Where Do We Stand with Immunotherapy for Advanced Pancreatic Ductal Adenocarcinoma: A Synopsis of Clinical Outcomes
Abstract
1. Introduction

2. Immune-Checkpoint Inhibitors
2.1. Blockade of Cytotoxic T-Lymphocyte-Associated Antigen 4
2.2. Blockade of Programmed Cell Death-1 with Its Ligands
3. Adoptive CAR T-Cell Therapy
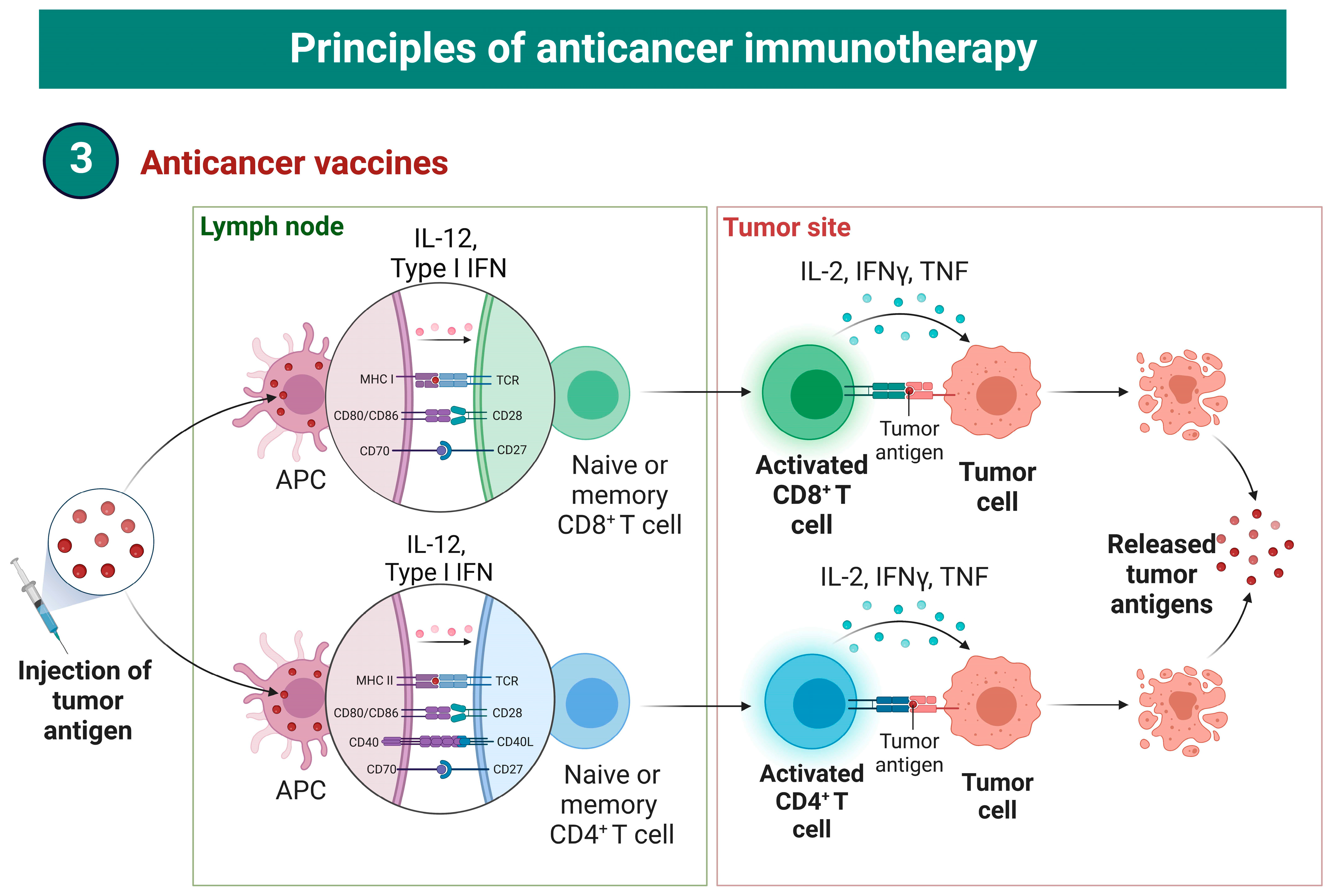
4. Cancer Vaccines
4.1. Cell-Based Vaccines
4.2. Peptide-Based Vaccines
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Huang, J.; Lok, V.; Ngai, C.H.; Zhang, L.; Yuan, J.; Lao, X.Q.; Ng, K.; Chong, C.; Zheng, Z.J.; Wong, M.C.S. Worldwide Burden of, Risk Factors for, and Trends in Pancreatic Cancer. Gastroenterology 2021, 160, 744–754. [Google Scholar] [CrossRef] [PubMed]
- Makohon-Moore, A.; Iacobuzio-Donahue, C.A. Pancreatic cancer biology and genetics from an evolutionary perspective. Nat. Rev. Cancer 2016, 16, 553–565. [Google Scholar] [CrossRef] [PubMed]
- Rishi, A.; Goggins, M.; Wood, L.D.; Hruban, R.H. Pathological and molecular evaluation of pancreatic neoplasms. Semin. Oncol. 2015, 42, 28–39. [Google Scholar] [CrossRef]
- Bliss, L.A.; Witkowski, E.R.; Yang, C.J.; Tseng, J.F. Outcomes in operative management of pancreatic cancer. J. Surg. Oncol. 2014, 110, 592–598. [Google Scholar] [CrossRef]
- Vincent, A.; Herman, J.; Schulick, R.; Hruban, R.H.; Goggins, M. Pancreatic cancer. Lancet 2011, 378, 607–620. [Google Scholar] [CrossRef] [PubMed]
- Ducreux, M.; Cuhna, A.S.; Caramella, C.; Hollebecque, A.; Burtin, P.; Goere, D.; Seufferlein, T.; Haustermans, K.; Van Laethem, J.L.; Conroy, T.; et al. Cancer of the pancreas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2015, 26 (Suppl. S5), v56–v68. [Google Scholar] [CrossRef]
- Conroy, T.; Hammel, P.; Hebbar, M.; Ben Abdelghani, M.; Wei, A.C.; Raoul, J.L.; Chone, L.; Francois, E.; Artru, P.; Biagi, J.J.; et al. FOLFIRINOX or Gemcitabine as Adjuvant Therapy for Pancreatic Cancer. N. Engl. J. Med. 2018, 379, 2395–2406. [Google Scholar] [CrossRef]
- Von Hoff, D.D.; Ervin, T.; Arena, F.P.; Chiorean, E.G.; Infante, J.; Moore, M.; Seay, T.; Tjulandin, S.A.; Ma, W.W.; Saleh, M.N.; et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N. Engl. J. Med. 2013, 369, 1691–1703. [Google Scholar] [CrossRef]
- Pham, T.; Roth, S.; Kong, J.; Guerra, G.; Narasimhan, V.; Pereira, L.; Desai, J.; Heriot, A.; Ramsay, R. An Update on Immunotherapy for Solid Tumors: A Review. Ann. Surg. Oncol. 2018, 25, 3404–3412. [Google Scholar] [CrossRef]
