Effectiveness of Physiotherapy in the Treatment of Temporomandibular Joint Dysfunction and the Relationship with Cervical Spine
Abstract
1. Introduction
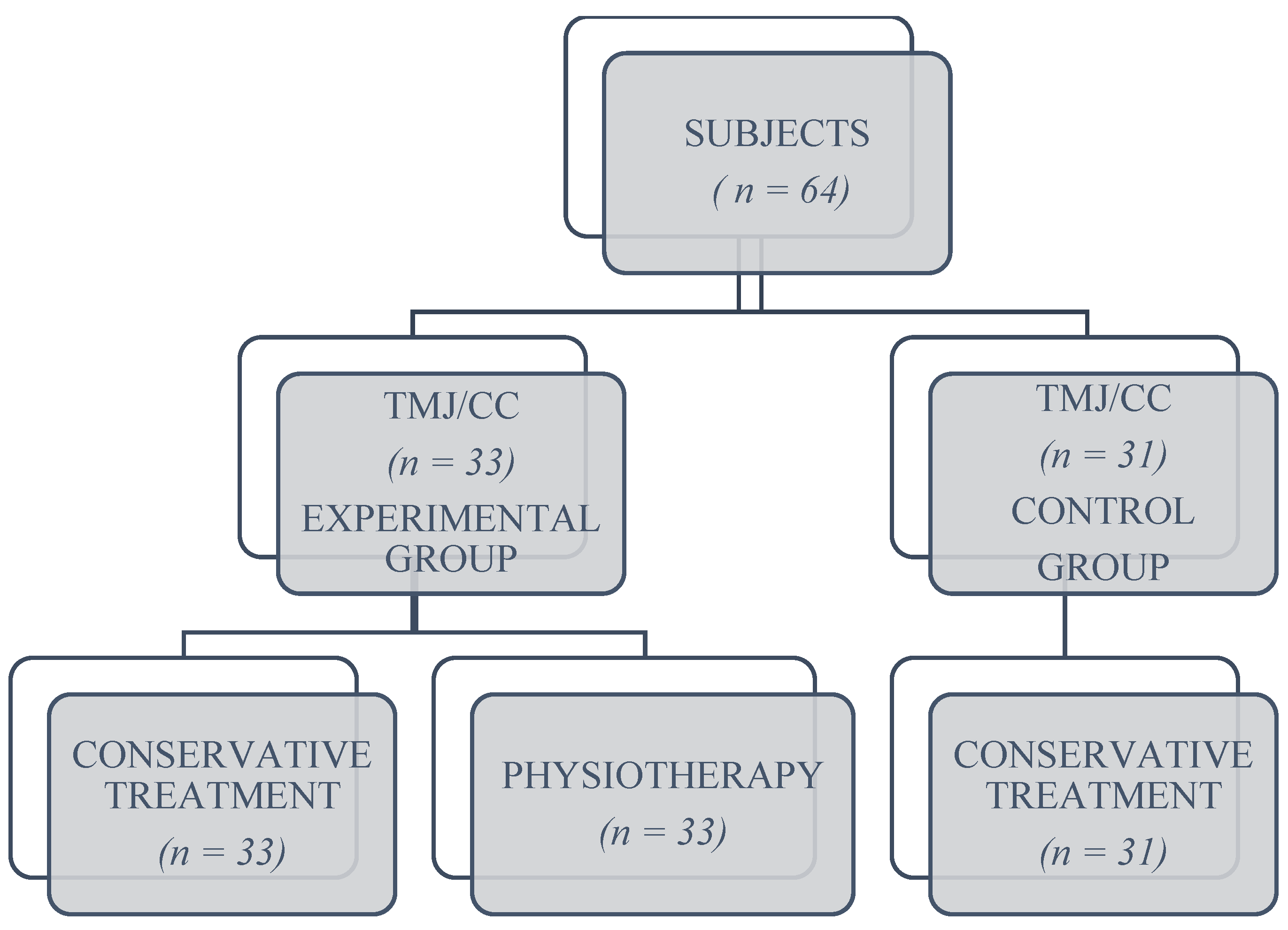
2. Materials and Methods
- Three sessions per week/first 2 weeks;
- Two sessions per week/next 2 weeks; and
- One session per week/up to 3 months.
- Evaluation and treatment of muscle spasms and myalgia for the following muscles: masseter, temporalis, internal pterygoid, external pterygoid, sternocleidomastoid (SCM); upper trapezius, splenius and semispinalis, head and neck massage, detection and treatment of the trigger points, passive, passive–active and active stretching exercises [12,20,21];
- techniques for manipulating the cervical spine to increase range of motion by performing flexion, extension, lateral flexion, and head and neck rotation [24];
- proprioceptive techniques at the TMJ: isometric coordination exercises, with mouth closed, half-open, and open; exercises to correct joint and muscle asymmetries by manual control applied at the cranio–mandibular level, and exercises to open the mouth with the trunk inverted [21];
- techniques to correct deglutition: swallowing a small amount of water, swallowing and speaking while holding a semi-hard small object between the dental arches [27];
- diaphragmatic respiration: patients were instructed on how to breathe for this technique in supine position, breathing properly from the sitting position, and finally from orthostatic position alternating the position of the head and neck;
- corrective techniques for the head, neck and torso from supine position, prone position, sitting position and standing, permanently maintaining visual control of the gesture performed [27,28]. Exercises to stabilize the deep cervical flexors (along the head and neck) maintain the correct posture of the cervical spine, improving muscle control at the craniofacial level [7,25].
