Role of Chemerin in Cardiovascular Diseases
Abstract
1. Introduction
Search Strategy
2. Cardiovascular Disease (CVDs)
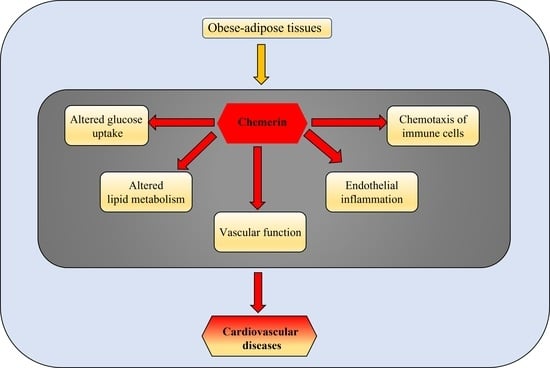
3. Chemerin
3.1. Chemerin and CVDs
3.1.1. Evidence from Human Studies Supporting Chemerin’s Role in CVDs
3.1.2. Chemerin Roles in CVDs: Evidence from Animal Studies
4. Perspectives for the Development of Chemerin-Targeting Therapeutic Agents
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vos, T.; Lim, S.S.; Abbafati, C.; Abbas, K.M.; Abbasi, M.; Abbasifard, M.; Abbasi-Kangevari, M.; Abbastabar, H.; Abd-Allah, F.; Abdelalim, A.; et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef]
- Powell-Wiley, T.M.; Poirier, P.; Burke, L.E.; Després, J.P.; Gordon-Larsen, P.; Lavie, C.J.; Lear, S.A.; Ndumele, C.E.; Neeland, I.J.; Sanders, P.; et al. Obesity and Cardiovascular Disease: A Scientific Statement from the American Heart Association. Circulation 2021, 143, e984–e1010. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, A.A.; Stoian, A.P. Metabolic Syndrome: From Molecular Mechanisms to Novel Therapies. Int. J. Mol. Sci. 2021, 22, 10038. [Google Scholar] [CrossRef] [PubMed]
- Karastergiou, K.; Mohamed-Ali, V. The autocrine and paracrine roles of adipokines. Mol. Cell. Endocrinol. 2010, 318, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Pardo, M.; Roca-Rivada, A.; Seoane, L.M.; Casanueva, F.F. Obesidomics: Contribution of adipose tissue secretome analysis to obesity research. Endocrine 2012, 41, 374–383. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Fuster, J.J.; Walsh, K. Adipokines: A link between obesity and cardiovascular disease. J. Cardiol. 2014, 63, 250–259. [Google Scholar] [CrossRef]
- Rizvi, A.A.; Nikolic, D.; Sallam, H.S.; Montalto, G.; Rizzo, M.; Abate, N. Adipokines and lipoproteins: Modulation by antihyperglycemic and hypolipidemic agents. Metab. Syndr. Relat. Disord. 2014, 12, 250–259. [Google Scholar] [CrossRef]
- Ouchi, N.; Parker, J.L.; Lugus, J.J.; Walsh, K. Adipokines in inflammation and metabolic disease. Nat. Rev. Immunol. 2011, 11, 85–97. [Google Scholar] [CrossRef]
- Kaur, J.; Mattu, H.S.; Chatha, K.; Randeva, H.S. Chemerin in human cardiovascular disease. Vasc. Pharmacol. 2018, 110, 1–6. [Google Scholar] [CrossRef]
- Abate, N.; Sallam, H.S.; Rizzo, M.; Nikolic, D.; Obradovic, M.; Bjelogrlic, P.; Isenovic, E.R. Resistin: An inflammatory cytokine. Role in cardiovascular diseases, diabetes and the metabolic syndrome. Curr. Pharm. Des. 2014, 20, 4961–4969. [Google Scholar] [CrossRef]
- Stepien, M.; Stepien, A.; Wlazel, R.N.; Paradowski, M.; Rizzo, M.; Banach, M.; Rysz, J. Predictors of insulin resistance in patients with obesity: A pilot study. Angiology 2014, 65, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Goralski, K.B.; McCarthy, T.C.; Hanniman, E.A.; Zabel, B.A.; Butcher, E.C.; Parlee, S.D.; Muruganandan, S.; Sinal, C.J. Chemerin, a novel adipokine that regulates adipogenesis and adipocyte metabolism. J. Biol. Chem. 2007, 282, 28175–28188. [Google Scholar] [CrossRef] [PubMed]
- Zabel, B.A.; Kwitniewski, M.; Banas, M.; Zabieglo, K.; Murzyn, K.; Cichy, J. Chemerin regulation and role in host defense. Am. J. Clin. Exp. Immunol. 2014, 3, 1–19. [Google Scholar] [PubMed]
- Yoshimura, T.; Oppenheim, J.J. Chemerin reveals its chimeric nature. J. Exp. Med. 2008, 205, 2187–2190. [Google Scholar] [CrossRef]
- Ernst, M.C.; Sinal, C.J. Chemerin: At the crossroads of inflammation and obesity. Trends Endocrinol. Metab. TEM 2010, 21, 660–667. [Google Scholar] [CrossRef] [PubMed]
- Rourke, J.L.; Dranse, H.J.; Sinal, C.J. Towards an integrative approach to understanding the role of chemerin in human health and disease. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2013, 14, 245–262. [Google Scholar] [CrossRef]
- Yan, Q.; Zhang, Y.; Hong, J.; Gu, W.; Dai, M.; Shi, J.; Zhai, Y.; Wang, W.; Li, X.; Ning, G. The association of serum chemerin level with risk of coronary artery disease in Chinese adults. Endocrine 2012, 41, 281–288. [Google Scholar] [CrossRef]
- Dong, B.; Ji, W.; Zhang, Y. Elevated serum chemerin levels are associated with the presence of coronary artery disease in patients with metabolic syndrome. Intern. Med. 2011, 50, 1093–1097. [Google Scholar] [CrossRef]
- Wang, B.; Kou, W.; Ji, S.; Shen, R.; Ji, H.; Zhuang, J.; Zhao, Y.; Li, B.; Peng, W.; Yu, X.; et al. Prognostic value of plasma adipokine chemerin in patients with coronary artery disease. Front. Cardiovasc. Med. 2022, 9, 968349. [Google Scholar] [CrossRef]
- Spiroglou, S.G.; Kostopoulos, C.G.; Varakis, J.N.; Papadaki, H.H. Adipokines in periaortic and epicardial adipose tissue: Differential expression and relation to atherosclerosis. J. Atheroscler. Thromb. 2010, 17, 115–130. [Google Scholar] [CrossRef]
- Yoo, H.J.; Choi, H.Y.; Yang, S.J.; Kim, H.Y.; Seo, J.A.; Kim, S.G.; Kim, N.H.; Choi, K.M.; Choi, D.S.; Baik, S.H. Circulating chemerin level is independently correlated with arterial stiffness. J. Atheroscler. Thromb. 2012, 19, 59–66; discussion 67–68. [Google Scholar] [CrossRef] [PubMed]
- Lehrke, M.; Becker, A.; Greif, M.; Stark, R.; Laubender, R.P.; von Ziegler, F.; Lebherz, C.; Tittus, J.; Reiser, M.; Becker, C.; et al. Chemerin is associated with markers of inflammation and components of the metabolic syndrome but does not predict coronary atherosclerosis. Eur. J. Endocrinol. 2009, 161, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Flora, G.D.; Nayak, M.K. A Brief Review of Cardiovascular Diseases, Associated Risk Factors and Current Treatment Regimes. Curr. Pharm. Des. 2019, 25, 4063–4084. [Google Scholar] [CrossRef] [PubMed]
- Barquera, S.; Pedroza-Tobías, A.; Medina, C.; Hernández-Barrera, L.; Bibbins-Domingo, K.; Lozano, R.; Moran, A.E. Global Overview of the Epidemiology of Atherosclerotic Cardiovascular Disease. Arch. Med. Res. 2015, 46, 328–338. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, A.A.; Popovic, D.S.; Papanas, N.; Pantea Stoian, A.; Al Mahmeed, W.; Sahebkar, A.; Janez, A.; Rizzo, M. Current and emerging drugs for the treatment of atherosclerosis: The evidence to date. Expert Rev. Cardiovasc. Ther. 2022, 20, 515–527. [Google Scholar] [CrossRef]
- Watson, A.D.; Leitinger, N.; Navab, M.; Faull, K.F.; Hörkkö, S.; Witztum, J.L.; Palinski, W.; Schwenke, D.; Salomon, R.G.; Sha, W.; et al. Structural identification by mass spectrometry of oxidized phospholipids in minimally oxidized low density lipoprotein that induce monocyte/endothelial interactions and evidence for their presence in vivo. J. Biol. Chem. 1997, 272, 13597–13607. [Google Scholar] [CrossRef]