- Brower, V. Checkpoint blockade immunotherapy for cancer comes of age. J. Natl. Cancer Inst. 2015, 107, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Disis, M.L. Mechanism of action of immunotherapy. Semin Oncol. 2014, 41 (Suppl. S5), S3–S13. [Google Scholar] [CrossRef]
- Herzberg, B.; Campo, M.J.; Gainor, J.F. Immune Checkpoint Inhibitors in Non-Small Cell Lung Cancer. Oncologist 2017, 22, 81–88. [Google Scholar] [CrossRef]
- Shek, D.; Akhuba, L.; Carlino, M.S.; Nagrial, A.; Moujaber, T.; Read, S.A.; Gao, B.; Ahlenstiel, G. Immune-Checkpoint Inhibitors for Metastatic Colorectal Cancer: A Systematic Review of Clinical Outcomes. Cancers 2021, 13, 4345. [Google Scholar] [CrossRef] [PubMed]
- Shek, D.; Read, S.A.; Nagrial, A.; Carlino, M.S.; Gao, B.; George, J.; Ahlenstiel, G. Immune-Checkpoint Inhibitors for Advanced Hepatocellular Carcinoma: A Synopsis of Response Rates. Oncologist 2021, 26, e1216–e1225. [Google Scholar] [CrossRef]
- Schuster, S.J.; Bishop, M.R.; Tam, C.S.; Waller, E.K.; Borchmann, P.; McGuirk, J.P.; Jager, U.; Jaglowski, S.; Andreadis, C.; Westin, J.R.; et al. Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma. N. Engl. J. Med. 2019, 380, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Munoz, J.; Goy, A.; Locke, F.L.; Jacobson, C.A.; Hill, B.T.; Timmerman, J.M.; Holmes, H.; Jaglowski, S.; Flinn, I.W.; et al. KTE-X19 CAR T-Cell Therapy in Relapsed or Refractory Mantle-Cell Lymphoma. N. Engl. J. Med. 2020, 382, 1331–1342. [Google Scholar] [CrossRef]
- Brahmer, J.R.; Tykodi, S.S.; Chow, L.Q.; Hwu, W.J.; Topalian, S.L.; Hwu, P.; Drake, C.G.; Camacho, L.H.; Kauh, J.; Odunsi, K.; et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N. Engl. J. Med. 2012, 366, 2455–2465. [Google Scholar] [CrossRef]
- Schizas, D.; Charalampakis, N.; Kole, C.; Economopoulou, P.; Koustas, E.; Gkotsis, E.; Ziogas, D.; Psyrri, A.; Karamouzis, M.V. Immunotherapy for pancreatic cancer: A 2020 update. Cancer Treat Rev. 2020, 86, 102016. [Google Scholar] [CrossRef]
- Fan, J.Q.; Wang, M.F.; Chen, H.L.; Shang, D.; Das, J.K.; Song, J. Current advances and outlooks in immunotherapy for pancreatic ductal adenocarcinoma. Mol. Cancer 2020, 19, 32. [Google Scholar] [CrossRef]
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Meyer, L.; Gress, D.M.; Byrd, D.R.; Winchester, D.P. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more "personalized" approach to cancer staging. CA Cancer J. Clin. 2017, 67, 93–99. [Google Scholar] [CrossRef]