Statistical Analysis
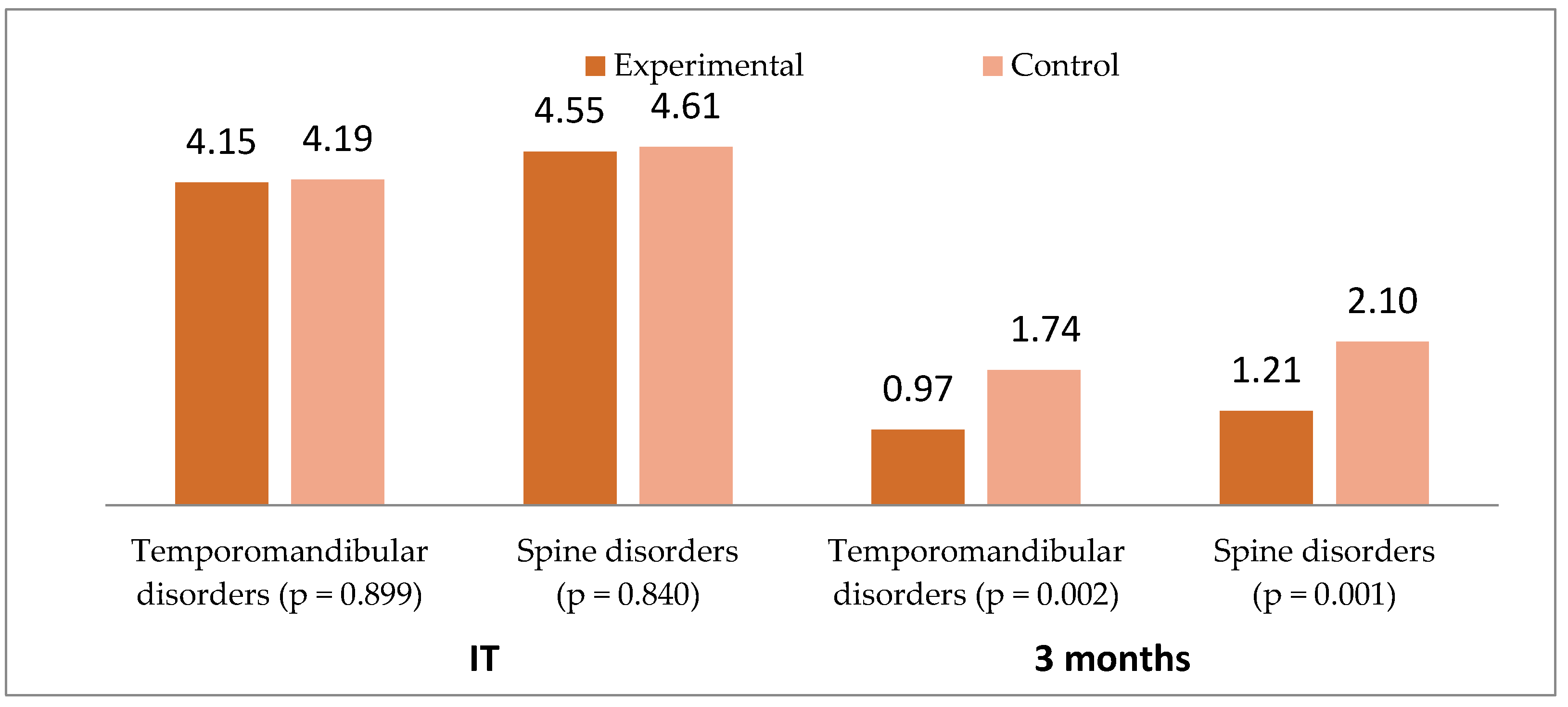
3. Results
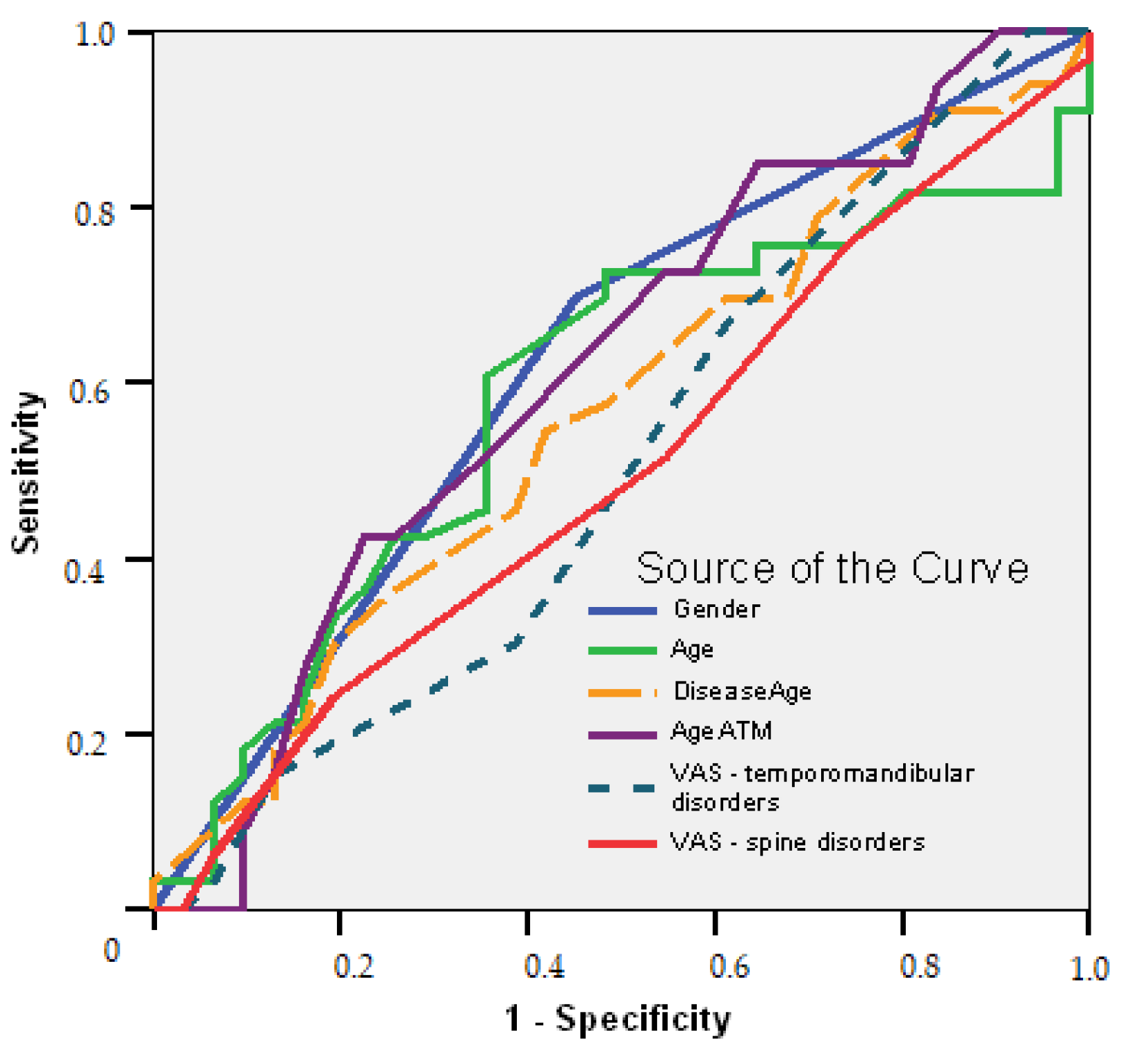
- Female gender is a good predictor of initiation treatment (AUC = 0.623; 95% CI: 0.484–0.761; p = 0.092); long time suffering of the temporomandibular area over 1.5 years is a good predictor to determine the treatment plans, with a sensitivity of 73% and a specificity of 45% (AUC = 0.612; 95% CI: 0.472–0.752; p = 0.124).