- Libby, P.; Theroux, P. Pathophysiology of coronary artery disease. Circulation 2005, 111, 3481–3488. [Google Scholar] [CrossRef]
- Park, Y.M. CD36, a scavenger receptor implicated in atherosclerosis. Exp. Mol. Med. 2014, 46, e99. [Google Scholar] [CrossRef]
- Rizzo, M.; Corrado, E.; Coppola, G.; Muratori, I.; Novo, G.; Novo, S. Markers of inflammation are strong predictors of subclinical and clinical atherosclerosis in women with hypertension. Coron. Artery Dis. 2009, 20, 15–20. [Google Scholar] [CrossRef]
- Sardu, C.; Paolisso, G.; Marfella, R. Inflammatory Related Cardiovascular Diseases: From Molecular Mechanisms to Therapeutic Targets. Curr. Pharm. Des. 2020, 26, 2565–2573. [Google Scholar] [CrossRef]
- Alfaddagh, A.; Martin, S.S.; Leucker, T.M.; Michos, E.D.; Blaha, M.J.; Lowenstein, C.J.; Jones, S.R.; Toth, P.P. Inflammation and cardiovascular disease: From mechanisms to therapeutics. Am. J. Prev. Cardiol. 2020, 4, 100130. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Wu, N.; Du, W.; Wang, S.; Wang, J.; Zhang, C.; Chen, X. Visfatin Amplifies Cardiac Inflammation and Aggravates Cardiac Injury via the NF-κB p65 Signaling Pathway in LPS-Treated Mice. Mediat. Inflamm. 2022, 2022, 3306559. [Google Scholar] [CrossRef] [PubMed]
- Trpkovic, A.; Obradovic, M.; Petrovic, N.; Davidovic, R.; Sudar-Milovanovic, E.; Isenovic, E.R. C-Reactive Protein. In Encyclopedia of Signaling Molecules; Choi, S., Ed.; Springer: New York, NY, USA, 2016; pp. 1–5. [Google Scholar]
- LaPointe, M.C.; Isenović, E. Interleukin-1beta regulation of inducible nitric oxide synthase and cyclooxygenase-2 involves the p42/44 and p38 MAPK signaling pathways in cardiac myocytes. Hypertension 1999, 33, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Marfella, R.; Grella, R.; Rizzo, M.R.; Barbieri, M.; Grella, R.; Ferraraccio, F.; Cacciapuoti, F.; Mazzarella, G.; Ferraro, N.; D’Andrea, F.; et al. Role of subcutaneous abdominal fat on cardiac function and proinflammatory cytokines in premenopausal obese women. Ann. Plast. Surg. 2009, 63, 490–495. [Google Scholar] [CrossRef]
- Sardu, C.; Pieretti, G.; D’Onofrio, N.; Ciccarelli, F.; Paolisso, P.; Passavanti, M.B.; Marfella, R.; Cioffi, M.; Mone, P.; Dalise, A.M.; et al. Inflammatory Cytokines and SIRT1 Levels in Subcutaneous Abdominal Fat: Relationship With Cardiac Performance in Overweight Pre-diabetics Patients. Front. Physiol. 2018, 9, 1030. [Google Scholar] [CrossRef]
- Houtkooper, R.H.; Pirinen, E.; Auwerx, J. Sirtuins as regulators of metabolism and healthspan. Nat. Rev. Mol. Cell Biol. 2012, 13, 225–238. [Google Scholar] [CrossRef]
- Sardu, C.; Carreras, G.; Katsanos, S.; Kamperidis, V.; Pace, M.C.; Passavanti, M.B.; Fava, I.; Paolisso, P.; Pieretti, G.; Nicoletti, G.F.; et al. Metabolic syndrome is associated with a poor outcome in patients affected by outflow tract premature ventricular contractions treated by catheter ablation. BMC Cardiovasc. Disord. 2014, 14, 176. [Google Scholar] [CrossRef]
- Stein, J.H.; Korcarz, C.E.; Hurst, R.T.; Lonn, E.; Kendall, C.B.; Mohler, E.R.; Najjar, S.S.; Rembold, C.M.; Post, W.S. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: A consensus statement from the American Society of Echocardiography Carotid Intima-Media Thickness Task Force. Endorsed by the Society for Vascular Medicine. J. Am. Soc. Echocardiogr. 2008, 21, 93–111. [Google Scholar] [CrossRef]
- Lorente-Cebrián, S.; González-Muniesa, P.; Milagro, F.I.; Martínez, J.A. MicroRNAs and other non-coding RNAs in adipose tissue and obesity: Emerging roles as biomarkers and therapeutic targets. Clin. Sci. 2019, 133, 23–40. [Google Scholar] [CrossRef]
- Kuryłowicz, A.; Wicik, Z.; Owczarz, M.; Jonas, M.I.; Kotlarek, M.; Świerniak, M.; Lisik, W.; Jonas, M.; Noszczyk, B.; Puzianowska-Kuźnicka, M. NGS Reveals Molecular Pathways Affected by Obesity and Weight Loss-Related Changes in miRNA Levels in Adipose Tissue. Int. J. Mol. Sci. 2017, 19, 66. [Google Scholar] [CrossRef]
- Chen, T.; Zhang, Y.; Liu, Y.; Zhu, D.; Yu, J.; Li, G.; Sun, Z.; Wang, W.; Jiang, H.; Hong, Z. MiR-27a promotes insulin resistance and mediates glucose metabolism by targeting PPAR-γ-mediated PI3K/AKT signaling. Aging 2019, 11, 7510–7524. [Google Scholar] [CrossRef] [PubMed]
- Mortuza, R.; Feng, B.; Chakrabarti, S. miR-195 regulates SIRT1-mediated changes in diabetic retinopathy. Diabetologia 2014, 57, 1037–1046. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Qin, D.; Shi, H.; Zhang, Y.; Li, H.; Han, Q. MiR-195-5p Promotes Cardiomyocyte Hypertrophy by Targeting MFN2 and FBXW7. BioMed Res. Int. 2019, 2019, 1580982. [Google Scholar] [CrossRef] [PubMed]
- Sardu, C.; Trotta, M.C.; Pieretti, G.; Gatta, G.; Ferraro, G.; Nicoletti, G.F.; Onofrio, N.D.; Balestrieri, M.L.; Amico, M.D.; Abbatecola, A.; et al. MicroRNAs modulation and clinical outcomes at 1 year of follow-up in obese patients with pre-diabetes treated with metformin vs. placebo. Acta Diabetol. 2021, 58, 1381–1393. [Google Scholar] [CrossRef]
- Kivimäki, M.; Kuosma, E.; Ferrie, J.E.; Luukkonen, R.; Nyberg, S.T.; Alfredsson, L.; Batty, G.D.; Brunner, E.J.; Fransson, E.; Goldberg, M.; et al. Overweight, obesity, and risk of cardiometabolic multimorbidity: Pooled analysis of individual-level data for 120,813 adults from 16 cohort studies from the USA and Europe. Lancet Public Health 2017, 2, e277–e285. [Google Scholar] [CrossRef]
- Saklayen, M.G. The Global Epidemic of the Metabolic Syndrome. Curr. Hypertens. Rep. 2018, 20, 12. [Google Scholar] [CrossRef] [PubMed]
- Rohde, K.; Keller, M.; la Cour Poulsen, L.; Blüher, M.; Kovacs, P.; Böttcher, Y. Genetics and epigenetics in obesity. Metab. Clin. Exp. 2019, 92, 37–50. [Google Scholar] [CrossRef]
- Wallis, N.; Raffan, E. The Genetic Basis of Obesity and Related Metabolic Diseases in Humans and Companion Animals. Genes 2020, 11, 1378. [Google Scholar] [CrossRef]
- Obradovic, M.; Sudar-Milovanovic, E.; Soskic, S.; Essack, M.; Arya, S.; Stewart, A.J.; Gojobori, T.; Isenovic, E.R. Leptin and Obesity: Role and Clinical Implication. Front. Endocrinol. 2021, 12, 585887. [Google Scholar] [CrossRef]
- Norouzirad, R.; González-Muniesa, P.; Ghasemi, A. Hypoxia in Obesity and Diabetes: Potential Therapeutic Effects of Hyperoxia and Nitrate. Oxidative Med. Cell. Longev. 2017, 2017, 5350267. [Google Scholar] [CrossRef]
