The Association of CD8+ Cytotoxic T Cells and Granzyme B+ Lymphocytes with Immunosuppressive Factors, Tumor Stage and Prognosis in Cutaneous Melanoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Histological Specimens
2.2. Immunohistochemistry
2.3. GrB + FoxP3 Immunofluorescence Double Stainings
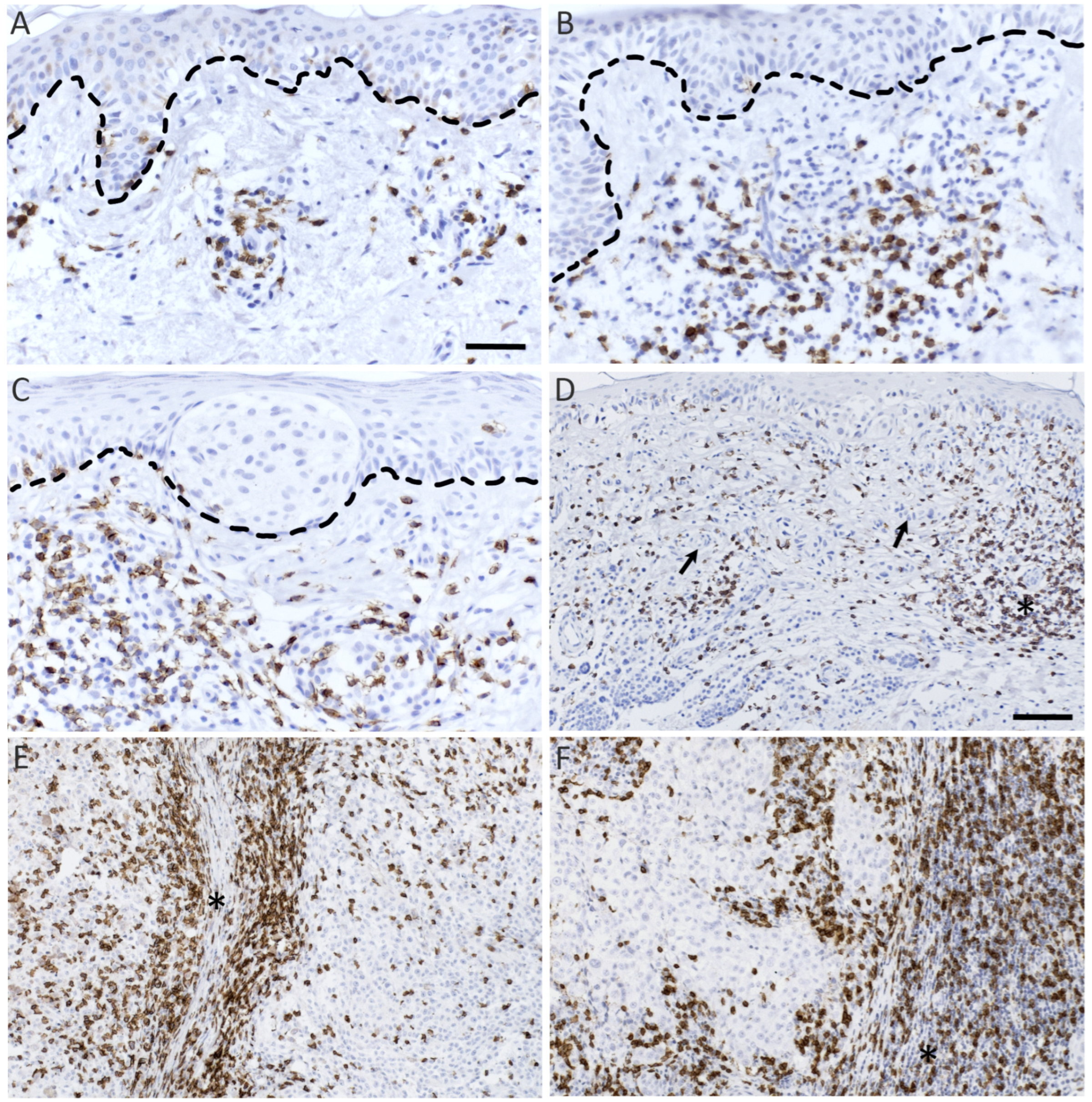
2.4. Analysis of CD8 and GrB Stained Samples
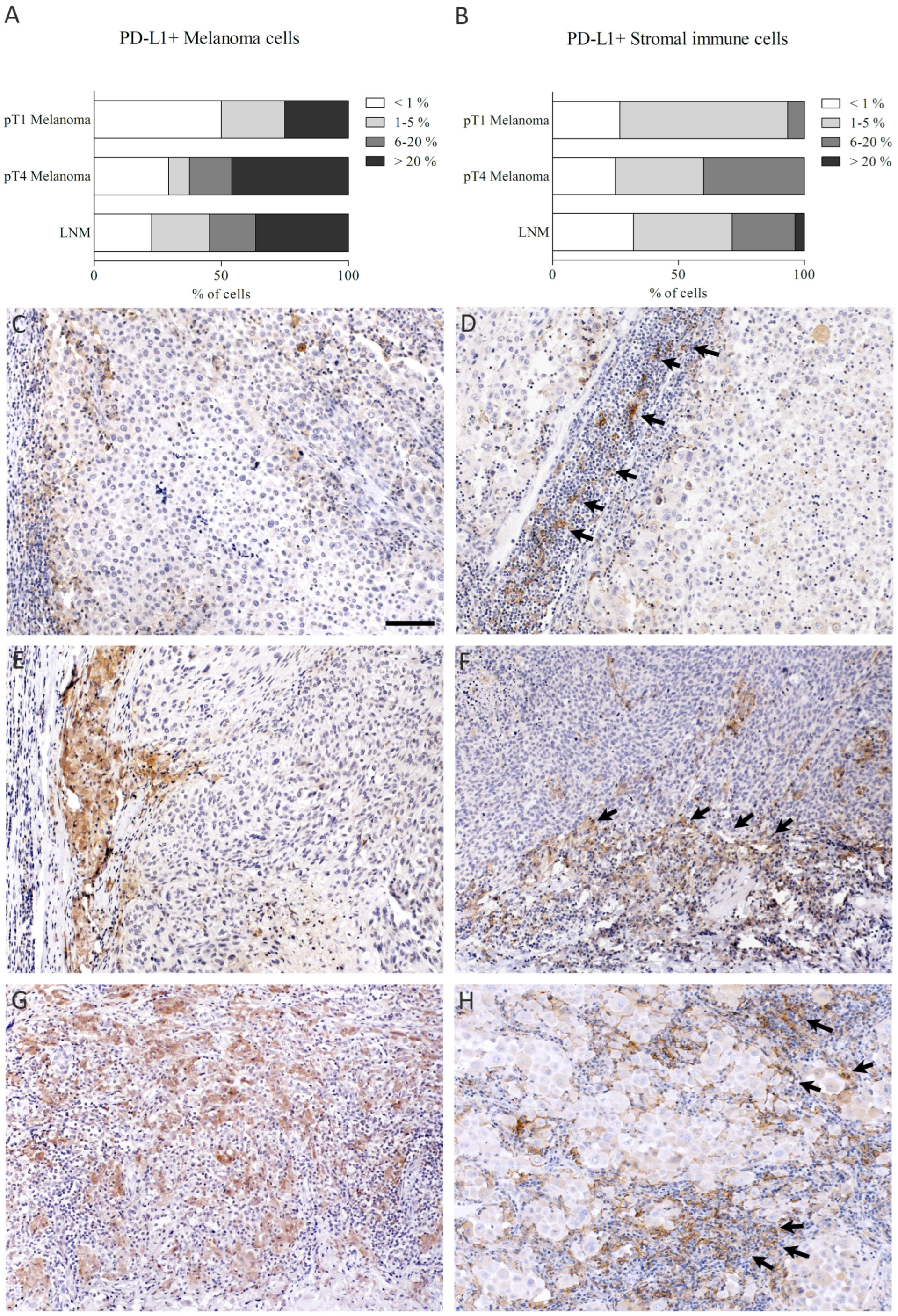
2.5. Analysis of PD-L1 Stainings
2.6. Statistical Analysis
3. Results
3.1. Patient Characteristics
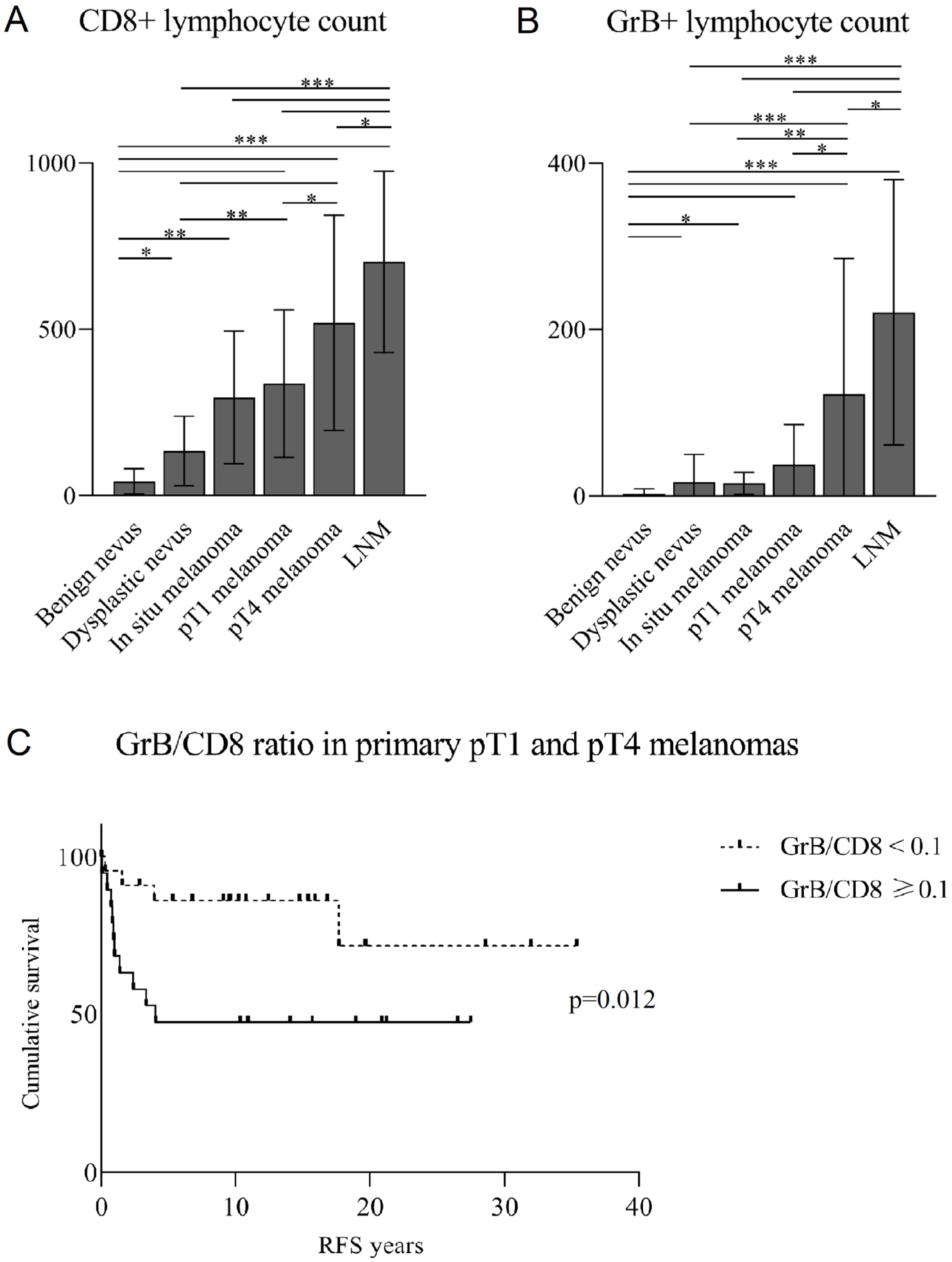
3.2. CD8+ and GrB+ Lymphocytes Associate Positively with Breslow’s Depth
3.3. The Association of CD8+ and GrB+ Lymphocytes with Clinicopathological Parameters
3.4. A Low GrB/CD8 Ratio Is an Independent Positive Prognostic Factor for Non-Immunotherapy-Related RFS in Primary Melanomas
3.5. The Association of PD-L1 Expression with Tumor Stage and Clinicopathological Parameters
3.6. The Association of CD8+ and GrB+ Lymphocytes with Immunosuppressive Factors
3.7. The Association of Tumor and Stromal Immune Cell PD-L1 Expression with Other Immunosuppressive Factors
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Davis, L.E.; Shalin, S.C.; Tackett, A.J. Current state of melanoma diagnosis and treatment. Cancer. Biol. Ther. 2019, 20, 1366–1379. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, F.; Shields, B.; Makhoul, I.; Avaritt, N.; Wong, H.K.; Hutchins, L.F.; Shalin, S.; Tackett, A.J. Immune surveillance in melanoma: From immune attack to melanoma escape and even counterattack. Cancer. Biol. Ther. 2017, 18, 451–469. [Google Scholar] [CrossRef]
- Davoodzadeh Gholami, M.; Kardar, G.A.; Saeedi, Y.; Heydari, S.; Garssen, J.; Falak, R. Exhaustion of T lymphocytes in the tumor microenvironment: Significance and effective mechanisms. Cell Immunol. 2017, 322, 1–14. [Google Scholar] [CrossRef]
- Passarelli, A.; Mannavola, F.; Stucci, L.S.; Tucci, M.; Silvestris, F. Immune system and melanoma biology: A balance between immunosurveillance and immune escape. Oncotarget 2017, 8, 106132–106142. [Google Scholar] [CrossRef]