4. Discussions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guarda-Nardini, L.; Cadorin, C.; Frizziero, A.; Masiero, S.; Manfredini, D. Interrelationship between temporomandibular joint osteoarthritis (OA) and cervical spine pain: Effects of intra-articular injection with hyaluronic acid. Cranio 2017, 35, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Havriş, M.D.; Iordache, C.; Ancuta, C.; Chirieac, R.M. Contributions to the methodology of study in the functional assessment of temporomandibular joint dysfunctional syndrome. Rev. Med. Chir. Soc. Med. Nat. 2012, 116, 588–594. [Google Scholar]
- Armijo-Olivo, S.; Magee, D. Cervical musculoskeletal impairments and temporomandibular disorders. J. Oral Maxillofac. Res. 2012, 3, e4. [Google Scholar] [CrossRef] [PubMed]
- De Laat, A.; Meuleman, H.; Stevens, A.; Verbeke, G. Correlation between cervical spine and temporomandibular disorders. Clin. Oral Investig. 1998, 2, 54–57. [Google Scholar] [CrossRef]
- Matheus, R.A.; Ramos-Perez, F.M.D.M.; Menezes, A.V.; Ambrosano, G.M.B.; Neto, F.H.; Bóscolo, F.N.; De Almeida, S.M. The relationship between temporomandibular dysfunction and head and cervical posture. J. Appl. Oral Sci. 2009, 17, 204–208. [Google Scholar] [CrossRef]
- Stiesch-Scholz, M.; Fink, M.; Tschemitschek, H.; Rossbach, A. Medical and physical therapy of temporamandibualr joint disk displacement without reduction. J. Craniomandib. Pract. 2002, 20, 85–90. [Google Scholar] [CrossRef]
- Lewis, F.; Naude, B. The effectiveness of physiotherapy in cervicogenic headache and concurring temporomandibular dysfunction: A case report. S. Afr. J. Physiother. 2010, 66, 26–30. [Google Scholar] [CrossRef][Green Version]
- Corum, M. Evaluation of Cervical Dysfunctions in Temporomandibular Disorders. Med. J. Bakirkoy 2021, 17, 72–78. [Google Scholar]
- Réus, J.C.; Polmann, H.; Mendes Souza, B.D.; Flores-Mir, C.; Trevisol Bittencourt, P.C.; Winocur, E.; Okeson, J.; Canto, G.D.L. Association Between Primary Headache and Bruxism: An Updated Systematic Review. J. Oral Facial Pain Headache 2021, 35, 129–138. [Google Scholar] [CrossRef]
- Graff-Radford, S.B.; Abbott, J.J. Temporomandibular disorders and headache. Oral Maxillofac. Surg. Clin. 2016, 28, 335–349. [Google Scholar] [CrossRef]
- Paço, M.; Peleteiro, B.; Duarte, J.; Pinho, T. The Effectiveness of Physiotherapy in the Management of Temporomandibular Disorders: A Systematic Review and Meta-analysis. J. Oral Facial Pain Headache 2016, 30, 210–220. [Google Scholar] [CrossRef] [PubMed]
- Idáñez-Robles, A.M.; Obrero-Gaitán, E.; Lomas-Vega, R.; Osuna-Pérez, M.C.; Cortés-Pérez, I.; Zagalaz-Anula, N. Exercise therapy improves pain and mouth opening in temporomandibular disorders: A systematic review with meta-analysis. Clin. Rehabil. 2022. [Google Scholar] [CrossRef] [PubMed]
- Schiffman, E.; Ohrbach, R.; Truelove, E.; Look, J.; Anderson, G.; Goulet, J.P.; List, T.; Svensson, P.; Gonzalez, Y.; Lobbezoo, F.; et al. International RDC/TMD Consortium Network, International association for Dental Research; Orofacial Pain Special Interest Group, International Association for the Study of Pain. Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) for Clinical and Research Applications: Recommendations of the International RDC/TMD Consortium Network* and Orofacial Pain Special Interest Group. J. Oral Facial Pain Headache 2014, 28, 6–27. [Google Scholar] [CrossRef] [PubMed]
- Peck, C.C.; Goulet, J.P.; Lobbezoo, F.; Schiffman, E.L.; Alstergren, P.; Anderson, G.C.; de Leeuw, R.; Jensen, R.; Michelotti, A.; Ohrbach, R.; et al. Expanding the taxonomy of the diagnostic criteria for temporomandibular disorders. J. Oral Rehabil. 2014, 41, 2–23. [Google Scholar] [CrossRef] [PubMed]