- Katsiki, N.; Athyros, V.G.; Karagiannis, A.; Mikhailidis, D.P. Characteristics other than the diagnostic criteria associated with metabolic syndrome: An overview. Curr. Vasc. Pharmacol. 2014, 12, 627–641. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, M.; van Greevenbroek, M.M.; van der Kallen, C.J.; Ferreira, I.; Blaak, E.E.; Feskens, E.J.; Jansen, E.H.; Schalkwijk, C.G.; Stehouwer, C.D. Low-grade inflammation can partly explain the association between the metabolic syndrome and either coronary artery disease or severity of peripheral arterial disease: The CODAM study. Eur. J. Clin. Investig. 2009, 39, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Tsalamandris, S.; Antonopoulos, A.S.; Oikonomou, E.; Papamikroulis, G.-A.; Vogiatzi, G.; Papaioannou, S.; Deftereos, S.; Tousoulis, D. The Role of Inflammation in Diabetes: Current Concepts and Future Perspectives. Eur. Cardiol. 2019, 14, 50–59. [Google Scholar] [CrossRef] [PubMed]
- De Rosa, S.; Arcidiacono, B.; Chiefari, E.; Brunetti, A.; Indolfi, C.; Foti, D.P. Type 2 Diabetes Mellitus and Cardiovascular Disease: Genetic and Epigenetic Links. Front. Endocrinol. 2018, 9, 2. [Google Scholar] [CrossRef] [PubMed]
- Bays, H.E.; Toth, P.P.; Kris-Etherton, P.M.; Abate, N.; Aronne, L.J.; Brown, W.V.; Gonzalez-Campoy, J.M.; Jones, S.R.; Kumar, R.; La Forge, R.; et al. Obesity, adiposity, and dyslipidemia: A consensus statement from the National Lipid Association. J. Clin. Lipidol. 2013, 7, 304–383. [Google Scholar] [CrossRef]
- Aguilera, C.M.; Gil-Campos, M.; Canete, R.; Gil, A. Alterations in plasma and tissue lipids associated with obesity and metabolic syndrome. Clin. Sci. 2008, 114, 183–193. [Google Scholar] [CrossRef]
- Benjamin, E.J.; Blaha, M.J.; Chiuve, S.E.; Cushman, M.; Das, S.R.; Deo, R.; de Ferranti, S.D.; Floyd, J.; Fornage, M.; Gillespie, C.; et al. Heart Disease and Stroke Statistics-2017 Update: A Report from the American Heart Association. Circulation 2017, 135, e146–e603. [Google Scholar] [CrossRef]
- Navar-Boggan, A.M.; Peterson, E.D.; D’Agostino, R.B.; Neely, B.; Sniderman, A.D.; Pencina, M.J. Hyperlipidemia in early adulthood increases long-term risk of coronary heart disease. Circulation 2015, 131, 451–458. [Google Scholar] [CrossRef]
- Zaric, B.; Obradovic, M.; Trpkovic, A.; Banach, M.; Mikhailidis, D.P.; Isenovic, E.R. Endothelial Dysfunction in Dyslipidaemia: Molecular Mechanisms and Clinical Implications. Curr. Med. Chem. 2020, 27, 1021–1040. [Google Scholar] [CrossRef]
- Macvanin, M.; Obradovic, M.; Zafirovic, S.; Stanimirovic, J.; Isenovic, E.R. The role of miRNAs in metabolic diseases. Curr. Med. Chem. 2022, 29, 1. [Google Scholar] [CrossRef]
- Rizvi, A.A.; Stoian, A.P. Lipoproteins and Cardiovascular Disease: An Update on the Clinical Significance of Atherogenic Small, Dense LDL and New Therapeutical Options. Biomedicines 2021, 9, 1579. [Google Scholar] [CrossRef] [PubMed]
- Obradovic, M.; Sudar, E.; Zafirovic, S.; Stanimirovic, J.; Labudovic-Borovic, M.; Isenovic, E.R. Estradiol in vivo induces changes in cardiomyocytes size in obese rats. Angiology 2015, 66, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Solinas, G.; Karin, M. JNK1 and IKKbeta: Molecular links between obesity and metabolic dysfunction. Faseb J. 2010, 24, 2596–2611. [Google Scholar] [CrossRef] [PubMed]
- Stienstra, R.; Tack, C.J.; Kanneganti, T.D.; Joosten, L.A.; Netea, M.G. The inflammasome puts obesity in the danger zone. Cell Metab. 2012, 15, 10–18. [Google Scholar] [CrossRef]
- Jia, G.; Aroor, A.R.; Martinez-Lemus, L.A.; Sowers, J.R. Overnutrition, mTOR signaling, and cardiovascular diseases. Am. J. Physiol. Integr. Comp. Physiol. 2014, 307, R1198–R1206. [Google Scholar] [CrossRef]
- Zafirovic, S.; Obradovic, M.; Sudar-Milovanovic, E.; Jovanovic, A.; Stanimirovic, J.; Stewart, A.J.; Pitt, S.J.; Isenovic, E.R. 17β-Estradiol protects against the effects of a high fat diet on cardiac glucose, lipid and nitric oxide metabolism in rats. Mol. Cell. Endocrinol. 2017, 446, 12–20. [Google Scholar] [CrossRef]
- Cao, H. Adipocytokines in obesity and metabolic disease. J. Endocrinol. 2014, 220, 13–0339. [Google Scholar] [CrossRef]
- Gimbrone, M.A., Jr.; Garcia-Cardena, G. Endothelial Cell Dysfunction and the Pathobiology of Atherosclerosis. Circ. Res. 2016, 118, 620–636. [Google Scholar] [CrossRef]
- Pignatelli, P.; Menichelli, D.; Pastori, D.; Violi, F. Oxidative stress and cardiovascular disease: New insights. Kardiol. Pol. 2018, 14, 713–722. [Google Scholar] [CrossRef]
- Toth, P.P. Insulin resistance, small LDL particles, and risk for atherosclerotic disease. Curr. Vasc. Pharmacol. 2014, 12, 653–657. [Google Scholar] [CrossRef]
- Vekic, J.; Zeljkovic, A. Atherosclerosis Development and Progression: The Role of Atherogenic Small, Dense LDL. Medicina 2022, 58, 299. [Google Scholar] [CrossRef] [PubMed]
- Treeck, O.; Buechler, C.; Ortmann, O. Chemerin and Cancer. Int. J. Mol. Sci. 2019, 20, 3750. [Google Scholar] [CrossRef] [PubMed]
- Buechler, C.; Feder, S.; Haberl, E.M.; Aslanidis, C. Chemerin Isoforms and Activity in Obesity. Int. J. Mol. Sci. 2019, 20, 1128. [Google Scholar] [CrossRef] [PubMed]
- Nagpal, S.; Patel, S.; Jacobe, H.; DiSepio, D.; Ghosn, C.; Malhotra, M.; Teng, M.; Duvic, M.; Chandraratna, R.A. Tazarotene-induced gene 2 (TIG2), a novel retinoid-responsive gene in skin. J. Investig. Dermatol. 1997, 109, 91–95. [Google Scholar] [CrossRef]
- Shin, W.J.; Zabel, B.A.; Pachynski, R.K. Mechanisms and Functions of Chemerin in Cancer: Potential Roles in Therapeutic Intervention. Front. Immunol. 2018, 9, 2772. [Google Scholar] [CrossRef]
- Zabel, B.A.; Allen, S.J.; Kulig, P.; Allen, J.A.; Cichy, J.; Handel, T.M.; Butcher, E.C. Chemerin activation by serine proteases of the coagulation, fibrinolytic, and inflammatory cascades. J. Biol. Chem. 2005, 280, 34661–34666. [Google Scholar] [CrossRef]
- Ferland, D.J.; Mullick, A.E.; Watts, S.W. Chemerin as a Driver of Hypertension: A Consideration. Am. J. Hypertens. 2020, 33, 975–986. [Google Scholar] [CrossRef]
- Wittamer, V.; Franssen, J.-D.; Vulcano, M.; Mirjolet, J.-F.; Le Poul, E.; Migeotte, I.; Brézillon, S.; Tyldesley, R.; Blanpain, C.; Detheux, M. Specific recruitment of antigen-presenting cells by chemerin, a novel processed ligand from human inflammatory fluids. J. Exp. Med. 2003, 198, 977–985. [Google Scholar] [CrossRef]
- Fagerberg, L.; Hallström, B.M.; Oksvold, P.; Kampf, C.; Djureinovic, D.; Odeberg, J.; Habuka, M.; Tahmasebpoor, S.; Danielsson, A.; Edlund, K.; et al. Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol. Cell Proteom. MCP 2014, 13, 397–406. [Google Scholar] [CrossRef]
- Kwiecien, K.; Brzoza, P. The methylation status of the chemerin promoter region located from - 252 to + 258 bp regulates constitutive but not acute-phase cytokine-inducible chemerin expression levels. Sci. Rep. 2020, 10, 13702. [Google Scholar] [CrossRef]