- Boon, T.; Coulie, P.G.; Van den Eynde Benoît, J.; van der Bruggen, P. Human T cell responses against melanoma. Annu. Rev. Immunol. 2006, 24, 175–208. [Google Scholar] [CrossRef] [PubMed]
- Oble, D.A.; Loewe, R.; Yu, P.; Mihm, M.C. Focus on TILs: Prognostic significance of tumor infiltrating lymphocytes in human melanoma. Cancer Immun. 2009, 9, 3. [Google Scholar] [PubMed]
- Olbryt, M.; Rajczykowski, M.; Widłak, W. Biological Factors behind Melanoma Response to Immune Checkpoint Inhibitors. Int. J. Mol. Sci. 2020, 21, 4071. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Chen, N.; Ge, C.; Li, R.; Li, Z.; Zeng, B.; Li, C.; Wang, Y.; Xue, Y.; Song, X.; et al. Prognostic value of tumor-infiltrating lymphocytes in melanoma: A systematic review and meta-analysis. Oncoimmunology 2019, 8, 1593806. [Google Scholar] [CrossRef]
- Gartrell, R.D.; Marks, D.K.; Hart, T.D.; Li, G.; Davari, D.R.; Wu, A.; Blake, Z.; Lu, Y.; Askin, K.N.; Monod, A.; et al. Quantitative Analysis of Immune Infiltrates in Primary Melanoma. Cancer Immunol. Res. 2018, 6, 481–493. [Google Scholar] [CrossRef]
- Erdag, G.; Schaefer, J.T.; Smolkin, M.E.; Deacon, D.H.; Shea, S.M.; Dengel, L.T.; Patterson, J.W.; Slingluff, C.L. Immunotype and immunohistologic characteristics of tumor-infiltrating immune cells are associated with clinical outcome in metastatic melanoma. Cancer Res. 2012, 72, 1070–1080. [Google Scholar] [CrossRef]
- Kakavand, H.; Vilain, R.E.; Wilmott, J.S.; Burke, H.; Yearley, J.H.; Thompson, J.F.; Hersey, P.; Long, G.V.; Scolyer, R.A. Tumor PD-L1 expression, immune cell correlates and PD-1+ lymphocytes in sentinel lymph node melanoma metastases. Mod. Pathol. 2015, 28, 1535–1544. [Google Scholar] [CrossRef]
- Tumeh, P.C.; Harview, C.L.; Yearley, J.H.; Shintaku, I.P.; Taylor, E.J.M.; Robert, L.; Chmielowski, B.; Spasic, M.; Henry, G.; Ciobanu, V.; et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014, 515, 568–571. [Google Scholar] [CrossRef]