- Schiffman, E.; Ohrbach, R. Executive summary of the Diagnostic Criteria for Temporomandibular Disorders for clinical and research applications. J. Am. Dent. Assoc. 2016, 147, 438–445. [Google Scholar] [CrossRef]
- Ohrbach, R.; Dworkin, S.F. The Evolution of TMD Diagnosis: Past, Present, Future. J. Dent. Res. 2016, 95, 1093–1101. [Google Scholar] [CrossRef]
- Ibáñez-Vera, A.J.; Alonso-Royo, R.; Sánchez-Torrelo, C.M.; Zagalaz-Anula, N.; López-Collantes, J.; Lomas-Vega, R. Psychometric Evaluation of the Krogh-Poulsen Test for the Diagnosis of the Temporomandibular Disorders. Diagnostics 2021, 11, 1876. [Google Scholar] [CrossRef]
- Ouanounou, A.; Goldberg, M.; Haas, D.A. Pharmacotherapy in Temporomandibular Disorders: A Review. J. Can. Dent. Assoc. 2017, 83, h7. [Google Scholar] [PubMed]
- Wu, M.; Cai, J.; Yu, Y.; Hu, S.; Wang, Y.; Wu, M. Therapeutic Agents for the Treatment of Temporomandibular Joint Disorders: Progress and Perspective. Front Pharmacol. 2021, 11, 596099. [Google Scholar] [CrossRef]
- McNealy, M.L.; Armijo Olivo, S.; Magee, D.J. A systematic review of the effectiveness of physical therapy interventions for temporomandibular disorders. Phys. Ther. 2006, 86, 710–725. [Google Scholar] [CrossRef]
- Crăciun, M.D.; Sabău, A. Means of evaluation and treatment in the orofacial myogenic pain for rheumatic patients. In Proceedings of the 2015 E-Health and Bioengineering Conference (EHB), Iasi, Romania, 19–21 November 2015; pp. 1–4. [Google Scholar] [CrossRef]
- Kwon, J.; Yu, W. Comparison of the effects of temporomandibular joint and cervical vertebra treatment on pain and functional improvement in persons with tension-type headaches. Phys. Ther. Rehabil. Sci. 2019, 8, 202–209. [Google Scholar] [CrossRef]
- Armijo-Olivo, S.; Pitance, L.; Singh, V.; Neto, F.; Thie, N.; Michelotti, A. Effectiveness of Manual Therapy and Therapeutic Exercise for Temporomandibular Disorders: Systematic Review and Meta-Analysis. Phys. Ther. 2016, 96, 9–25. [Google Scholar] [CrossRef] [PubMed]
- La Touche, R.; Fernández-de-las-Peñas, C.; Fernández Carnero, J.; Escalante, K.; Angulo-Díaz-Parreño, S.; Paris-Alemany, A.; Cleland, J.A. The effects of manual therapy and exercise directed at the cervical spine on pain and pressure pain sensitivity in patients with myofascial temporomandibular disorders. J. Oral Rehabil. 2009, 36, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Crăciun, M.D.; Chiriac, R.M. Possibilities and limitations in the kinetic treatment of the temporomandibular joint dysfunction in rheumatic patients. In Proceedings of the 2015 E-Health and Bioengineering Conference (EHB), Iasi, Romania, 19–21 November 2015; pp. 1–4. [Google Scholar] [CrossRef]
- Kalladka, M.; Young, A.; Khan, J. Myofascial pain in temporomandibular disorders: Updates on etiopathogenesis and management. J. Bodyw. Mov. Ther. 2021, 28, 104–113. [Google Scholar] [CrossRef]
- Wright, E.F.; North, S.L. Management and Treatment of Temporomandibular Disorders: A Clinical Perspective. J. Man. Manip. Ther. 2009, 17, 247–254. [Google Scholar] [CrossRef]
- Grossi, D.B.; Chaves, T.C. Physiotherapeutic treatment for temporomandibular disorders (TMD). Braz. J. Oral Sci. 2004, 3, 492–497. [Google Scholar] [CrossRef]
- Landi, N.; Manfredini, D.; Tognini, F.; Romagnoli, M.; Bosco, M. Quantification of the relative risk of multiple occlusal variables for muscle disorders of the stomatognathic system. J. Prosthet. Dent. 2004, 92, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Von Piekartz, H.; Lüdtke, K. Effect of treatment of temporomandibular disorders (TMD) in patients with cervicogenic headache: A single-blind, randomized controlled study. Cranio 2011, 29, 43–56. [Google Scholar] [CrossRef]