- Uhlén, M.; Fagerberg, L.; Hallström, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, Å.; Kampf, C.; Sjöstedt, E.; Asplund, A.; et al. Proteomics. Tissue-based map of the human proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef] [PubMed]
- Skrzeczyńska-Moncznik, J.; Stefańska, A.; Zabel, B.A.; Kapińska-Mrowiecka, M.; Butcher, E.C.; Cichy, J. Chemerin and the recruitment of NK cells to diseased skin. Acta Biochim. Pol. 2009, 56, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Pachynski, R.K.; Wang, P.; Salazar, N.; Zheng, Y.; Nease, L.; Rosalez, J.; Leong, W.I.; Virdi, G.; Rennier, K.; Shin, W.J.; et al. Chemerin Suppresses Breast Cancer Growth by Recruiting Immune Effector Cells into the Tumor Microenvironment. Front. Immunol. 2019, 10, 983. [Google Scholar] [CrossRef]
- Niklowitz, P.; Rothermel, J.; Lass, N.; Barth, A.; Reinehr, T. Link between chemerin, central obesity, and parameters of the Metabolic Syndrome: Findings from a longitudinal study in obese children participating in a lifestyle intervention. Int. J. Obes. 2018, 42, 1743–1752. [Google Scholar] [CrossRef]
- Parlee, S.D.; Ernst, M.C.; Muruganandan, S.; Sinal, C.J.; Goralski, K.B. Serum chemerin levels vary with time of day and are modified by obesity and tumor necrosis factor-{alpha}. Endocrinology 2010, 151, 2590–2602. [Google Scholar] [CrossRef] [PubMed]
- Bauer, S.; Wanninger, J.; Schmidhofer, S.; Weigert, J.; Neumeier, M.; Dorn, C.; Hellerbrand, C.; Zimara, N.; Schäffler, A.; Aslanidis, C.; et al. Sterol regulatory element-binding protein 2 (SREBP2) activation after excess triglyceride storage induces chemerin in hypertrophic adipocytes. Endocrinology 2011, 152, 26–35. [Google Scholar] [CrossRef]
- Deng, Y.; Wang, H.; Lu, Y.; Liu, S.; Zhang, Q.; Huang, J.; Zhu, R.; Yang, J.; Zhang, R.; Zhang, D.; et al. Identification of chemerin as a novel FXR target gene down-regulated in the progression of nonalcoholic steatohepatitis. Endocrinology 2013, 154, 1794–1801. [Google Scholar] [CrossRef]
- Muruganandan, S.; Parlee, S.D.; Rourke, J.L.; Ernst, M.C.; Goralski, K.B.; Sinal, C.J. Chemerin, a novel peroxisome proliferator-activated receptor gamma (PPARgamma) target gene that promotes mesenchymal stem cell adipogenesis. J. Biol. Chem. 2011, 286, 23982–23995. [Google Scholar] [CrossRef]
- Mattern, A.; Zellmann, T.; Beck-Sickinger, A.G. Processing, signaling, and physiological function of chemerin. IUBMB Life 2014, 66, 19–26. [Google Scholar] [CrossRef]
- Zhao, L.; Yamaguchi, Y.; Shen, W.-J.; Morser, J.; Leung, L.L. Dynamic and tissue-specific proteolytic processing of chemerin in obese mice. PLoS ONE 2018, 13, e0202780. [Google Scholar] [CrossRef]
- Du, X.Y.; Zabel, B.A.; Myles, T.; Allen, S.J.; Handel, T.M.; Lee, P.P.; Butcher, E.C.; Leung, L.L. Regulation of chemerin bioactivity by plasma carboxypeptidase N, carboxypeptidase B (activated thrombin-activable fibrinolysis inhibitor), and platelets. J. Biol. Chem. 2009, 284, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.S.; Eisenberg, D.; Zhao, L.; Adams, C.; Leib, R.; Morser, J.; Leung, L. Chemerin activation in human obesity. Obesity 2016, 24, 1522–1529. [Google Scholar] [CrossRef] [PubMed]
- Ferland, D.J.; Seitz, B.; Darios, E.S.; Thompson, J.M.; Yeh, S.T.; Mullick, A.E.; Watts, S.W. Whole-Body but Not Hepatic Knockdown of Chemerin by Antisense Oligonucleotide Decreases Blood Pressure in Rats. J. Pharmacol. Exp. Ther. 2018, 365, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Leung, L.L.; Morser, J. Chemerin Forms: Their Generation and Activity. Biomedicines 2022, 10, 2018. [Google Scholar] [CrossRef]
- Ge, X.; Yamaguchi, Y.; Zhao, L.; Bury, L.; Gresele, P.; Berube, C.; Leung, L.L.; Morser, J. Prochemerin cleavage by factor XIa links coagulation and inflammation. Blood 2018, 131, 353–364. [Google Scholar] [CrossRef]
- Watts, S.W.; Dorrance, A.M.; Penfold, M.E.; Rourke, J.L.; Sinal, C.J.; Seitz, B.; Sullivan, T.J.; Charvat, T.T.; Thompson, J.M.; Burnett, R.; et al. Chemerin connects fat to arterial contraction. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 1320–1328. [Google Scholar] [CrossRef]
- Darios, E.S.; Winner, B.M.; Charvat, T.; Krasinksi, A.; Punna, S.; Watts, S.W. The adipokine chemerin amplifies electrical field-stimulated contraction in the isolated rat superior mesenteric artery. Am. J. Physiol. Heart Circ. Physiol. 2016, 311, H498–H507. [Google Scholar] [CrossRef]
- Kennedy, A.J.; Yang, P.; Read, C.; Kuc, R.E.; Yang, L.; Taylor, E.J.; Taylor, C.W.; Maguire, J.J.; Davenport, A.P. Chemerin Elicits Potent Constrictor Actions via Chemokine-Like Receptor 1 (CMKLR1), not G-Protein-Coupled Receptor 1 (GPR1), in Human and Rat Vasculature. J. Am. Heart Assoc. 2016, 5, e004421. [Google Scholar] [CrossRef]
- Muruganandan, S.; Roman, A.A.; Sinal, C.J. Role of chemerin/CMKLR1 signaling in adipogenesis and osteoblastogenesis of bone marrow stem cells. J. Bone Miner. Res. 2010, 25, 222–234. [Google Scholar] [CrossRef]
- Bondue, B.; Wittamer, V.; Parmentier, M. Chemerin and its receptors in leukocyte trafficking, inflammation and metabolism. Cytokine Growth Factor Rev. 2011, 22, 331–338. [Google Scholar] [CrossRef]
- Yang, Y.-L.; Ren, L.-R.; Sun, L.-F.; Huang, C.; Xiao, T.-X.; Wang, B.-B.; Chen, J.; Zabel, B.A.; Ren, P.; Zhang, J.V. The role of GPR1 signaling in mice corpus luteum. J. Endocrinol. 2016, 230, 55. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zabel, B.A.; Nakae, S.; Zúñiga, L.; Kim, J.-Y.; Ohyama, T.; Alt, C.; Pan, J.; Suto, H.; Soler, D.; Allen, S.J. Mast cell–expressed orphan receptor CCRL2 binds chemerin and is required for optimal induction of IgE-mediated passive cutaneous anaphylaxis. J. Exp. Med. 2008, 205, 2207–2220. [Google Scholar] [CrossRef]
- De Henau, O.; Degroot, G.-N.; Imbault, V.; Robert, V.; De Poorter, C.; McHeik, S.; Galés, C.; Parmentier, M.; Springael, J.-Y. Signaling Properties of Chemerin Receptors CMKLR1, GPR1 and CCRL2. PLoS ONE 2016, 11, e0164179. [Google Scholar] [CrossRef] [PubMed]
- Rourke, J.L.; Muruganandan, S.; Dranse, H.J.; McMullen, N.M.; Sinal, C.J. Gpr1 is an active chemerin receptor influencing glucose homeostasis in obese mice. J. Endocrinol. 2014, 222, 201–215. [Google Scholar] [CrossRef] [PubMed]
- Fischer, T.F.; Czerniak, A.S.; Weiß, T.; Schoeder, C.T.; Wolf, P.; Seitz, O.; Meiler, J.; Beck-Sickinger, A.G. Ligand-binding and -scavenging of the chemerin receptor GPR1. Cell. Mol. Life Sci. 2021, 78, 6265–6281. [Google Scholar] [CrossRef]