- Sebestyén, T.; Mohos, A.; Liszkay, G.; Somlai, B.; Gaudi, I.; Ladányi, A. Correlation with lymphocyte infiltration, but lack of prognostic significance of MECA-79-positive high endothelial venules in primary malignant melanoma. Melanoma Res. 2018, 28, 304–310. [Google Scholar] [CrossRef]
- Voskoboinik, I.; Whisstock, J.C.; Trapani, J.A. Perforin and granzymes: Function, dysfunction and human pathology. Nat. Rev. Immunol. 2015, 15, 388–400. [Google Scholar] [CrossRef]
- Prager, I.; Liesche, C.; van Ooijen, H.; Urlaub, D.; Verron, Q.; Sandström, N.; Fasbender, F.; Claus, M.; Eils, R.; Beaudouin, J.; et al. NK cells switch from granzyme B to death receptor-mediated cytotoxicity during serial killing. J. Exp. Med. 2019, 216, 2113–2127. [Google Scholar] [CrossRef]
- Prizment, A.E.; Vierkant, R.A.; Smyrk, T.C.; Tillmans, L.S.; Nelson, H.H.; Lynch, C.F.; Pengo, T.; Thibodeau, S.N.; Church, T.R.; Cerhan, J.R.; et al. Cytotoxic T Cells and Granzyme B Associated with Improved Colorectal Cancer Survival in a Prospective Cohort of Older Women. Cancer Epidemiol. Biomark. Prev. 2017, 26, 622–631. [Google Scholar] [CrossRef]
- Vycital, O.; Dubova, M.; Palek, R.; Hosek, P.; Branzovsky, J.; Treska, V.; Daum, O.; Liska, V. The Impact of Immune Interaction on the Metastatic Infiltration of Colorectal Carcinoma to Lymph Nodes. Anticancer Res. 2018, 38, 4159–4167. [Google Scholar] [CrossRef]
- Wang, W.; Zou, R.; Qiu, Y.; Liu, J.; Xin, Y.; He, T.; Qiu, Z. Interaction Networks Converging on Immunosuppressive Roles of Granzyme B: Special Niches Within the Tumor Microenvironment. Front. Immunol. 2021, 12, 670324. [Google Scholar] [CrossRef]
- van Houdt, I.S.; Sluijter, B.J.R.; van Leeuwen, P.M.; Moesbergen, L.M.; Hooijberg, E.; Meijer CJ, L.M.; de Gruijl, T.D.; Oudejans, J.J.; Boven, E. Absence of Granzyme B positive tumour-infiltrating lymphocytes in primary melanoma excisional biopsies is strongly associated with the presence of sentinel lymph node metastasis. Cell Oncol. 2009, 31, 407–413. [Google Scholar] [CrossRef]