- Walczyńska-Dragon, K.; Baron, S.; Nitecka-Buchta, A.; Tkacz, E. Correlation between TMD and Cervical Spine Pain and Mobility: Is the Whole Body Balance TMJ Related? BioMed Res. Int. 2014, 2014, 582414. [Google Scholar] [CrossRef]
- Ozdemir-Karatas, M.; Peker, K.; Balık, A.; Uysal, O.; Tuncer, E.B. Identifying potential predictors of pain-related disability in Turkish patients with chronic temporomandibular disorder pain. J. Headache Pain 2013, 14, 17. [Google Scholar] [CrossRef]
- Van der Meer, H.; Calixtre, L.; Engelbert, R.H.H.; Visscher, C.; Nijhuis van der Sanden, M.; Speksnijder, C. Effects of physical therapy for temporomandibular disorders on headache pain intensity: A systematic review. Sci. Pract. 2020, 50, 102277. [Google Scholar] [CrossRef] [PubMed]
- López-de-Uralde-Villanueva, I.; Beltran-Alacreu, H.; Paris-Alemany, A.; Angulo-Díaz-Parreño, S.; La Touche, R. Relationships between craniocervical posture and pain-related disability in patients with cervico-craniofacial pain. J. Pain Res. 2015, 8, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Packer, A.C.; Dibai-Filho, A.V.; de Souza Costa, A.C.; dos Santos Berni, K.C.; Rodrigues-Bigaton, D. Relationship between neck disability and mandibular range of motion. J. Back Musculoskelet Rehabil. 2014, 27, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Wiesinger, B.; Malker, H.; Englund, E.; Wänman, A. Does a dose-response relation exist between spinal pain and temporomandibular disorders? BMC Musculoskelet Disord 2009, 10, 28. [Google Scholar] [CrossRef]
- Cuenca-Martínez, F.; Herranz-Gómez, A.; Madroñero-Miguel, B.; Reina-Varona, Á.; La Touche, R.; Angulo-Díaz-Parreño, S.; Pardo-Montero, J.; Del Corral, T.; López-de-Uralde-Villanueva, I. Craniocervical and Cervical Spine Features of Patients with Temporomandibular Disorders: A Systematic Review and Meta-Analysis of Observational Studies. J. Clin. Med. 2020, 9, 2806. [Google Scholar] [CrossRef]
- Paço, M.; Duarte, J.A.; Pinho, T. Orthodontic Treatment and Craniocervical Posture in Patients with Temporomandibular Disorders: An Observational Study. Int. J. Environ. Res. Public Health. 2021, 18, 3295. [Google Scholar] [CrossRef]
- Di Giacomo, P.; Ferrara, V.; Accivile, E.; Ferrato, G.; Polimeni, A.; Di Paolo, C. Relationship between Cervical Spine and Skeletal Class II in Subjects with and without Temporomandibular Disorders. Pain Res. Manag. 2018, 2018, 4286796. [Google Scholar] [CrossRef]
- Wänman, A.; Marklund, S. Treatment outcome of supervised exercise, home exercise and bite splint therapy, respectively, in patients with symptomatic disc displacement with reduction: A randomised clinical trial. J. Oral Rehabil. 2020, 47, 143–149. [Google Scholar] [CrossRef]
- Ferrillo, M.; Nucci, L.; Giudice, A.; Calafiore, D.; Marotta, N.; Minervini, G.; d’Apuzzo, F.; Ammendolia, A.; Perillo, L.; de Sire, A. Efficacy of conservative approaches on pain relief in patients with temporomandibular joint disorders: A systematic review with network meta-analysis. Cranio 2022. epub ahead of print. [Google Scholar] [CrossRef]

| SELECTION CRITERIA | |
|---|---|
| Inclusion Criteria | Exclusion Criteria |
| neck pain | acute inflammation in the cervical area or temporomandibular level |
| pain in the temporomandibular region | feverish states |
| limiting the amplitude of movement at the cervical level | neoplasms at the level of the cephalic extremity or at other levels |
| limiting the amplitude of movement at the temporomandibular level | recent dental treatments- in the last 3 months |
| intra-articular noises in the last 30 days | recent head trauma—in the last 3 months |
| myalgia or spasms of the orofacial and cervical | drug specific treatment—in the last month |