- Yu, J.X.; Hubbard-Lucey, V.M.; Tang, J. Immuno-oncology drug development goes global. Nat. Rev. Drug Discov. 2019, 18, 899–900. [Google Scholar] [CrossRef] [PubMed]
- Carlino, M.S.; Larkin, J.; Long, G.V. Immune checkpoint inhibitors in melanoma. Lancet 2021, 398, 1002–1014. [Google Scholar] [CrossRef] [PubMed]
- Robert, C. A decade of immune-checkpoint inhibitors in cancer therapy. Nat. Commun. 2020, 11, 3801. [Google Scholar] [CrossRef]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef]
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Royal, R.E.; Levy, C.; Turner, K.; Mathur, A.; Hughes, M.; Kammula, U.S.; Sherry, R.M.; Topalian, S.L.; Yang, J.C.; Lowy, I.; et al. Phase 2 trial of single agent Ipilimumab (anti-CTLA-4) for locally advanced or metastatic pancreatic adenocarcinoma. J. Immunother. 2010, 33, 828–833. [Google Scholar] [CrossRef]
- Kamath, S.D.; Kalyan, A.; Kircher, S.; Nimeiri, H.; Fought, A.J.; Benson, A.; Mulcahy, M., 3rd. Ipilimumab and Gemcitabine for Advanced Pancreatic Cancer: A Phase Ib Study. Oncologist 2020, 25, e808–e815. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Dirix, L.; Vos, F.Y.F.L.D.; Allison, J.P.; Decoster, L.; Zaucha, R.; Park, J.O.; Vanderwalde, A.M.; Kataria, R.S.; Ferro, S.; et al. Efficacy and tolerability of tremelimumab in patients with metastatic pancreatic ductal adenocarcinoma. J. Clin. Oncol. 2018, 36 (Suppl. S4), 470. [Google Scholar] [CrossRef]
- Aglietta, M.; Barone, C.; Sawyer, M.B.; Moore, M.J.; Miller, W.H.; Bagala, C., Jr.; Colombi, F.; Cagnazzo, C.; Gioeni, L.; Wang, E.; et al. A phase I dose escalation trial of tremelimumab (CP-675,206) in combination with gemcitabine in chemotherapy-naive patients with metastatic pancreatic cancer. Ann. Oncol. 2014, 25, 1750–1755. [Google Scholar] [CrossRef]
- O’Reilly, E.M.; Oh, D.Y.; Dhani, N.; Renouf, D.J.; Lee, M.A.; Sun, W.; Fisher, G.; Hezel, A.; Chang, S.C.; Vlahovic, G.; et al. Durvalumab With or Without Tremelimumab for Patients With Metastatic Pancreatic Ductal Adenocarcinoma: A Phase 2 Randomized Clinical Trial. JAMA Oncol. 2019, 5, 1431–1438. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.Gov: Gemcitabine and Nab-Paclitaxel vs. Gemcitabine, Nab-Paclitaxel, Durvalumab and Tremelimumab as 1st Line Therapy in Metastatic Pancreatic Adenocarcinoma. Available online: https://clinicaltrialsgov/ct2/show/results/NCT02879318 (accessed on 6 November 2022).