- Sun, C.; Mezzadra, R.; Schumacher, T.N. Regulation and Function of the PD-L1 Checkpoint. Immunity 2018, 48, 434–452. [Google Scholar] [CrossRef]
- Hino, R.; Kabashima, K.; Kato, Y.; Yagi, H.; Nakamura, M.; Honjo, T.; Okazaki, T.; Tokura, Y. Tumor cell expression of programmed cell death-1 ligand 1 is a prognostic factor for malignant melanoma. Cancer 2010, 116, 1757–1766. [Google Scholar] [CrossRef]
- Ren, M.; Dai, B.; Kong, Y.; Lv, J.; Cai, X. PD-L1 expression in tumour-infiltrating lymphocytes is a poor prognostic factor for primary acral melanoma patients. Histopathology 2018, 73, 386–396. [Google Scholar] [CrossRef]
- Gadiot, J.; Hooijkaas, A.I.; Kaiser, A.D.M.; van Tinteren, H.; van Boven, H.; Blank, C. Overall survival and PD-L1 expression in metastasized malignant melanoma. Cancer 2011, 117, 2192–2201. [Google Scholar] [CrossRef]
- Salmi, S.; Siiskonen, H.; Sironen, R.; Tyynelä-Korhonen, K.; Hirschovits-Gerz, B.; Valkonen, M.; Auvinen, P.; Pasonen-Seppänen, S. The number and localization of CD68+ and CD163+ macrophages in different stages of cutaneous melanoma. Melanoma Res. 2019, 29, 237–247. [Google Scholar] [CrossRef]
- Salmi, S.; Lin, A.; Hirschovits-Gerz, B.; Valkonen, M.; Aaltonen, N.; Sironen, R.; Siiskonen, H.; Pasonen-Seppänen, S. The role of FoxP3+ regulatory T cells and IDO+ immune and tumor cells in malignant melanoma—An immunohistochemical study. BMC Cancer 2021, 21, 641. [Google Scholar] [CrossRef]
- Wong, P.F.; Wei, W.; Smithy, J.W.; Acs, B.; Toki, M.I.; Blenman, K.R.M.; Zelterman, D.; Kluger, H.M.; Rimm, D.L. Multiplex Quantitative Analysis of Tumor-Infiltrating Lymphocytes and Immunotherapy Outcome in Metastatic Melanoma. Clin. Cancer Res. 2019, 25, 2442–2449. [Google Scholar] [CrossRef]