| degenerative joint disease | physiotherapeutic specific treatment—in the last month |
| mental disorders and mental illness | |
| neurological disorders | |
| ENT disorders | |
| systemic inflammatory conditions | |
| fibromyalgia | |
| Parameters | Group | Tests Significance | ||
|---|---|---|---|---|
| Experimental | Control | p Value | 95% CI | |
| (n = 33) | (n = 31) | |||
| Mouth opening/closing | ||||
| Initial test n (%) | 28 (84.8) | 25 (80.6) | 0.656 ns | 1.16 * (0.58–2.33) |
| After 3 months n (%) | 11 (33.3) | 21 (66.7) | 0.005 b | 0.24 * (0.08–0.68) |
| Left–right laterality | ||||
| Initial test n (%) | 20 (60.6) | 21 (67.7) | 0.552 ns | 0.73 * (0.26–2.05) |
| After 3 months n (%) | 5 (15.2) | 17 (54.8) | 0.001 a | 0.15 * (0.05–0.48) |
| Protrusion–Retrusion | ||||
| Initial test n (%) | 14 (42.4) | 14 (45.2) | 0.825 ns | 0.90 * (0.33–2.40) |
| After 3 months n (%) | 4 (12.1) | 12 (38.7) | 0.013 b | 0.22 * (0.06–0.78) |
| Parameters | Group | Tests Significance | ||
|---|---|---|---|---|
| Experimental (n = 33) | Control (n = 31) | p Value | 95% CI | |
| SCM/Right | ||||
| Initial test | ||||
| Pain n (%) | 20 (60.6) | 20 (64.5) | 0.747 | 0.92 * (0.57–1.49) |
| Spasm n (%) | 8 (24.2) | 6 (19.4) | 0.636 | 1.14 * (0.67–1.95) |
| After 3 months | ||||
| Pain n (%) | 6 (18.2) a | 12 (38.7) b | 0.066 | 0.57 * (0.28–1.14) |
| Spasm n (%) | 0 (0.0) b | 0 (0.0) b | - | - |
| SCM/Left | ||||
| Initial test | ||||
| Pain n (%) | 19 (57.6) | 22 (71.0) | 0.263 | 0.76 * (0.48–1.21) |
| Spasm n (%) | 6 (18.2) | 6 (19.4) | 0.904 | 0.96 * (0.52–1.80) |
| Aftee 3 months | ||||
| Pain n (%) | 6 (18.2) b | 11 (35.5) a | 0.116 | 0.61 * (0.31–1.22) |
| Spasm n (%) | 0 (0.0) b | 0 (0.0) b | - | - |
| Right–Upper Trapezoid | ||||
| Initial test | ||||
| Pain n (%) | 23 (69.7) | 23 (74.2) | 0.689 | 0.90 * (0.54–1.49) |
| Spasm n (%) | 2 (6.1) | 5 (16.1) | 0.192 | 0.53 * (0.16–1.74) |
| After 3 months | ||||
| Pain n (%) | 7 (21.2) a | 13 (41.9) a | 0.072 | 0.59 * (0.31–1.13) |
| Spasm n (%) | 0 (0.0) ns | 0 (0.0) b | - | - |
| Left–Upper Trapezoid | ||||
| Initial test | ||||
| Pain n (%) | 20 (60.6) | 23 (74.2) | 0.245 | 0.75 * (0.47–1.20) |
| Spasm n (%) | 4 (12.1) | 6 (19.4) | 0.425 | 0.75 * (0.34–1.66) |
| After 3 months | ||||
| Pain n (%) | 6 (18.2) a | 14 (45.2) a | 0.019b | 0.49 * (0.24–0.99) |
| Spasm n (%) | 0 (0.0) b | 0 (0.0) b | - | - |
| Right–Splenius and Semispinal of the Head–Right | ||||
| Initial test | ||||
| Pain n (%) | 13 (39.4) | 19 (61.3) | 0.079 | 0.65 * (0.40–1.07) |
| Spasm n (%) | 3 (9.1) | 7 (22.6) | 0.134 | 0.54 * (0.20–1.43) |
| After 3 months | ||||
| Pain n (%) | 3 (9.1) b | 13 (41.9) ns | 0.002b | 0.30 *(0.11–0.85) |
| Spasm n (%) | 0 (0.0) ns | 3 (9.7) ns | 0.034b | - |
| Left Splenius and Semispinal left head | ||||
| Initial test | ||||
| Pain n (%) | 13 (39.4) | 19 (61.3) | 0.079 | 0.65 *(0.40–1.07) |
| Spasm n (%) | 3 (9.1) | 8 (25.8) | 0.073 | 0.48 *(0.18–1.30) |
| After 3 months | ||||
| Pain n (%) | 3 (9.1) b | 14 (45.2) ns | 0.001b | 0.28 *(0.10–0.79) |
| Spasm n (%) | 0 (0.0) ns | 2 (6.5) b | 0.085 | - |
| Parameters | Group | Tests Significance | ||
|---|---|---|---|---|
| Experimental (n = 33) | Control (n = 31) | p Value | 95% CI | |
| Masseter–Right | ||||
| Initial test | ||||
| Pain n (%) | 28 (84.8) | 27 (87.1) | 0.796 | 0.92 * (0.48–1.74) |
| Spasm n (%) | 15 (45.5) | 14 (45.2) | 0.981 | 1.01 * (0.62–1.62) |
| After 3 months | ||||
| Pain n (%) | 8 (24.2) a | 19 (61.3) | 0.002b | 0.44 * (0.24–0.82) |
| Spasm n (%) | 3 (9.1) a | 2 (6.5) | 0.693 | 1.18 * (0.55–2.52) |
| Masseter–Left | ||||
| Initial test | ||||
| Pain n (%) | 27 (81.8) | 27 (87.1) | 0.56 | 0.83 * (0.47–1.48) |
| Spasm n (%) | 13 (39.4) | 15 (48.4) | 0.468 | 0.84 * (0.51–1.37) |