- Renouf, D.J.; Loree, J.M.; Knox, J.J.; Topham, J.T.; Kavan, P.; Jonker, D.; Welch, S.; Couture, F.; Lemay, F.; Tehfe, M.; et al. The CCTG PA.7 phase II trial of gemcitabine and nab-paclitaxel with or without durvalumab and tremelimumab as initial therapy in metastatic pancreatic ductal adenocarcinoma. Nat. Commun. 2022, 13, 5020. [Google Scholar] [CrossRef] [PubMed]
- Lipson, E.J.; Sharfman, W.H.; Drake, C.G.; Wollner, I.; Taube, J.M.; Anders, R.A.; Xu, H.; Yao, S.; Pons, A.; Chen, L.; et al. Durable cancer regression off-treatment and effective reinduction therapy with an anti-PD-1 antibody. Clin. Cancer Res. 2013, 19, 462–468. [Google Scholar] [CrossRef]
- Le, D.T.; Uram, J.N.; Wang, H.; Bartlett, B.R.; Kemberling, H.; Eyring, A.D.; Skora, A.D.; Luber, B.S.; Azad, N.S.; Laheru, D.; et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N. Engl. J. Med. 2015, 372, 2509–2520. [Google Scholar] [CrossRef] [PubMed]
- Humphris, J.L.; Patch, A.M.; Nones, K.; Bailey, P.J.; Johns, A.L.; McKay, S.; Chang, D.K.; Miller, D.K.; Pajic, M.; Kassahn, K.S.; et al. Hypermutation In Pancreatic Cancer. Gastroenterology 2017, 152, 68–74 e62. [Google Scholar] [CrossRef]
- Le, D.T.; Durham, J.N.; Smith, K.N.; Wang, H.; Bartlett, B.R.; Aulakh, L.K.; Lu, S.; Kemberling, H.; Wilt, C.; Luber, B.S.; et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017, 357, 409–413. [Google Scholar] [CrossRef]
- Marabelle, A.; Le, D.T.; Ascierto, P.A.; Di Giacomo, A.M.; De Jesus-Acosta, A.; Delord, J.P.; Geva, R.; Gottfried, M.; Penel, N.; Hansen, A.R.; et al. Efficacy of Pembrolizumab in Patients With Noncolorectal High Microsatellite Instability/Mismatch Repair-Deficient Cancer: Results From the Phase II KEYNOTE-158 Study. J. Clin. Oncol. 2020, 38, 1–10. [Google Scholar] [CrossRef]
- Ueno, M.; Morizane, C.; Ikeda, M.; Sudo, K.; Hirashima, Y.; Kuroda, M.; Fukuyama, Y.; Okusaka, T.; Furuse, J. A phase II study of nivolumab in combination with modified FOLFIRINOX for metastatic pancreatic cancer. J. Clin. Oncol. 2022, 40 (Suppl. S4), 553. [Google Scholar] [CrossRef]
- Padron, L.J.; Maurer, D.M.; O’Hara, M.H.; O’Reilly, E.M.; Wolff, R.A.; Wainberg, Z.A.; Ko, A.H.; Fisher, G.; Rahma, O.; Lyman, J.P.; et al. Sotigalimab and/or nivolumab with chemotherapy in first-line metastatic pancreatic cancer: Clinical and immunologic analyses from the randomized phase 2 PRINCE trial. Nat. Med. 2022, 28, 1167–1177. [Google Scholar] [CrossRef]
- Wainberg, Z.A.; Hochster, H.S.; Kim, E.J.; George, B.; Kaylan, A.; Chiorean, E.G.; Waterhouse, D.M.; Guiterrez, M.; Parikh, A.; Jain, R.; et al. Open-label, Phase I Study of Nivolumab Combined with nab-Paclitaxel Plus Gemcitabine in Advanced Pancreatic Cancer. Clin. Cancer Res 2020, 26, 4814–4822. [Google Scholar] [CrossRef]
- Chen, I.M.; Johansen, J.S.; Theile, S.; Hjaltelin, J.X.; Novitski, S.I.; Brunak, S.; Hasselby, J.P.; Willemoe, G.L.; Lorentzen, T.; Madsen, K.; et al. Randomized Phase II Study of Nivolumab With or Without Ipilimumab Combined With Stereotactic Body Radiotherapy for Refractory Metastatic Pancreatic Cancer (CheckPAC). J. Clin. Oncol. 2022, 40, 3180–3189. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Roca, C.; Cassier, P.; Zamarin, D.; Machiels, J.P.; Luis Perez Gracia, J.; Stephen Hodi, F.; Taus, A.; Martinez Garcia, M.; Boni, V.; Eder, J.P.; et al. Anti-CSF-1R emactuzumab in combination with anti-PD-L1 atezolizumab in advanced solid tumor patients naive or experienced for immune checkpoint blockade. J. Immunother. Cancer 2022, 10, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Rodon, J.; Tan, D.W.; Laguna, I.G.; Harb, W.; Thaddeus Beck, J.; Bahary, N.; Rottey, S.; Zhu, Z.; Deng, S.; Kowalski, K.; et al. 344 Avelumab + binimetinib in metastatic pancreatic ductal adenocarcinoma (mPDAC): Dose-escalation results from the phase 1b/2 JAVELIN PARP MEKi trial. J. Immuno. Therapy Cancer 2021, 9 (Suppl. S2), A371. [Google Scholar] [CrossRef]