- Lindner, S.; Dahlke, K.; Sontheimer, K.; Hagn, M.; Kaltenmeier, C.; Barth, T.F.E.; Beyer, T.; Reister, F.; Fabricius, D.; Lotfi, R.; et al. Interleukin 21-induced granzyme B-expressing B cells infiltrate tumors and regulate T cells. Cancer Res. 2013, 73, 2468–2479. [Google Scholar] [CrossRef]
- Sabbatino, F.; Scognamiglio, G.; Liguori, L.; Marra, A.; Anniciello, A.M.; Polcaro, G.; Dal Col, J.; Caputo, A.; Peluso, A.L.; Botti, G.; et al. Peritumoral Immune Infiltrate as a Prognostic Biomarker in Thin Melanoma. Front. Immunol. 2020, 11, 561390. [Google Scholar] [CrossRef]
- Wu, X.; Wang, X.; Zhao, Y.; Li, K.; Yu, B.; Zhang, J. Granzyme family acts as a predict biomarker in cutaneous melanoma and indicates more benefit from anti-PD-1 immunotherapy. Int. J. Med. Sci. 2021, 18, 1657–1669. [Google Scholar] [CrossRef]
- Giavina-Bianchi, M.; Giavina-Bianchi, P.; Sotto, M.N.; Rodig, S.; Mihm, M.; Festa Neto, C.; Duncan, L.M.; Kalil, J. Nodular primary cutaneous melanoma is associated with PD-L1 expression. Eur. J. Dermatol. 2020, 30, 352–357. [Google Scholar] [CrossRef]
- Škuciová, V.; Drahošová, S.; Výbohová, D.; Cígerová, V.; Adamkov, M. The relationships between PD-L1 expression, CD8+ TILs and clinico-histomorphological parameters in malignant melanomas. Pathol. Res. Pract. 2020, 216, 153071. [Google Scholar] [CrossRef] [PubMed]
- Kluger, H.M.; Zito, C.R.; Barr, M.L.; Baine, M.K.; Chiang, V.L.S.; Sznol, M.; Rimm, D.L.; Chen, L.; Jilaveanu, L.B. Characterization of PD-L1 Expression and Associated T-cell Infiltrates in Metastatic Melanoma Samples from Variable Anatomic Sites. Clin. Cancer Res. 2015, 21, 3052–3060. [Google Scholar] [CrossRef] [PubMed]
- Spranger, S.; Spaapen, R.M.; Zha, Y.; Williams, J.; Meng, Y.; Ha, T.T.; Gajewski, T.F. Up-regulation of PD-L1, IDO, and T(regs) in the melanoma tumor microenvironment is driven by CD8(+) T cells. Sci. Transl. Med. 2013, 5, 200ra116. [Google Scholar] [CrossRef] [PubMed]