- Mazzotti, C.; Gagliostro, V.; Bosisio, D.; Del Prete, A.; Tiberio, L.; Thelen, M.; Sozzani, S. The atypical receptor CCRL2 (CC Chemokine Receptor-Like 2) does not act as a decoy receptor in endothelial cells. Front. Immunol. 2017, 8, 1233. [Google Scholar] [CrossRef]
- Schioppa, T.; Sozio, F.; Barbazza, I.; Scutera, S.; Bosisio, D.; Sozzani, S.; Del Prete, A. Molecular Basis for CCRL2 Regulation of Leukocyte Migration. Front. Cell Dev. Biol. 2020, 8, 615031. [Google Scholar] [CrossRef]
- Zabel, B.A.; Zuniga, L.; Ohyama, T.; Allen, S.J.; Cichy, J.; Handel, T.M.; Butcher, E.C. Chemoattractants, extracellular proteases, and the integrated host defense response. Exp. Hematol. 2006, 34, 1021–1032. [Google Scholar] [CrossRef]
- Jia, J.; Yu, F.; Xiong, Y.; Wei, W.; Ma, H.; Nisi, F.; Song, X.; Yang, L.; Wang, D.; Yuan, G.; et al. Chemerin enhances the adhesion and migration of human endothelial progenitor cells and increases lipid accumulation in mice with atherosclerosis. Lipids Health Dis. 2020, 19, 207. [Google Scholar] [CrossRef]
- Samson, M.; Edinger, A.L.; Stordeur, P.; Rucker, J.; Verhasselt, V.; Sharron, M.; Govaerts, C.; Mollereau, C.; Vassart, G.; Doms, R.W.; et al. ChemR23, a putative chemoattractant receptor, is expressed in monocyte-derived dendritic cells and macrophages and is a coreceptor for SIV and some primary HIV-1 strains. Eur. J. Immunol. 1998, 28, 1689–1700. [Google Scholar] [CrossRef]
- Zabel, B.A.; Silverio, A.M.; Butcher, E.C. Chemokine-like receptor 1 expression and chemerin-directed chemotaxis distinguish plasmacytoid from myeloid dendritic cells in human blood. J. Immunol. 2005, 174, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Yamawaki, H.; Kameshima, S.; Usui, T.; Okada, M.; Hara, Y. A novel adipocytokine, chemerin exerts anti-inflammatory roles in human vascular endothelial cells. Biochem. Biophys. Res. Commun. 2012, 423, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Kaur, J.; Adya, R.; Tan, B.K.; Chen, J.; Randeva, H.S. Identification of chemerin receptor (ChemR23) in human endothelial cells: Chemerin-induced endothelial angiogenesis. Biochem. Biophys. Res. Commun. 2010, 391, 1762–1768. [Google Scholar] [CrossRef]
- Bozaoglu, K.; Curran, J.E.; Stocker, C.J.; Zaibi, M.S.; Segal, D.; Konstantopoulos, N.; Morrison, S.; Carless, M.; Dyer, T.D.; Cole, S.A.; et al. Chemerin, a novel adipokine in the regulation of angiogenesis. J. Clin. Endocrinol. Metab. 2010, 95, 2476–2485. [Google Scholar] [CrossRef] [PubMed]
- Ferland, D.J.; Watts, S.W. Chemerin: A comprehensive review elucidating the need for cardiovascular research. Pharmacol. Res. 2015, 99, 351–361. [Google Scholar] [CrossRef]
- Neves, K.B.; Lobato, N.S.; Lopes, R.A.; Filgueira, F.P.; Zanotto, C.Z.; Oliveira, A.M.; Tostes, R.C. Chemerin reduces vascular nitric oxide/cGMP signalling in rat aorta: A link to vascular dysfunction in obesity? Clin. Sci. 2014, 127, 111–122. [Google Scholar] [CrossRef]
- Didion, S.P.; Heistad, D.D.; Faraci, F.M. Mechanisms That Produce Nitric Oxide–Mediated Relaxation of Cerebral Arteries during Atherosclerosis. Stroke 2001, 32, 761–766. [Google Scholar] [CrossRef]
- Siragusa, M.; Fleming, I. The eNOS signalosome and its link to endothelial dysfunction. Pflug. Arch. Eur. J. Physiol. 2016, 468, 1125–1137. [Google Scholar] [CrossRef]
- Xie, Y.; Liu, L. Role of Chemerin/ChemR23 axis as an emerging therapeutic perspective on obesity-related vascular dysfunction. J. Transl. Med. 2022, 20, 141. [Google Scholar] [CrossRef]
- Nakamura, N.; Naruse, K.; Kobayashi, Y.; Miyabe, M.; Saiki, T.; Enomoto, A.; Takahashi, M.; Matsubara, T. Chemerin promotes angiogenesis in vivo. Physiol. Rep. 2018, 6, e13962. [Google Scholar] [CrossRef]
- Corre, I.; Paris, F.; Huot, J. The p38 pathway, a major pleiotropic cascade that transduces stress and metastatic signals in endothelial cells. Oncotarget 2017, 8, 55684–55714. [Google Scholar] [CrossRef] [PubMed]
- Luangsay, S.; Wittamer, V.; Bondue, B.; De Henau, O.; Rouger, L.; Brait, M.; Franssen, J.D.; de Nadai, P.; Huaux, F.; Parmentier, M. Mouse ChemR23 is expressed in dendritic cell subsets and macrophages, and mediates an anti-inflammatory activity of chemerin in a lung disease model. J. Immunol. 2009, 183, 6489–6499. [Google Scholar] [CrossRef] [PubMed]
- Haybar, H.; Shahrabi, S.; Rezaeeyan, H.; Shirzad, R.; Saki, N. Endothelial Cells: From Dysfunction Mechanism to Pharmacological Effect in Cardiovascular Disease. Cardiovasc. Toxicol. 2019, 19, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Dimitriadis, G.K.; Kaur, J.; Adya, R.; Miras, A.D.; Mattu, H.S.; Hattersley, J.G.; Kaltsas, G.; Tan, B.K.; Randeva, H.S. Chemerin induces endothelial cell inflammation: Activation of nuclear factor-kappa beta and monocyte-endothelial adhesion. Oncotarget 2018, 9, 16678–16690. [Google Scholar] [CrossRef]
- Neves, K.B.; Nguyen Dinh Cat, A.; Lopes, R.A.; Rios, F.J.; Anagnostopoulou, A.; Lobato, N.S.; de Oliveira, A.M.; Tostes, R.C.; Montezano, A.C.; Touyz, R.M. Chemerin Regulates Crosstalk Between Adipocytes and Vascular Cells Through Nox. Hypertension 2015, 66, 657–666. [Google Scholar] [CrossRef]
- Landgraf, K.; Friebe, D.; Ullrich, T.; Kratzsch, J.; Dittrich, K.; Herberth, G.; Adams, V.; Kiess, W.; Erbs, S.; Körner, A. Chemerin as a mediator between obesity and vascular inflammation in children. J. Clin. Endocrinol. Metab. 2012, 97, E556–E564. [Google Scholar] [CrossRef]
- Shen, W.; Tian, C.; Chen, H.; Yang, Y.; Zhu, D.; Gao, P.; Liu, J. Oxidative stress mediates chemerin-induced autophagy in endothelial cells. Free Radic. Biol. Med. 2013, 55, 73–82. [Google Scholar] [CrossRef]
- Shi, N.; Mei, X.; Chen, S.Y. Smooth Muscle Cells in Vascular Remodeling. Arterioscler. Thromb. Vasc. Biol. 2019, 39, e247–e252. [Google Scholar] [CrossRef]
- Wen, J.; Wang, J.; Guo, L.; Cai, W.; Wu, Y.; Chen, W.; Tang, X. Chemerin stimulates aortic smooth muscle cell proliferation and migration via activation of autophagy in VSMCs of metabolic hypertension rats. Am. J. Transl. Res. 2019, 11, 1327–1342. [Google Scholar]
- Kunimoto, H.; Kazama, K.; Takai, M.; Oda, M.; Okada, M.; Yamawaki, H. Chemerin promotes the proliferation and migration of vascular smooth muscle and increases mouse blood pressure. Am. J. Physiol. Heart Circ. Physiol. 2015, 309, H1017–H1028. [Google Scholar] [CrossRef]
- Hanthazi, A.; Jespers, P.; Vegh, G.; Dubois, C.; Hubesch, G.; Springael, J.Y.; Dewachter, L.; Mc Entee, K. Chemerin Added to Endothelin-1 Promotes Rat Pulmonary Artery Smooth Muscle Cell Proliferation and Migration. Front. Physiol. 2020, 11, 926. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, Z.; Yang, B.; Yi, J.; Zhu, S.; Lu, S.; Liu, Y.; Li, Y.; Li, Y.; Mehmood, K.; Hussain, R.; et al. Exposure to Fluoride induces apoptosis in liver of ducks by regulating Cyt-C/Caspase 3/9 signaling pathway. Ecotoxicol. Environ. Saf. 2021, 224, 112662. [Google Scholar] [CrossRef] [PubMed]