- Yeo, D.; Giardina, C.; Saxena, P.; Rasko, J.E.J. The next wave of cellular immunotherapies in pancreatic cancer. Mol. Ther. Oncolytics 2022, 24, 561–576. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Guo, Y.; Wu, Z.; Feng, K.; Tong, C.; Wang, Y.; Dai, H.; Shi, F.; Yang, Q.; Han, W. Anti-EGFR chimeric antigen receptor-modified T cells in metastatic pancreatic carcinoma: A phase I clinical trial. Cytotherapy 2020, 22, 573–580. [Google Scholar] [CrossRef]
- Feng, K.; Liu, Y.; Guo, Y.; Qiu, J.; Wu, Z.; Dai, H.; Yang, Q.; Wang, Y.; Han, W. Phase I study of chimeric antigen receptor modified T cells in treating HER2-positive advanced biliary tract cancers and pancreatic cancers. Protein Cell 2018, 9, 838–847. [Google Scholar] [CrossRef]
- Beatty, G.L.; O’Hara, M.H.; Lacey, S.F.; Torigian, D.A.; Nazimuddin, F.; Chen, F.; Kulikovskaya, I.M.; Soulen, M.C.; McGarvey, M.; Nelson, A.M.; et al. Activity of Mesothelin-Specific Chimeric Antigen Receptor T Cells Against Pancreatic Carcinoma Metastases in a Phase 1 Trial. Gastroenterology 2018, 155, 29–32. [Google Scholar] [CrossRef]
- Haas, A.R.; Tanyi, J.L.; O’Hara, M.H.; Gladney, W.L.; Lacey, S.F.; Torigian, D.A.; Soulen, M.C.; Tian, L.; McGarvey, M.; Nelson, A.M.; et al. Phase I Study of Lentiviral-Transduced Chimeric Antigen Receptor-Modified T Cells Recognizing Mesothelin in Advanced Solid Cancers. Mol. Ther. 2019, 27, 1919–1929. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, M.; Wu, Z.; Tong, C.; Dai, H.; Guo, Y.; Liu, Y.; Huang, J.; Lv, H.; Luo, C.; et al. CD133-directed CAR T cells for advanced metastasis malignancies: A phase I trial. Oncoimmunology 2018, 7, e1440169. [Google Scholar] [CrossRef]
- Jang, J.E.; Hajdu, C.H.; Liot, C.; Miller, G.; Dustin, M.L.; Bar-Sagi, D. Crosstalk between Regulatory T Cells and Tumor-Associated Dendritic Cells Negates Anti-tumor Immunity in Pancreatic Cancer. Cell Rep. 2017, 20, 558–571. [Google Scholar] [CrossRef]
- Bailey, P.; Chang, D.K.; Forget, M.A.; Lucas, F.A.; Alvarez, H.A.; Haymaker, C.; Chattopadhyay, C.; Kim, S.H.; Ekmekcioglu, S.; Grimm, E.A.; et al. Exploiting the neoantigen landscape for immunotherapy of pancreatic ductal adenocarcinoma. Sci. Rep. 2016, 6, 35848. [Google Scholar] [CrossRef]
- Liu, J.; Fu, M.; Wang, M.; Wan, D.; Wei, Y.; Wei, X. Cancer vaccines as promising immuno-therapeutics: Platforms and current progress. J. Hematol. Oncol. 2022, 15, 28. [Google Scholar] [CrossRef] [PubMed]
- Tsujikawa, T.; Crocenzi, T.; Durham, J.N.; Sugar, E.A.; Wu, A.A.; Onners, B.; Nauroth, J.M.; Anders, R.A.; Fertig, E.J.; Laheru, D.A.; et al. Evaluation of Cyclophosphamide/GVAX Pancreas Followed by Listeria-Mesothelin (CRS-207) with or without Nivolumab in Patients with Pancreatic Cancer. Clin. Cancer Res. 2020, 26, 3578–3588. [Google Scholar] [CrossRef] [PubMed]
- Quezada, S.A.; Peggs, K.S.; Curran, M.A.; Allison, J.P. CTLA4 blockade and GM-CSF combination immunotherapy alters the intratumor balance of effector and regulatory T cells. J. Clin. Investig. 2006, 116, 1935–1945. [Google Scholar] [CrossRef]