- Toker, A.; Nguyen, L.T.; Stone, S.C.; Yang, S.Y.C.; Katz, S.R.; Shaw, P.A.; Clarke, B.A.; Ghazarian, D.; Al-Habeeb, A.; Easson, A.; et al. Regulatory T Cells in Ovarian Cancer Are Characterized by a Highly Activated Phenotype Distinct from that in Melanoma. Clin. Cancer Res. 2018, 24, 5685–5696. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.Y.; Garcet, S.; Gulati, N.; Kiecker, F.; Fuentes-Duculan, J.; Gilleaudeau, P.; Sullivan-Whalen, M.; Shemer, A.; Mitsui, H.; Krueger, J.G. Novel immune signatures associated with dysplastic naevi and primary cutaneous melanoma in human skin. Exp. Dermatol. 2019, 28, 35–44. [Google Scholar] [CrossRef] [PubMed]

| CD8 Stainings n (%) | GrB Stainings n (%) | PD-L1 Stainings n (%) | |
|---|---|---|---|
| Benign nevi | 27 (13) | 27 (16) | |
| Dysplastic nevi | 24 (12) | 25 (15) | |
| In situ melanoma | 10 (5) | 10 (6) | |
| pT1 melanoma | 36 (18) | 29 (17) | 14 (21) |
| pT4 melanoma | 46 (23) | 37 (22) | 28 (41) |
| LNM | 60 (30) | 44 (26) | 26 (38) |
| Total | 203 (100) | 172 (100) | 68 (100) |
| CD8 Stainings n (%) | GrB Stainings n (%) | |
|---|---|---|
| pT1 melanoma | 28 (27) | 22 (28) |
| pT4 melanoma | 31 (30) | 26 (33) |
| LNM | 44 (43) | 31 (39) |
| Total | 103 (100) | 79 (100) |
| Characteristics | n (%) | |
|---|---|---|
| Total number of patients | 129 | |
| T and N classification | ||
| pT1 | 41 (32) | |
| pT4 | 41 (32) | |
| pN1 | 47 (36) | |
| Age | ||
| Mean ± SD | 59.1 ± 16.6 | |
| Range | 5–92 | |
| Sex | ||
| Female | 55 (43) | |
| Male | 74 (57) | |
| Breslow’s depth (mm) | ||
| Mean ± SD | 4.5 ± 7.5 | |
| Range | 0.2–60.0 | |
| Relapse | ||
| Yes | 66 (51) | |
| No | 55 (43) | |
| Spread at diagnosis | 6 (5) | |
| Missing | 2 (2) | |
| Anatomic site of primary melanoma | ||
| Head and neck | 27 (21) | |
| Trunk | 18 (14) | |
| Back | 34 (26) | |
| Upper limbs | 13 (10) | |
| Lower limbs | 22 (17) | |
| Feet | 7 (5) | |
| Hands | 1 (1) | |
| Fingers or toes | 2 (2) | |
| Not found | 4 (3) | |
| Cause of death | ||
| Malignant melanoma | 55 (43) | |
| Other | 17 (13) | |
| Alive | 40 (31) | |
| Unknown | 17 (13) |
| Variables | IDO+ Stromal Immune Cells | FoxP3+ Tregs | CD68+ TAMs | CD163+ TAMs | GrB+ Lymphocytes | |
