossRef] [PubMed]
- Ari, F.; Cevatemre, B.; Armutak, E.I.I.; Aztopal, N.; Yilmaz, V.T.; Ulukaya, E. Apoptosis-Inducing Effect of a Palladium(II) Saccharinate Complex of Terpyridine on Human Breast Cancer Cells in Vitro and in Vivo. Bioorg. Med. Chem. 2014, 22, 4948–4954. [Google Scholar] [CrossRef] [PubMed]
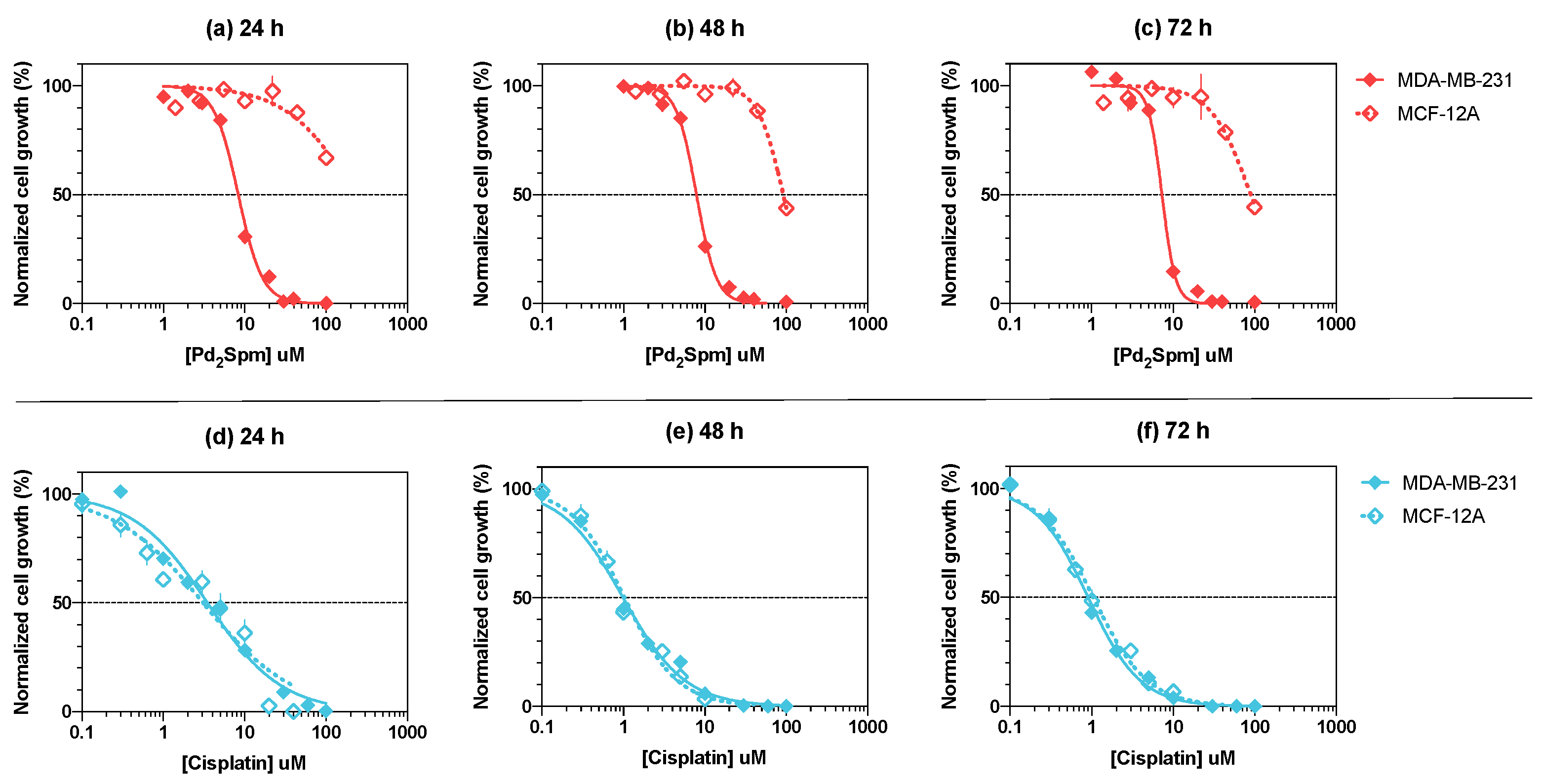



| Drug | Time | MDA-MB-231 | MCF-12A | p-Value a | ||
|---|---|---|---|---|---|---|
| IC50 in µM [95% CI] | logIC50 ± SEM | IC50 in µM [95% CI] | logIC50 ± SEM | |||
| Pd2Spm | 24 h | 8.3 [7.4–9.3] | −5.081 ± 0.024 | 228.9 [107.9–114412] | −3.640 ± 0.215 | 0.0006 |
| 48 h | 7.9 [7.1–8.8] | −5.105 ± 0.022 | 91.5 [81.5–104.3] | −4.039 ± 0.023 | <0.0001 | |
| 72 h | 7.3 [6.6–8.0] | −5.137 ± 0.021 | 89.5 [66.4–159.0] | −4.048 ± 0.068 | <0.0001 | |
| Cisplatin | 24 h | 3.5 [2.9–4.1] | −5.459 ± 0.036 | 3.1 [2.3–4.1] | −5.510 ± 0.062 | 0.5308 |
| 48 h | 1.0 [0.9–1.1] | −6.005 ± 0.027 | 1.0 [0.9–1.1] | −5.988 ± 0.029 | 0.6954 | |
| 72 h | 0.9 [0.8–1.0] | −6.043 ± 0.020 | 1.0 [0.9–1.2] | −5.981 ± 0.026 | 0.1176 | |
| Drug | logEC50 | p-Value |
|---|---|---|
| Pd2Spm | −4.243 ± 0.113 (57.2) | 0.631 |
| Cisplatin | −4.329 ± 0.130 (46.9) |
| Relative Organ Weight (%) a | |||
|---|---|---|---|
| Organ | Vehicle | Cisplatin 2 mg/kg/day | Pd2Spm 5 mg/kg/day |
| (n = 5) | (n = 7) | (n = 7) | |
| Liver | 6.08 ± 0.55 | 6.50 ± 0.34 | 6.14 ± 0.29 |
| Heart | 0.53 ± 0.06 | 0.53 ± 0.06 | 0.53 ± 0.06 |
| Lungs | 0.73 ± 0.11 | 0.77 ± 0.08 | 0.77 ± 0.08 |
| Spleen | 0.31 ± 0.10 | 0.33 ± 0.08 | 0.32 ± 0.11 |
| Brain | 1.85 ± 0.10 | 1.79 ± 0.14 | 1.79 ± 0.16 |