- Le, D.T.; Brockstedt, D.G.; Nir-Paz, R.; Hampl, J.; Mathur, S.; Nemunaitis, J.; Sterman, D.H.; Hassan, R.; Lutz, E.; Moyer, B.; et al. A live-attenuated Listeria vaccine (ANZ-100) and a live-attenuated Listeria vaccine expressing mesothelin (CRS-207) for advanced cancers: Phase I studies of safety and immune induction. Clin. Cancer Res. 2012, 18, 858–868. [Google Scholar] [CrossRef] [PubMed]
- Le, D.T.; Picozzi, V.J.; Ko, A.H.; Wainberg, Z.A.; Kindler, H.; Wang-Gillam, A.; Oberstein, P.; Morse, M.A.; Zeh, H.J., 3rd; Weekes, C.; et al. Results from a Phase IIb, Randomized, Multicenter Study of GVAX Pancreas and CRS-207 Compared with Chemotherapy in Adults with Previously Treated Metastatic Pancreatic Adenocarcinoma (ECLIPSE Study). Clin. Cancer Res. 2019, 25, 5493–5502. [Google Scholar] [CrossRef] [PubMed]
- Hewitt, D.B.; Nissen, N.; Hatoum, H.; Musher, B.; Seng, J.; Coveler, A.L.; Al-Rajabi, R.; Yeo, C.J.; Leiby, B.; Banks, J.; et al. A Phase 3 Randomized Clinical Trial of Chemotherapy With or Without Algenpantucel-L (HyperAcute-Pancreas) Immunotherapy in Subjects With Borderline Resectable or Locally Advanced Unresectable Pancreatic Cancer. Ann. Surg. 2022, 275, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Hardacre, J.M.; Mulcahy, M.; Small, W.; Talamonti, M.; Obel, J.; Krishnamurthi, S.; Rocha-Lima, C.S.; Safran, H.; Lenz, H.J.; Chiorean, E.G. Addition of algenpantucel-L immunotherapy to standard adjuvant therapy for pancreatic cancer: A phase 2 study. J. Gastrointest Surg. 2013, 17, 94–100; discussion 100–101. [Google Scholar] [CrossRef] [PubMed]
- Mehrotra, S.; Britten, C.D.; Chin, S.; Garrett-Mayer, E.; Cloud, C.A.; Li, M.; Scurti, G.; Salem, M.L.; Nelson, M.H.; Thomas, M.B.; et al. Vaccination with poly(IC:LC) and peptide-pulsed autologous dendritic cells in patients with pancreatic cancer. J. Hematol. Oncol. 2017, 10, 82. [Google Scholar] [CrossRef]
- Taniuchi, K.; Nakagawa, H.; Nakamura, T.; Eguchi, H.; Ohigashi, H.; Ishikawa, O.; Katagiri, T.; Nakamura, Y. Down-regulation of RAB6KIFL/KIF20A, a kinesin involved with membrane trafficking of discs large homologue 5, can attenuate growth of pancreatic cancer cell. Cancer Res. 2005, 65, 105–112. [Google Scholar] [CrossRef]
- Asahara, S.; Takeda, K.; Yamao, K.; Maguchi, H.; Yamaue, H. Phase I/II clinical trial using HLA-A24-restricted peptide vaccine derived from KIF20A for patients with advanced pancreatic cancer. J. Transl. Med. 2013, 11, 291. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Hazama, S.; Iguchi, H.; Uesugi, K.; Tanaka, H.; Hirakawa, K.; Aruga, A.; Hatori, T.; Ishizaki, H.; Umeda, Y.; et al. Phase II clinical trial of peptide cocktail therapy for patients with advanced pancreatic cancer: VENUS-PC study. Cancer Sci. 2017, 108, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Zhang, G.; Tang, T.Y.; Gao, X.; Liang, T.B. Personalized pancreatic cancer therapy: From the perspective of mRNA vaccine. Mil. Med. Res. 2022, 9, 53. [Google Scholar] [CrossRef] [PubMed]
- Middleton, G.; Silcocks, P.; Cox, T.; Valle, J.; Wadsley, J.; Propper, D.; Coxon, F.; Ross, P.; Madhusudan, S.; Roques, T.; et al. Gemcitabine and capecitabine with or without telomerase peptide vaccine GV1001 in patients with locally advanced or metastatic pancreatic cancer (TeloVac): An open-label, randomised, phase 3 trial. Lancet Oncol. 2014, 15, 829–840. [Google Scholar] [CrossRef]
- Ho, W.J.; Jaffee, E.M.; Zheng, L. The tumour microenvironment in pancreatic cancer—clinical challenges and opportunities. Nat. Rev. Clin. Oncol. 2020, 17, 527–540. [Google Scholar] [CrossRef] [PubMed]

| NCT | Phase | Rand. | Sponsor | ICI | Endpoint(s) | Estimated Date for Primary Results |