|---|---|---|---|---|---|---|
| CD8+ lymphocytes | rs | 0.599 | 0.357 | 0.380 | 0.266 | 0.829 |
| p-value | <0.001 | <0.001 | <0.001 | 0.020 | <0.001 | |
| GrB+ lymphocytes | rs | 0.542 | 0.479 | 0.429 | 0.310 | |
| p-value | <0.001 | <0.001 | <0.001 | 0.009 |
| Variables | CD8+ Lymphocytes | GrB+ Lymphocytes | |||||
|---|---|---|---|---|---|---|---|
| Low n (%) | High n (%) | p-Value | Low n (%) | High n (%) | p-Value | ||
| Ulceration | <0.001 | 0.046 | |||||
| Yes | 8 (38) | 13 (62) | 8 (53) | 7 (47) | |||
| No | 32 (87) | 5 (14) | 26 (81) | 6 (19) | |||
| Growth pattern | <0.001 | 0.023 | |||||
| Nodular | 12 (43) | 16 (57) | 14 (58) | 10 (42) | |||
| Other | 28 (90) | 3 (9) | 21 (88) | 3 (13) | |||
| Presence of mitoses | 0.005 | 0.043 | |||||
| Yes | 27 (59) | 19 (41) | 26 (67) | 13 (33) | |||
| No | 13 (100) | 0 (0) | 9 (100) | 0 (0) | |||
| Lymph node capsule rupture | 0.003 | 0.035 | |||||
| Yes | 8 (57) | 6 (43) | 3 (33) | 6 (67) | |||
| No | 4 (14) | 25 (86) | 1 (5) | 20 (95) | |||
| Overall recurrence | <0.001 | <0.001 | |||||
| Yes | 18 (33) | 36 (67) | 14 (34) | 27 (66) | |||
| No | 30 (71) | 12 (28) | 24 (73) | 9 (27) | |||
| Locoregional recurrence | <0.001 | <0.001 | |||||
| Yes | 13 (29) | 32 (71) | 8 (25) | 24 (75) | |||
| No | 37 (66) | 19 (34) | 31 (69) | 14 (31) | |||
| Distal recurrence | 0.021 | 0.053 | |||||
| Yes | 19 (38) | 31 (62) | 15 (40) | 23 (61) | |||
| No | 30 (61) | 19 (39) | 24 (62) | 15 (39) | |||
| Variables | PD-L1+ Tumor Cells | PD-L1+ Stromal Immune Cells | |||||
|---|---|---|---|---|---|---|---|
| <5% n (%) | >5% n (%) | p-Value | <5% n (%) | >5% n (%) | p-Value | ||
| CD8+ lymphocytes | 0.015 | <0.001 | |||||
| Low | 18 (69) | 8 (31) | 22 (100) | 0 (0) | |||
| High | 15 (39) | 24 (62) | 20 (54) | 17 (46) | |||
| GrB+ lymphocytes | 0.009 | 0.027 | |||||
| Low | 20 (69) | 9 (31) | 22 (85) | 4 (15) | |||
| High | 10 (35) | 19 (66) | 16 (57) | 12 (43) | |||
| pT classification | 0.037 | 0.034 | |||||