| Kidneys | 1.46 ± 0.05 | 1.29 ± 0.10 * | 1.43 ± 0.10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vojtek, M.; Gonçalves-Monteiro, S.; Šeminská, P.; Valová, K.; Bellón, L.; Dias-Pereira, P.; Marques, F.; Marques, M.P.M.; Batista de Carvalho, A.L.M.; Mota-Filipe, H.; et al. Pd2Spermine Complex Shows Cancer Selectivity and Efficacy to Inhibit Growth of Triple-Negative Breast Tumors in Mice. Biomedicines 2022, 10, 210. https://doi.org/10.3390/biomedicines10020210
Vojtek M, Gonçalves-Monteiro S, Šeminská P, Valová K, Bellón L, Dias-Pereira P, Marques F, Marques MPM, Batista de Carvalho ALM, Mota-Filipe H, et al. Pd2Spermine Complex Shows Cancer Selectivity and Efficacy to Inhibit Growth of Triple-Negative Breast Tumors in Mice. Biomedicines. 2022; 10(2):210. https://doi.org/10.3390/biomedicines10020210
Chicago/Turabian StyleVojtek, Martin, Salomé Gonçalves-Monteiro, Patrícia Šeminská, Katarína Valová, Loreto Bellón, Patrícia Dias-Pereira, Franklim Marques, Maria P. M. Marques, Ana L. M. Batista de Carvalho, Helder Mota-Filipe, and et al. 2022. "Pd2Spermine Complex Shows Cancer Selectivity and Efficacy to Inhibit Growth of Triple-Negative Breast Tumors in Mice" Biomedicines 10, no. 2: 210. https://doi.org/10.3390/biomedicines10020210
APA StyleVojtek, M., Gonçalves-Monteiro, S., Šeminská, P., Valová, K., Bellón, L., Dias-Pereira, P., Marques, F., Marques, M. P. M., Batista de Carvalho, A. L. M., Mota-Filipe, H., Ferreira, I. M. P. L. V. O., & Diniz, C. (2022). Pd2Spermine Complex Shows Cancer Selectivity and Efficacy to Inhibit Growth of Triple-Negative Breast Tumors in Mice. Biomedicines, 10(2), 210. https://doi.org/10.3390/biomedicines10020210