|---|---|---|---|---|---|---|
| NCT03816358 | 1/2 | No | National Cancer Institute | Arm I: Anetumab + NIVO. Arm II: Anetumab + IPI + NIVO. Arm III: Anetumab + Gemcitabine + NIVO. | Primary: maximum tolerated dose. Secondary: biomarker analysis. | January 2024 |
| NCT04666688 | 1/2 | No | PureTech | Dose-expansion arm: LYT-200 (Galectin-9 inhibitor) + PD-1 inhibitor + Gemcitabine + Nab-paclitaxel. | Primary: Incidence of TRAEs, incidence of DLTs, PFS and ORR. Secondary: Pharmacokinetics and pharmacodynamics of LYT-200. | December 2022 |
| NCT04140526 | 1/2 | No | National Cancer Institute; OncoC4 Inc. | Arm I: ONC-392 (CTLA-4 inhibitor). Arm II: ONC-392 + Pembrolizumab. | Primary: DLT, maximum tolerated dose, incidence of TRAEs. Secondary: ORR, PFS, OS. | December 2023 |
| NCT04152018 | 1 | No | Pfizer | Dose-escalation arm: PF-06940434 (PD-1 inhibitor). Dose-finding arm: PF-06940434 + PF-06801591 (PD-1 inhibitor). Dose-expansion form A: PF-06940434 + PF-06801591 in SCCHN. Dose-expansion form B: PF-06940434 + PF-06801591 in RCC. | Primary: DLT, PFS and incidence of TRAEs. Secondary: Pharmacokinetics and pharmacodynamics of PF-06940434 and PF-06801591, DOR. | September 2023 |
| NCT04336098 | 1 | No | Surface Oncology; MSD LLC | Arm III: SRF617 + PEMBRO. Arm IV: SRF617 + PEMBRO + Gemcitabine + albumin-bound Paclitaxel. | Primary: DLT Secondary: Pharmacokinetics and pharmacodynamics of SRF617, PFS. | November 2022 |
| NCT04332653 | 1/2 | No | NeoImmune Tech | NT-I7 (Efineptakin Alfa, long-acting human interleukin-7) + PEMBRO | Primary: Safety and tolerability of NT-I7 Secondary: DOR, PFS, OS, ORR, incidence of irAEs | May 2024 |
| NCT05293496 | 1 | No | MacroGenics | MGC018 (B7-H3 inhibitor) + Lorigerlimab (bispecific CTLA-4/PD-1 inhibitor) | Primary: incidence of irAEs Secondary: Pharmacokinetics and pharmacodynamics of MGC018, DOR, OS, PFS, ORR. | March 2024 |
| NCT03915678 | 2 | No | Institut Bergonie | ATEZO + BDB001 (Toll-like receptor 7/8 agonist) + Radiotherapy | Primary: assessment of antitumor activity Secondary: PFS, ORR | September 2023 |
| NCT04548752 | 2 | Yes | National Cancer Institute | Control arm: Olaparib. Experimental arm: Olaparib + PEMBRO. | Primary: PFS Secondary: Incidence of irAEs, OS, ORR, DOR | March 2025 |
| NCT03485209 | 2 | No | Seagen Inc.; MSD LLC | Arm IV: Tisotumab vedotin + PEMBRO + carboplatin + cisplatin. | Primary: ORR Secondary: incidence of irAEs, DOR, TTR, PFS, OS | November 2023 |
| NCT04429542 | 1 | No | Bicara Therapeutics | Arm II: BCA101 (EGFR and TGFβ fusion mAb + PEMBRO. | Primary: Safety and incidence of DLTs Secondary: ORR, PFS, DOR, OS | December 2023 |
| NCT04561362 | 1/2 | No | BicycleTx Limited | Dose-escalation cohort A2: BT8009 + NIVO. Dose-expansion B2: BT8009 + NIVO. | Primary: DLTs, ORR, PFS, OS Secondary: DOR | June 2023 |
| NCT02834013 | 2 | No | National Cancer Institute | Arm I: IPI + NIVO. Arm II: NIVO. | Primary: ORR Secondary: incidence of irAEs, OS, PFS | October 2023 |
| NCT | Phase | Rand. | Sponsor | Intervention | Primary Endpoint(s) | Estimated Date for Primary Results |
|---|---|---|---|---|---|---|
| Adoptive cell therapies | ||||||
| NCT04404595 | 1/2 | No | CARsgen Therapeutics Co., Ltd. | CAR T cells (Claudin 18.2) | Incidence of TRAEs, ORR | June 2025 |
| NCT04660929 | 1 | No | Carisma Therapeutics Inc | CAR macrophages (HER-2) | Incidence of TRAEs | February 2023 |
| NCT05239143 | 1 | No | Poseida Therapeutics, Inc. | CAR T cells (MUC1) | MTD; ORR and incidence of TRAEs | April 2026 |