| pT1 | 11 (79) | 3 (21) | 13 (93) | 1 (7) | |||
| pT4 | 12 (44) | 15 (56) | 14 (61) | 9 (39) | |||
| Ulceration | 0.069 | 0.018 | |||||
| Yes | 6 (38) | 10 (63) | 7 (50) | 7 (50) | |||
| No | 16 (67) | 8 (33) | 19 (86) | 3 (14) | |||
| Growth pattern | 0.009 | 0.001 | |||||
| Nodular | 10 (40) | 15 (60) | 11 (52) | 10 (48) | |||
| Other | 13 (81) | 3 (19) | 16 (100) | 0 (0) | |||
| Lymph node capsule rupture | 0.032 | 0.134 | |||||
| Yes | 7 (70) | 3 (30) | 8 (80) | 2 (20) | |||
| No | 4 (27) | 11 (73) | 7 (50) | 7 (50) | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salmi, S.; Hälinen, K.; Lin, A.; Suikkanen, S.; Jokelainen, O.; Rahunen, E.; Siiskonen, H.; Pasonen-Seppänen, S. The Association of CD8+ Cytotoxic T Cells and Granzyme B+ Lymphocytes with Immunosuppressive Factors, Tumor Stage and Prognosis in Cutaneous Melanoma. Biomedicines 2022, 10, 3209. https://doi.org/10.3390/biomedicines10123209
Salmi S, Hälinen K, Lin A, Suikkanen S, Jokelainen O, Rahunen E, Siiskonen H, Pasonen-Seppänen S. The Association of CD8+ Cytotoxic T Cells and Granzyme B+ Lymphocytes with Immunosuppressive Factors, Tumor Stage and Prognosis in Cutaneous Melanoma. Biomedicines. 2022; 10(12):3209. https://doi.org/10.3390/biomedicines10123209
Chicago/Turabian StyleSalmi, Satu, Kaisla Hälinen, Anton Lin, Sanna Suikkanen, Otto Jokelainen, Eija Rahunen, Hanna Siiskonen, and Sanna Pasonen-Seppänen. 2022. "The Association of CD8+ Cytotoxic T Cells and Granzyme B+ Lymphocytes with Immunosuppressive Factors, Tumor Stage and Prognosis in Cutaneous Melanoma" Biomedicines 10, no. 12: 3209. https://doi.org/10.3390/biomedicines10123209
APA StyleSalmi, S., Hälinen, K., Lin, A., Suikkanen, S., Jokelainen, O., Rahunen, E., Siiskonen, H., & Pasonen-Seppänen, S. (2022). The Association of CD8+ Cytotoxic T Cells and Granzyme B+ Lymphocytes with Immunosuppressive Factors, Tumor Stage and Prognosis in Cutaneous Melanoma. Biomedicines, 10(12), 3209. https://doi.org/10.3390/biomedicines10123209