- Satoh, K.; Nigro, P.; Berk, B.C. Oxidative stress and vascular smooth muscle cell growth: A mechanistic linkage by cyclophilin A. Antioxid. Redox Signal. 2010, 12, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Zhang, E.; Senapati, P.; Amaram, V.; Reddy, M.A.; Stapleton, K.; Leung, A.; Lanting, L.; Wang, M.; Chen, Z.; et al. A Novel Angiotensin II-Induced Long Noncoding RNA Giver Regulates Oxidative Stress, Inflammation, and Proliferation in Vascular Smooth Muscle Cells. Circ. Res. 2018, 123, 1298–1312. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Zhou, Q.; Zheng, X.; Sun, B.; Zhao, S. Mitoquinone attenuates vascular calcification by suppressing oxidative stress and reducing apoptosis of vascular smooth muscle cells via the Keap1/Nrf2 pathway. Free Radic. Biol. Med. 2020, 161, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Schurgers, L.J.; Shiels, P.G.; Stenvinkel, P. Early vascular ageing in chronic kidney disease: Impact of inflammation, vitamin K, senescence and genomic damage. Nephrol. Dial. Transplant. 2020, 35, ii31–ii37. [Google Scholar] [CrossRef]
- Er, L.K.; Hsu, L.A. Circulating Chemerin Levels, but not the RARRES2 Polymorphisms, Predict the Long-Term Outcome of Angiographically Confirmed Coronary Artery Disease. Int. J. Mol. Sci. 2019, 20, 1174. [Google Scholar] [CrossRef]
- Eichelmann, F.; Schulze, M.B.; Wittenbecher, C.; Menzel, J.; Weikert, C.; di Giuseppe, R.; Biemann, R.; Isermann, B.; Fritsche, A.; Boeing, H.; et al. Chemerin as a Biomarker Linking Inflammation and Cardiovascular Diseases. J. Am. Coll. Cardiol. 2019, 73, 378–379. [Google Scholar] [CrossRef]
- Kostopoulos, C.G.; Spiroglou, S.G.; Varakis, J.N.; Apostolakis, E.; Papadaki, H.H. Chemerin and CMKLR1 expression in human arteries and periadventitial fat: A possible role for local chemerin in atherosclerosis? BMC Cardiovasc. Disord. 2014, 14, 56. [Google Scholar] [CrossRef]
- Dessein, P.H.; Tsang, L.; Woodiwiss, A.J.; Norton, G.R.; Solomon, A. Circulating concentrations of the novel adipokine chemerin are associated with cardiovascular disease risk in rheumatoid arthritis. J. Rheumatol. 2014, 41, 1746–1754. [Google Scholar] [CrossRef]
- Zhang, G.; Xiao, M.; Zhang, L.; Zhao, Y.; Yang, Q. Association of serum chemerin concentrations with the presence of atrial fibrillation. Ann. Clin. Biochem. 2017, 54, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Tao, Y.; Chen, Y.; Xu, W.; Qian, Z.; Lu, X. Serum Chemerin as a Novel Prognostic Indicator in Chronic Heart Failure. J. Am. Heart Assoc. 2019, 8, e012091. [Google Scholar] [CrossRef] [PubMed]
- Kammerer, A.; Staab, H.; Herberg, M.; Kerner, C.; Klöting, N.; Aust, G. Increased circulating chemerin in patients with advanced carotid stenosis. BMC Cardiovasc. Disord. 2018, 18, 65. [Google Scholar] [CrossRef] [PubMed]
- Müssig, K.; Staiger, H.; Machicao, F.; Thamer, C.; Machann, J.; Schick, F.; Claussen, C.D.; Stefan, N.; Fritsche, A.; Häring, H.U. RARRES2, encoding the novel adipokine chemerin, is a genetic determinant of disproportionate regional body fat distribution: A comparative magnetic resonance imaging study. Metab. Clin. Exp. 2009, 58, 519–524. [Google Scholar] [CrossRef]
- Ministrini, S.; Ricci, M.A.; Nulli Migliola, E.; De Vuono, S.; D’Abbondanza, M.; Paganelli, M.T.; Vaudo, G.; Siepi, D.; Lupattelli, G. Chemerin predicts carotid intima-media thickening in severe obesity. Eur. J. Clin. Investig. 2020, 50, e13256. [Google Scholar] [CrossRef]
- El Dayem, S.M.; Battah, A.A.; El Bohy Ael, M.; El Shehaby, A.; El Ghaffar, E.A. Relationship of plasma level of chemerin and vaspin to early atherosclerotic changes and cardiac autonomic neuropathy in adolescent type 1 diabetic patients. J. Pediatr. Endocrinol. Metab. JPEM 2015, 28, 265–273. [Google Scholar] [CrossRef]
- Maghsoudi, Z.; Kelishadi, R.; Hosseinzadeh-Attar, M.J. The comparison of chemerin, adiponectin and lipid profile indices in obese and non-obese adolescents. Diabetes Metab. Syndr. 2016, 10, S43–S46. [Google Scholar] [CrossRef]
- Hah, Y.J.; Kim, N.K.; Kim, M.K.; Kim, H.S.; Hur, S.H.; Yoon, H.J.; Kim, Y.N.; Park, K.G. Relationship between Chemerin Levels and Cardiometabolic Parameters and Degree of Coronary Stenosis in Korean Patients with Coronary Artery Disease. Diabetes Metab. J. 2011, 35, 248–254. [Google Scholar] [CrossRef]
- Molina Mdel, C.; Faria, C.P.; Montero, M.P.; Cade, N.V.; Mill, J.G. Cardiovascular risk factors in 7-to-10-year-old children in Vitória, Espírito Santo State, Brazil. Cad. Saude Publica 2010, 26, 909–917. [Google Scholar] [CrossRef][Green Version]
- Ferranti, S.D.d.; Steinberger, J.; Ameduri, R.; Baker, A.; Gooding, H.; Kelly, A.S.; Mietus-Snyder, M.; Mitsnefes, M.M.; Peterson, A.L.; St-Pierre, J.; et al. Cardiovascular Risk Reduction in High-Risk Pediatric Patients: A Scientific Statement from the American Heart Association. Circulation 2019, 139, e603–e634. [Google Scholar] [CrossRef]
- Ji, Z.-S.; Jiang, H.; Xie, Y.; Wei, Q.-P.; Yin, X.-F.; Ye, J.-H.; Quan, X.-Z.; Lan, Y.-L.; Zhao, M.; Tian, X.-L.; et al. Chemerin promotes the pathogenesis of preeclampsia by activating CMKLR1/p-Akt/CEBPα axis and inducing M1 macrophage polarization. Cell Biol. Toxicol. 2022, 38, 611–628. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Wang, L.; Zhang, L.; Hong, Z.; Xia, R.; Zhao, Z.; Zhang, L. Analysis of the expression levels of chemerin, ox-LDL, MMP-9, and PAPP-A in ICVD patients and their relationship with the severity of neurological impairment. Brain Behav. 2022, 12, e2613. [Google Scholar] [CrossRef] [PubMed]
- Ji, Q.; Lin, Y.; Liang, Z.; Yu, K.; Liu, Y.; Fang, Z.; Liu, L.; Shi, Y.; Zeng, Q.; Chang, C.; et al. Chemerin is a novel biomarker of acute coronary syndrome but not of stable angina pectoris. Cardiovasc. Diabetol. 2014, 13, 145. [Google Scholar] [CrossRef] [PubMed]
- Gu, P.; Cheng, M.; Hui, X.; Lu, B.; Jiang, W.; Shi, Z. Elevating circulation chemerin level is associated with endothelial dysfunction and early atherosclerotic changes in essential hypertensive patients. J. Hypertens. 2015, 33, 1624–1632. [Google Scholar] [CrossRef] [PubMed]
- Wójcik, M.; Kozioł-Kozakowska, A.; Januś, D.; Furtak, A.; Małek, A.; Sztefko, K.; Starzyk, J.B. Circulating chemerin level may be associated with early vascular pathology in obese children without overt arterial hypertension—Preliminary results. J. Pediatr. Endocrinol. Metab. JPEM 2020, 33, 729–734. [Google Scholar] [CrossRef]