| NCT04157127 | 1 | No | Baylor College of Medicine | Autologous DC vaccine | MTD, DLTs | January 2024 |
| NCT04581473 | 1/2 | Yes | CARsgen Therapeutics Co., Ltd. | Experimental arm: CT041 (CAR T cells [Claudin 18.2]). Control arm: Chemotherapy or PD-1 inhibitor. | Incidence of TRAEs, MTD, PFS | June 2024 |
| NCT04348643 | 1/2 | No | Chongqing Precision Biotech Co., Ltd. | CAR T cells (CEA) | Incidence of TRAEs | January 2023 |
| Cancer vaccines | ||||||
| NCT03323944 | 1 | No | University of Pennsylvania | CAR T cells (Mesothelin) | Response rate, PFS, OS | September 2024 |
| NCT03953235 | 1/2 | No | Gritstone bio, Inc. and Bristol Myers Squibb | GRT-C903 + GRT-R904 + Nivolumab + Ipilimumab | Incidence of TRAEs, ORR | December 2023 |
| NCT04807972 | 2 | Yes | AbbVie | Control arm: FOLFIRINOX. Experimental arm I: FOLFIRINOX + ABBV-927 Experimental arm II: ABBV-927 + Budiglimab + mFOLFIRINOX. | OS | August 2024 |
| NCT04853017 | 1 | No | Elicio Therapeutics | ELI-002 (a lipid-conjugated immune-stimulatory oligonucleotide [Amph-CpG-7909] plus a mixture of lipid-conjugated peptide-based antigens [Amph-Peptides]) | MTD, safety | November 2024 |
| NCT02600949 | 1 | No | M.D. Anderson Cancer Center | Arm I: personalized vaccine + imiquimod. Arm II: personalized vaccine + imiquimod + pembrolizumab. Arms III and IV: vaccine + imiquimod + pembrolizumab + APX005M. | Incidence of TRAEs | May 2025 |
| NCT04111172 | 2 | Yes | Thomas Jefferson University | Experimental arm I: adenovirus 5/F35-human guanylyl cyclase C-PADRE vaccine (low dose) Experimental arm II: medium dose Experimental arm III: high dose | Incidence of TRAEs, Antigen-specific T-cell response to guanylyl cyclase C (GCC) | March 2024 |
| NCT02451982 | 2 | Yes | Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins and Bristol Myers Squibb | Experimental arm I: CY/GVAX alone. Experimental arm II: CY/GVAX with nivolumab. Experimental arm III: CY/GVAX with nivolumab and urelumab. Experimental arm IV: BMS-986253 and Nivolumab. | IL17A expression, Intratumoral CD8+ CD137+ cells, Intratumoral granzyme B PD-1+ CD137+ cells, Pathologic Response | June 2023 |
| NCT03767582 | 1/2 | Yes | Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins and Bristol Myers Squibb | Arm I: Nivolumab/CCR2/CCR5 dual antagonist. Arm II: Nivolumab/GVAX/CCR2/ CCR5 dual antagonist | Percentage of participants who have >80% increase in infiltration of CD8+ CD137+ T cells into the PDAC after treatment compared to baseline | March 2023 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akhuba, L.; Tigai, Z.; Shek, D. Where Do We Stand with Immunotherapy for Advanced Pancreatic Ductal Adenocarcinoma: A Synopsis of Clinical Outcomes. Biomedicines 2022, 10, 3196. https://doi.org/10.3390/biomedicines10123196
Akhuba L, Tigai Z, Shek D. Where Do We Stand with Immunotherapy for Advanced Pancreatic Ductal Adenocarcinoma: A Synopsis of Clinical Outcomes. Biomedicines. 2022; 10(12):3196. https://doi.org/10.3390/biomedicines10123196
Chicago/Turabian StyleAkhuba, Liia, Zhanna Tigai, and Dmitrii Shek. 2022. "Where Do We Stand with Immunotherapy for Advanced Pancreatic Ductal Adenocarcinoma: A Synopsis of Clinical Outcomes" Biomedicines 10, no. 12: 3196. https://doi.org/10.3390/biomedicines10123196
APA StyleAkhuba, L., Tigai, Z., & Shek, D. (2022). Where Do We Stand with Immunotherapy for Advanced Pancreatic Ductal Adenocarcinoma: A Synopsis of Clinical Outcomes. Biomedicines, 10(12), 3196. https://doi.org/10.3390/biomedicines10123196