| After 3 months | ||||
| Pain n (%) | 8 (24.2) a | 19 (61.3) | 0.002b | 0.44 * (0.24–0.82) |
| Spasm n (%) | 3 (9.1) a | 2 (6.5) | 0.693 | 1.18 * (0.55–2.52) |
| Temporalis–Right | ||||
| Initial test | ||||
| Pain n (%) | 21 (63.6) | 26 (83.9) | 0.064 | 0.63 * (0.41–0.99) |
| Spasm n (%) | 7 (21.2) | 5 (16.1) | 0.602 | 1.17 * (0.67–2.02) |
| After 3 months | ||||
| Pain n (%) | 8 (24.2) a | 16 (51.6) | 0.023b | 0.53 * (0.29–0.99) |
| Spasm n (%) | 0 (0.0) b | 0 (0.0) | - | - |
| Temporalis–Left | ||||
| Initial test | ||||
| Pain n (%) | 21 (63.6) | 25 (80.6) | 0.127 | 0.69 * (0.44–1.08) |
| Spasm n (%) | 7 (21.2) | 5 (16.1) | 0.602 | 1.17 * (0.67–2.02) |
| After 3 months | ||||
| Pain n (%) | 9 (27.3) a | 16 (51.6) | 0.045b | 0.59 * (0.33–1.04) |
| Spasm n (%) | 0 (0.0) b | 1 (3.2) | 0.226 | - |
| Right–Internal Pterygoid | ||||
| Initial test | ||||
| Pain n (%) | 15 (45.5) | 21 (67.7) | 0.071 | 0.65 * (0.40–1.04) |
| Spasm n (%) | 7 (21.2) | 9 (29.0) | 0.47 | 0.81 * (0.44–1.49) |
| After 3 months | ||||
| Pain n (%) | 4 (12.1) b | 14 (45.2) | 0.003b | 0.35 * (0.14–0.86) |
| Spasm n (%) | 0 (0.0) b | 1 (3.2) | 0.226 | - |
| Left–Internal Pterygoid | ||||
| Initial test | ||||
| Pain n (%) | 14 (42.4) | 21 (67.7) | 0.041b | 0.61 * (0.38–0.99) |
| Spasm n (%) | 7 (21.2) | 9 (29.0) | 0.47 | 0.81 * (0.44–1.49) |
| After 3 months | ||||
| Pain n (%) | 4 (12.1) b | 14 (45.2) | 0.003b | 0.35 * (0.14–0.86) |
| Spasm n (%) | 0 (0.0) b | 1 (3.2) | 0.226 | - |
| Right–External Pterygoid | ||||
| Initial test | ||||
| Pain n (%) | 31 (93.9) | 30 (96.8) | 0.588 | 0.76 * (0.33–1.76) |
| Spasm n (%) | 27 (81.8) | 16 (51.6) | 0.009b | 2.20 * (1.08–4.49) |
| After 3 months | ||||
| Pain n (%) | 10 (30.3) a | 17 (54.8) | 0.046b | 0.60 * (0.34–1.04) |
| Spasm n (%) | 2 (6.1) a | 4 (12.9) | 0.345 | 0.62 * (0.20–1.98) |
| Left–External Pterygoid | ||||
| Initial test | ||||
| Pain n (%) | 31 (93.9) | 30 (96.8) | 0.588 | 0.76 * (0.33–1.76) |
| Spasm n (%) | 27 (81.8) | 18 (58.1) | 0.036b | 1.90 * (0.94–3.84) |
| After 3 months | ||||
| Pain n (%) | 7 (21.2) a | 16 (51.6) | 0.011b | 0.48 * (0.25–0.93) |
| Spasm n (%) | 2 (6.1) a | 3 (9.7) | 0.589 | 0.76 * (0.25–2.29) |
| Groups | Range of Motion at the Cervical Spine | ||
|---|---|---|---|
| Initial | 3 Months | p Values for Paired Sample Test | |
| Flexion 1 | |||
| Experimental (n = 33) | 34.76 (±4.37) | 42.67 (±2.00) | 0.001 |
| Control (n = 31) | 33.97 (±3.42) | 38.90 (±2.27) | 0.001 |
| p values for t-Student test Experimental vs. Control | p = 0.426 | p = 0.001 | |
| Extension 1 | |||
| Experimental (n = 33) | 31.36 (±4.55) | 40.97 (±2.68) | 0.001 |
| Control (n = 31) | 31.81 (±3.01) | 36.32 (±2.89) | 0.001 |
| p values for t-Student test Experimental vs. Control | p = 0.650 | p = 0.001 | |
| Lateral flexion–Right 1 | |||
| Experimental (n = 33) | 35.15 (±4.57) | 42.55 (±2.05) | 0.001 |
| Control (n = 31) | 35.13 (±2.83) | 39.65 (±1.85) | 0.001 |
| p values for t-Student test Experimental vs. Control | p = 0.981 | p = 0.001 | |
| Lateral flexion–Left 1 | |||
| Experimental (n = 33) | 35.21 (±4.33) | 42.64 (±1.92) | 0.001 |
| Control (n = 31) | 35.39 (±3.00) | 39.77 (±1.93) | 0.001 |
| p values for t-Student test Experimental vs. Control | p = 0.852 | p = 0.001 | |
| Rotation–toward Right 1 | |||
| Experimental (n = 33) | 37.64 (±6.58) | 53.30 (±6.62) | 0.001 |
| Control (n = 31) | 36.74 (±3.44) | 41.52 (±2.41) | 0.001 |
| p values for t-Student test Experimental vs. Control | p = 0.502 | p = 0.001 | |
| Rotation–toward Left 1 | |||
| Experimental (n = 33) | 37.70 (±6.52) | 53.33 (±6.68) | 0.001 |
| Control (n = 31) | 36.90 (±3.50) | 41.45 (±2.46) | 0.001 |