- Thomas, G.D. Neural control of the circulation. Adv. Physiol. Educ. 2011, 35, 28–32. [Google Scholar] [CrossRef]
- Omori, A.; Goshima, M.; Kakuda, C.; Kodama, T.; Otani, K.; Okada, M.; Yamawaki, H. Chemerin-9-induced contraction was enhanced through the upregulation of smooth muscle chemokine-like receptor 1 in isolated pulmonary artery of pulmonary arterial hypertensive rats. Pflug. Arch. Eur. J. Physiol. 2020, 472, 335–342. [Google Scholar] [CrossRef]
- Aydın, K.; Canpolat, U.; Akın, Ş.; Dural, M.; Karakaya, J.; Aytemir, K.; Özer, N.; Gürlek, A. Chemerin is not associated with subclinical atherosclerosis markers in prediabetes and diabetes. Anatol. J. Cardiol. 2016, 16, 749–755. [Google Scholar] [CrossRef]
- Zylla, S.; Dörr, M.; Völzke, H.; Schminke, U.; Felix, S.B.; Nauck, M.; Friedrich, N. Association of Circulating Chemerin with Subclinical Parameters of Atherosclerosis: Results of a Population-Based Study. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 1656–1664. [Google Scholar] [CrossRef]
- Liang, Z.; Yu, K.; Wu, B.; Zhong, Y.; Zeng, Q. The elevated levels of plasma chemerin and C-reactive protein in patients with acute coronary syndrome. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi = Chin. J. Cell. Mol. Immunol. 2015, 31, 953–956. [Google Scholar]
- Aronis, K.N.; Sahin-Efe, A.; Chamberland, J.P.; Spiro, A., 3rd; Vokonas, P.; Mantzoros, C.S. Chemerin levels as predictor of acute coronary events: A case-control study nested within the veterans affairs normative aging study. Metab. Clin. Exp. 2014, 63, 760–766. [Google Scholar] [CrossRef] [PubMed]
- Borghetti, G.; von Lewinski, D.; Eaton, D.M.; Sourij, H.; Houser, S.R.; Wallner, M. Diabetic Cardiomyopathy: Current and Future Therapies. Beyond Glycemic Control. Front. Physiol. 2018, 9, 1514. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Liu, S.; Guo, B.; Chang, L.; Li, Y. Chemerin Induces Insulin Resistance in Rat Cardiomyocytes in Part through the ERK1/2 Signaling Pathway. Pharmacology 2014, 94, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Lamparter, J.; Raum, P.; Pfeiffer, N.; Peto, T.; Höhn, R.; Elflein, H.; Wild, P.; Schulz, A.; Schneider, A.; Mirshahi, A. Prevalence and associations of diabetic retinopathy in a large cohort of prediabetic subjects: The Gutenberg Health Study. J. Diabetes Its Complicat. 2014, 28, 482–487. [Google Scholar] [CrossRef]
- Li, J.; Song, L.-L.; Song, H. Chemerin promotes microangiopathy in diabetic retinopathy via activation of ChemR23 in rat primary microvascular endothelial cells. Mol. Vis. 2021, 27, 575–587. [Google Scholar]
- Rodríguez-Penas, D.; Feijóo-Bandín, S.; García-Rúa, V.; Mosquera-Leal, A.; Durán, D.; Varela, A.; Portolés, M.; Roselló-Lletí, E.; Rivera, M.; Diéguez, C.; et al. The Adipokine Chemerin Induces Apoptosis in Cardiomyocytes. Cell Physiol. Biochem. 2015, 37, 176–192. [Google Scholar] [CrossRef]
- Yamamoto, A.; Sagara, A.; Otani, K.; Okada, M.; Yamawaki, H. Chemerin-9 stimulates migration in rat cardiac fibroblasts in vitro. Eur. J. Pharmacol. 2021, 912, 174566. [Google Scholar] [CrossRef]
- Huang, X.; Fu, C.; Liu, W.; Liang, Y.; Li, P.; Liu, Z.; Sheng, Q.; Liu, P. Chemerin-induced angiogenesis and adipogenesis in 3 T3-L1 preadipocytes is mediated by lncRNA Meg3 through regulating Dickkopf-3 by sponging miR-217. Toxicol. Appl. Pharmacol. 2019, 385, 114815. [Google Scholar] [CrossRef]
- Ferland, D.J.; Garver, H.; Contreras, G.A.; Fink, G.D.; Watts, S.W. Chemerin contributes to in vivo adipogenesis in a location-specific manner. PLoS ONE 2020, 15, e0229251. [Google Scholar] [CrossRef]
- Watts, S.W.; Darios, E.S.; Mullick, A.E.; Garver, H.; Saunders, T.L.; Hughes, E.D.; Filipiak, W.E.; Zeidler, M.G.; McMullen, N.; Sinal, C.J.; et al. The chemerin knockout rat reveals chemerin dependence in female, but not male, experimental hypertension. Faseb J. 2018, 32, fj201800479. [Google Scholar] [CrossRef]
- Flood, E.D.; Watts, S.W. Endogenous Chemerin from PVAT Amplifies Electrical Field-Stimulated Arterial Contraction: Use of the Chemerin Knockout Rat. Int. J. Mol. Sci. 2020, 21, 6392. [Google Scholar] [CrossRef] [PubMed]
- van der Vorst, E.P.C.; Mandl, M.; Müller, M.; Neideck, C.; Jansen, Y.; Hristov, M.; Gencer, S.; Peters, L.J.F.; Meiler, S.; Feld, M.; et al. Hematopoietic ChemR23 (Chemerin Receptor 23) Fuels Atherosclerosis by Sustaining an M1 Macrophage-Phenotype and Guidance of Plasmacytoid Dendritic Cells to Murine Lesions-Brief Report. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Xiong, W.; Luo, Y.; Chen, H.; He, Y.; Cao, Y.; Dong, S. Adipokine Chemerin Stimulates Progression of Atherosclerosis in ApoE(-/-) Mice. Biomed. Res. Int. 2019, 2019, 7157865. [Google Scholar] [CrossRef] [PubMed]
- Lachine, N.A.; Elnekiedy, A.A.; Megallaa, M.H.; Khalil, G.I.; Sadaka, M.A.; Rohoma, K.H.; Kassab, H.S. Serum chemerin and high-sensitivity C reactive protein as markers of subclinical atherosclerosis in Egyptian patients with type 2 diabetes. Ther. Adv. Endocrinol. Metab. 2016, 7, 47–56. [Google Scholar] [CrossRef]
- Neves, K.B.; Nguyen Dinh Cat, A.; Alves-Lopes, R.; Harvey, K.Y.; Costa, R.M.D.; Lobato, N.S.; Montezano, A.C.; Oliveira, A.M.; Touyz, R.M.; Tostes, R.C. Chemerin receptor blockade improves vascular function in diabetic obese mice via redox-sensitive and Akt-dependent pathways. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H1851–H1860. [Google Scholar] [CrossRef]
- Jannaway, M.; Torrens, C.; Warner, J.A.; Sampson, A.P. Resolvin E1, resolvin D1 and resolvin D2 inhibit constriction of rat thoracic aorta and human pulmonary artery induced by the thromboxane mimetic U46619. Br. J. Pharmacol. 2018, 175, 1100–1108. [Google Scholar] [CrossRef]
- Goralski, K.B.; Sinal, C.J. Elucidation of chemerin and chemokine-like receptor-1 function in adipocytes by adenoviral-mediated shRNA knockdown of gene expression. Methods Enzymol. 2009, 460, 289–312. [Google Scholar] [CrossRef]
- Du, J.; Teng, R.J.; Guan, T.; Eis, A.; Kaul, S.; Konduri, G.G.; Shi, Y. Role of autophagy in angiogenesis in aortic endothelial cells. Am. J. Physiol. Cell Physiol. 2012, 302, C383–C391. [Google Scholar] [CrossRef]
- Carracedo, M.; Artiach, G.; Arnardottir, H.; Bäck, M. The resolution of inflammation through omega-3 fatty acids in atherosclerosis, intimal hyperplasia, and vascular calcification. Semin. Immunopathol. 2019, 41, 757–766. [Google Scholar] [CrossRef]