| p values for t-Student test (experimental vs. control) | p = 0.550 | p = 0.001 | |
| Groups | Range of Motion at the Temporomandibular Joint | ||
|---|---|---|---|
| Initial | 3 Months | p Values for Paired Sample Test | |
| Mouth opening/closing 1 | |||
| Experimental (n = 33) | 4.10 (±0.37) | 4.80 (±0.17) | 0.001 |
| Control (n = 31) | 4.11 (±0.30) | 4.38 (±0.76) | 0.001 |
| p value for t-Student test Experimental vs. Control | p = 0.966 | p = 0.003 | |
| Right laterality 1 | |||
| Experimental (n = 33) | 0.84 (±0.09) | 1.00 (±0.06) | 0.001 |
| Control (n = 31) | 0.86 (±0.08) | 0.92 (±0.07) | 0.001 |
| p values for t-Student test Experimental vs. Control | p = 0.320 | p = 0.005 | |
| Left laterality 1 | |||
| Experimental (n = 33) | 0.84 (±0.11) | 1.00 (±0.06) | 0.001 |
| Control (n = 31) | 0.86 (±0.10) | 0.91 (±0.07) | 0.001 |
| p values for t-Student test Experimental vs. Control | p = 0.411 | p = 0.001 | |
| Protrusion 1 | |||
| Experimental (n = 33) | 0.48 (±0.05) | 0.50 (±0.01) | 0.032 |
| Control (n = 31) | 0.48 (±0.04) | 0.49 (±0.03) | 0.083 |
| p values for t-Student test Experimental vs. Control | p = 0.915 | p = 0.069 | |
| Retrusion 1 | |||
| Experimental (n = 33) | 0.37 (±0.05) | 0.43 (±0.05) | 0.001 |
| Control (n = 31) | 0.39 (±0.03) | 0.41 (±0.03) | 0.012 |
| p values for t-Student test Experimental vs. Control | p = 0.015 | p = 0.022 | |
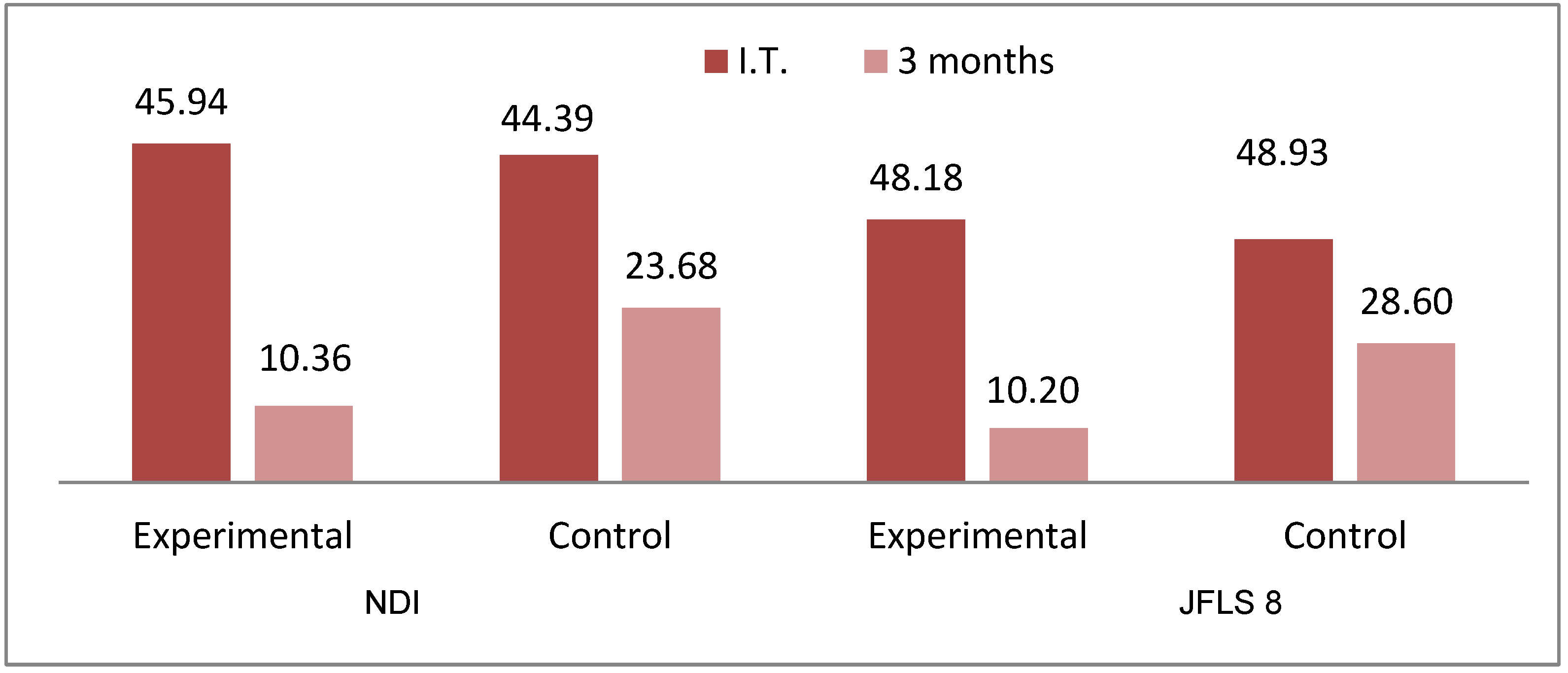
| Groups | NDI | ||
|---|---|---|---|
| IT | T 3 | p Values for Paired Sample Test | |
| Scores | |||
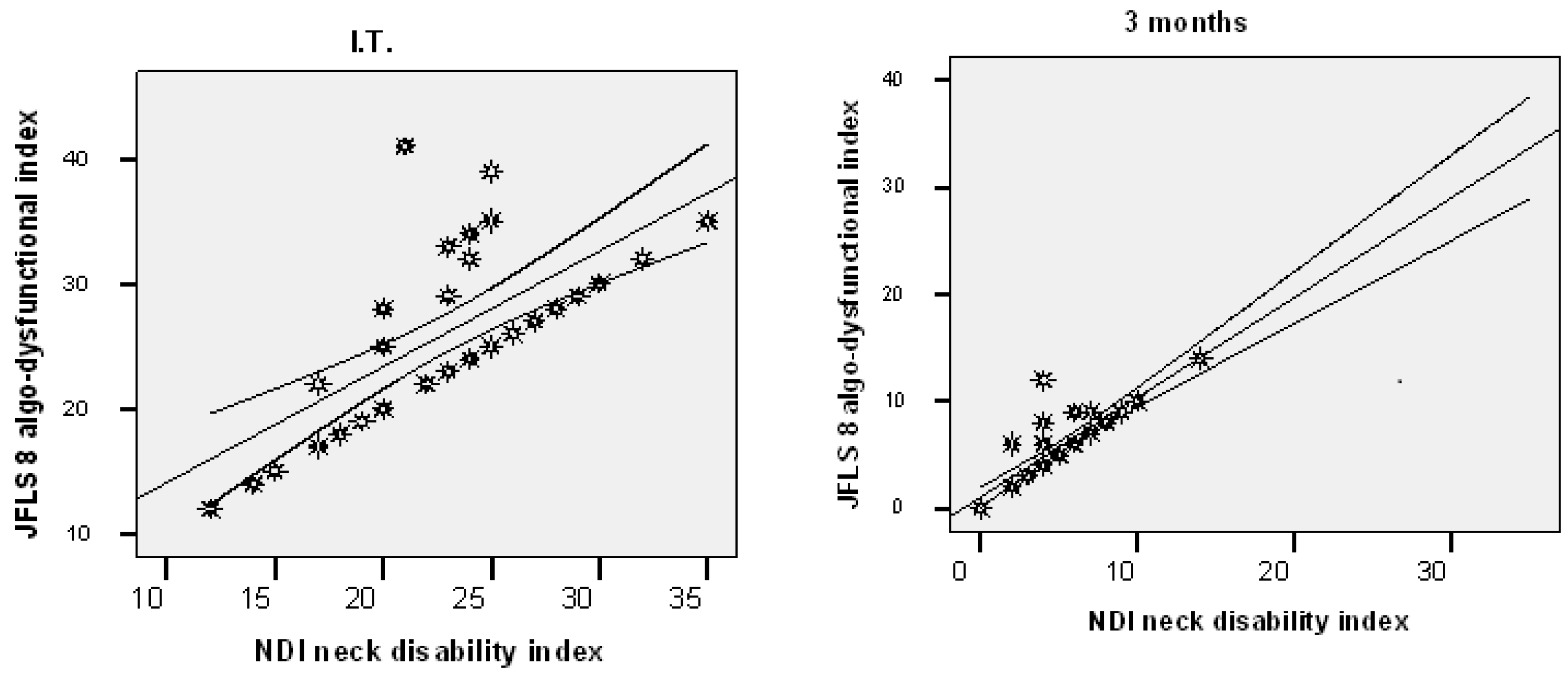
| Experimental (n = 33) | 22.97 (±5.18) | 5.18 (±3.29) | 0.001 |
| Control (n = 31) | 22.19 (±5.95) | 11.84 (±3.49) | 0.001 |
| p values for t-Student test Experimental vs. Control | p = 0.579 | p = 0.001 | |
| Percentage index | |||
| Experimental (n = 33) | 45.94 (±10.35) | 10.36 (±6.59) | 0.001 |
| Control (n = 31) | 44.39 (±11.89) | 23.68 (±6.99) | 0.001 |
| Groups | JFLS 8 | ||
|---|---|---|---|
| IT | T 3 | p Values for Paired Sample Test | |
| Scores | |||
| Experimental (n = 33) | 26.12 (±7.02) | 5.88 (±3.52) | 0.001 |
| Control (n = 31) | 26.39 (±6.17) | 15.10 (±3.72) | 0.001 |
| p values for t-Student test Experimental vs. Control | p = 0.873 | p = 0.001 | |
| Percentage index | |||
| Experimental (n = 33) | 32.65 (±8.77) | 7.35 (±4.41) | 0.001 |
| Control (n = 31) | 32.98 (±7.72) | 18.87 (±4.65) | 0.001 |
| p values for t-Student test Experimental vs. Control | p = 0.873 | p = 0.001 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crăciun, M.D.; Geman, O.; Leuciuc, F.V.; Holubiac, I.Ş.; Gheorghiţă, D.; Filip, F. Effectiveness of Physiotherapy in the Treatment of Temporomandibular Joint Dysfunction and the Relationship with Cervical Spine. Biomedicines 2022, 10, 2962. https://doi.org/10.3390/biomedicines10112962
Crăciun MD, Geman O, Leuciuc FV, Holubiac IŞ, Gheorghiţă D, Filip F. Effectiveness of Physiotherapy in the Treatment of Temporomandibular Joint Dysfunction and the Relationship with Cervical Spine. Biomedicines. 2022; 10(11):2962. https://doi.org/10.3390/biomedicines10112962
Chicago/Turabian StyleCrăciun, Maria Daniela, Oana Geman, Florin Valentin Leuciuc, Iulian Ştefan Holubiac, Daniela Gheorghiţă, and Florin Filip. 2022. "Effectiveness of Physiotherapy in the Treatment of Temporomandibular Joint Dysfunction and the Relationship with Cervical Spine" Biomedicines 10, no. 11: 2962. https://doi.org/10.3390/biomedicines10112962
APA StyleCrăciun, M. D., Geman, O., Leuciuc, F. V., Holubiac, I. Ş., Gheorghiţă, D., & Filip, F. (2022). Effectiveness of Physiotherapy in the Treatment of Temporomandibular Joint Dysfunction and the Relationship with Cervical Spine. Biomedicines, 10(11), 2962. https://doi.org/10.3390/biomedicines10112962