- Bäck, M.; Hansson, G.K. Omega-3 fatty acids, cardiovascular risk, and the resolution of inflammation. Faseb J. 2019, 33, 1536–1539. [Google Scholar] [CrossRef]
- Hasturk, H.; Abdallah, R.; Kantarci, A.; Nguyen, D.; Giordano, N.; Hamilton, J.; Van Dyke, T.E. Resolvin E1 (RvE1) Attenuates Atherosclerotic Plaque Formation in Diet and Inflammation-Induced Atherogenesis. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 1123–1133. [Google Scholar] [CrossRef] [PubMed]
- Carracedo, M.; Artiach, G.; Witasp, A.; Clària, J.; Carlström, M.; Laguna-Fernandez, A.; Stenvinkel, P.; Bäck, M. The G-protein coupled receptor ChemR23 determines smooth muscle cell phenotypic switching to enhance high phosphate-induced vascular calcification. Cardiovasc. Res. 2019, 115, 1557–1566. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Yoshizawa, H.; Seki, T.; Shirai, R.; Yamashita, T.; Okano, T.; Shibata, K.; Wakamatsu, M.J.; Mori, Y.; Morita, T. Chemerin-9, a potent agonist of chemerin receptor (ChemR23), prevents atherogenesis. Clin. Sci. 2019, 133, 1779–1796. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Han, C. Chemerin-9 Attenuates Experimental Abdominal Aortic Aneurysm Formation in ApoE(-/-) Mice. J. Oncol. 2021, 2021, 6629204. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Yamaguchi, Y.; Ge, X.; Robinson, W.H.; Morser, J. Chemerin 156F, generated by chymase cleavage of prochemerin, is elevated in joint fluids of arthritis patients. Arthritis Res. Ther. 2018, 20, 132. [Google Scholar] [CrossRef]
- Spirk, M.; Zimny, S.; Neumann, M.; McMullen, N.; Sinal, C.J.; Buechler, C. Chemerin-156 is the Active Isoform in Human Hepatic Stellate Cells. Int. J. Mol. Sci. 2020, 21, 7555. [Google Scholar] [CrossRef]
- Ferland, D.J.; Flood, E.D.; Garver, H.; Yeh, S.T.; Riney, S.; Mullick, A.E.; Fink, G.D.; Watts, S.W. Different blood pressure responses in hypertensive rats following chemerin mRNA inhibition in dietary high fat compared to dietary high-salt conditions. Physiol. Genom. 2019, 51, 553–561. [Google Scholar] [CrossRef]

| Patient’s Gender and Age (Mean ± SD or Median and Range) | CVDs | Chemerin Levels | CV- Associated Disorders/Parameters and Chemerin Correlation | Reference |
| male and female 11.6 ± 2.0 | ↑ BMI | ↑ (serum) | BMI, waist circumference, leptin, body fat insulin, HDL-C and TC | [85] |
| male and female 48.4 ± 10.9 | dyslipidemia, hypertension | ↑ (plasma) | RARRES2 gene polymorphism, hs-CRP | [138] |
| male and female 43.5 ± 13.0 | rheumatoid factor-positive, hypertension | ↑ (plasma) | Hs-CRP, leptin, vascular adhesion molecule, monocyte chemoattractant protein | [141] |
| male and female 60.54 ± 9.64 | arterial fibrillation | ↑ (serum) | arterial fibrillation, BMI, SBP, DBP, TC, LDL-C, creatinine, hs-CRP and left atrial diameter | [142] |
| male and female 66 (58–75) | hypertension, chronic heart failure, diabetes, hyperlipidemia | ↑ (serum) | heart failure, diabetes, hs-CRP, hypertension | [143] |
| male and female 66.9 ± 0.6 | coronary artery disease | ↑ (plasma) | TC, hsCRP, peripheral leukocyte count, TNF-α | [144] |
| male and female 45.5 (18–69) | ↑ BMI, impaired glucose tolerance | ↑RARRES2 expression (whole blood) | visceral fat mass | [145] |
| male and female 44.0 ± 10.1 | hypertension, diabetes, ↑ BMI | ↑ (plasma) | waist circumference, HOMA-IR, fat mass, HbA1c, cIMT | [146] |
| male and female 16.3 ± 1.5 | atherosclerotic lesions and cardiac autonomic neuropathy, diabetes type 1 | ↑ (serum) | vaspin and LDL-C | [147] |
| female 13.9 ± 1.8 | ↑ BMI | ↑ (serum) | TG, HDL-C, LDL-C and fat mass | [148] |
| male and female 62.2 ± 10.0 | coronary stenosis, hypertension, diabetes | ↑ (serum) | fasting glucose, TC, LDL-C, hs-CRP, degree of coronary artery stenosis | [149] |
| Cell Culture Model | Chemerin Concentrations | Duration of Stimulation | CV-Associated Disorders/Parameters–Chemerin Correlation | Reference |
| human microvascular endothelial cells | 10 nM | 2 h | ↑ endothelial cell adhesion, protein expression and secretion, activates NF-KB | [125] |
| human microvascular endothelial and vascular smooth muscle cells | 50 ng/mL | 5, 15, 30, 60 min and 2, 8, 24 h | ↑ O2·−, ↑ H2O2, ↑ Nox 1, ↑ Nox 4 and ↑ miRNA expression, ↑ phosphorylation of SAPK/JNK and ERK1/2, ↓ eNOS, ↓ NO and apoptosis | [126] |
| human peripheral blood mononuclear cells | 2.5, 25, 50 and 100 ng/mL | 12, 24, 36 and 48 h. | ↑ adhesion and migration abilities of endothelial progenitor cells | [138] |
| Animals (Gender) | Tissues | CV-Associated Disorders/Parameters–Chemerin Correlation | Reference | |
| chemerin knockout Sprague Dawley rat (female) | thoracic aorta | blood pressure modification | [171] | |
| chemerin knockout Sprague Dawley rat (female) | plasma, mesenteric adipocytes | ↓ visceral adiposity | [170] | |
| chemerin knockout Sprague Dawley rat (female) | superior mesenteric arteries | ↓ vascular tone | [172] | |
| Cell Culture Model | Chemerin Concentrations | Duration of Stimulation | CV-Associated Disorders/Parameters–Chemerin Correlation | Reference |
| rat vascular smooth muscle cells | 1–300 ng/mL | 24 h | ↑ vascular smooth muscle cells proliferation and migration | [131] |
| Sprague Dawley rat’s cardiomyocytes | 10 and 100 ng/mL | 24 h | impaired insulin signalling and induced insulin resistance | [164] |
| Sprague Dawley rat’s cardiomyocytes | 0.1, 1, 10 and 100 nM | 6–48 h | cardiomyocytes apoptosis | [167] |
| mouse 3T3-L1 preadipocytes | 0, 20, 40, 60, 80 and 100 ng/mL | 48 h | miRNA-217 suppression (correlated with fat accumulation), induced preadipocytes differentiation into adipocytes, ↑ Meg3 lncRNA | [169] |
| Wistar rat’s cardiac fibroblasts | 100 ng/mL | 12 h | fibroblast migration, ↑ ROS | [168] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Macvanin, M.T.; Rizzo, M.; Radovanovic, J.; Sonmez, A.; Paneni, F.; Isenovic, E.R. Role of Chemerin in Cardiovascular Diseases. Biomedicines 2022, 10, 2970. https://doi.org/10.3390/biomedicines10112970
Macvanin MT, Rizzo M, Radovanovic J, Sonmez A, Paneni F, Isenovic ER. Role of Chemerin in Cardiovascular Diseases. Biomedicines. 2022; 10(11):2970. https://doi.org/10.3390/biomedicines10112970
Chicago/Turabian StyleMacvanin, Mirjana T., Manfredi Rizzo, Jelena Radovanovic, Alper Sonmez, Francesco Paneni, and Esma R. Isenovic. 2022. "Role of Chemerin in Cardiovascular Diseases" Biomedicines 10, no. 11: 2970. https://doi.org/10.3390/biomedicines10112970
APA StyleMacvanin, M. T., Rizzo, M., Radovanovic, J., Sonmez, A., Paneni, F., & Isenovic, E. R. (2022). Role of Chemerin in Cardiovascular Diseases. Biomedicines, 10(11), 2970. https://doi.org/10.3390/biomedicines10112970