Detrusor Underactivity in Men with Bladder Outlet Obstruction
Abstract
1. Introduction
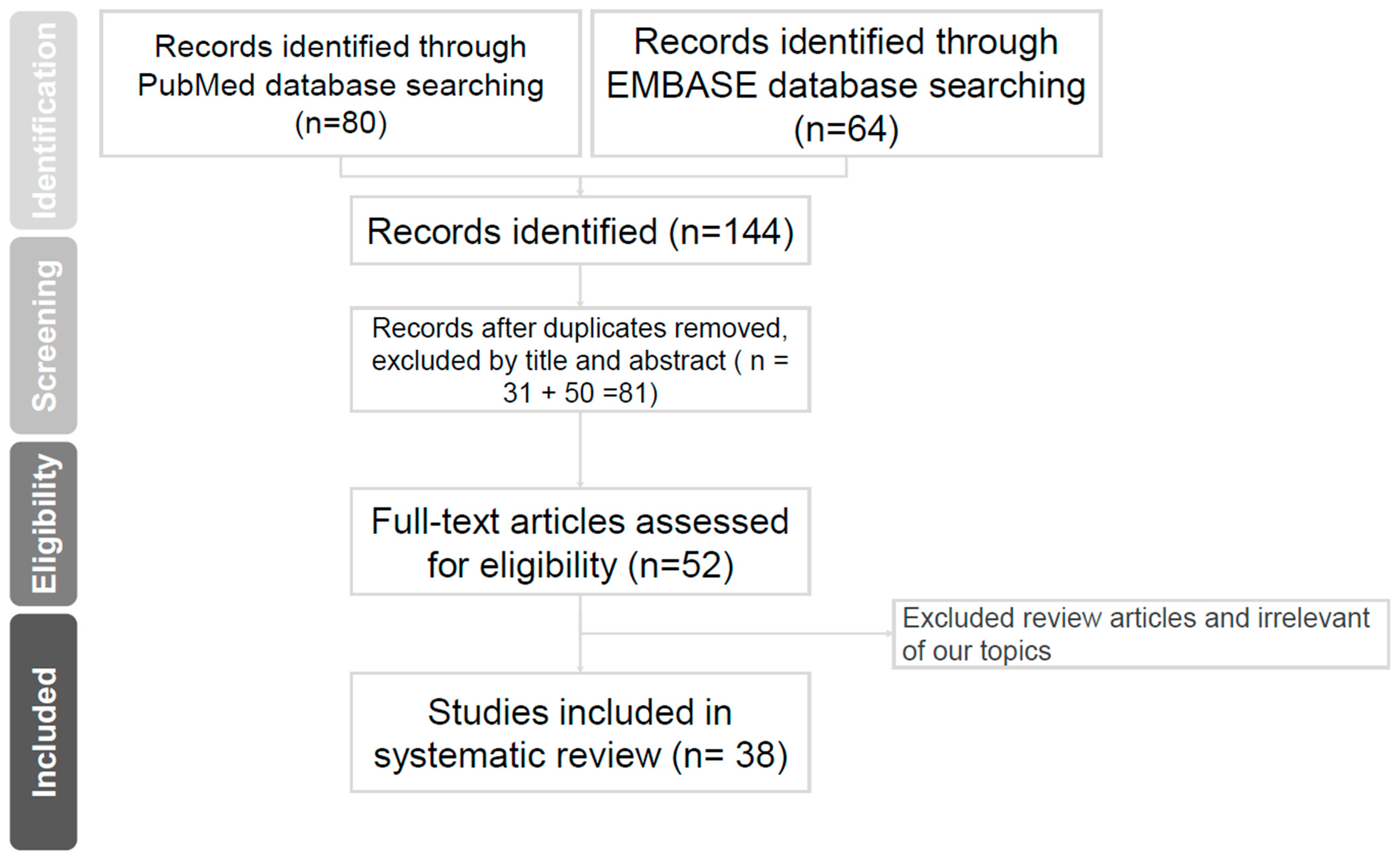
2. Evidence Acquisition
3. Pathophysiological Mechanisms
4. Diagnosis and Evaluation (Shown in Table 1)
| Study | Design | Participants | Inclusion Criteria | Noninvasive Parameters as DU Predictors | UDS Parameters as DU Predictors |
|---|---|---|---|---|---|
| Luo et al. [17] (2017) | Retrospective study | 704 men | BPH/LUTS patients who underwent urodynamic assessment | Smaller prostate volume (<30 mL), Higher post-void residual (PVR) (>400 mL) | |
| Nunzio et al. [18] (2020) | Retrospective analysis of a prospectively collected database | 448 men | Aged 45 years or older with LUTS | Bladder wall thickness (BWT, mm): measured with suprapubic ultrasound, 3.5 MHz Qmax (mL/s) Nomogram | |
| Rademakers et al. [20] (2016) | Prospective trial | 143 men | Treatment-naive men aged ≥40 years with uncomplicated LUTS | Detrusor wall thickness (DWT) ≤ 1.23 mm and bladder capacity >445 mL DWT: Anterior bladder wall with a 7.5 MHz ultrasound array and bladder filling ≥250 mL | |
| Gammie et al. [22] (2018) | Retrospective study | 1612 men | Comparing 129 DU and 60 DU + BOO(DU: BCI < 100, BVE < 90%, BOOI < 20. DU + BOO: BCI<100, BVE < 90%, BOOI ≧ 40) | Higher average urine flow rate Less decreasing urinary stream More history of ≧1 urinary tract infection | Higher abdominal pressure at maximum flow rate (PabdQmax): straining |
| Oelke et al. [23] (2016) | Retrospective study | 822 men | Aged ≧ 40 years with LUTs | WFmax-BOOI nomogram < 25th percentile groups Nomogram | |
| Oelke et al. [24] (2014) | Retrospective study | 786 men | Treatment-naive men aged ≥40 years with uncomplicated LUTS | BCI and WFmax rise with increasing BOO grade | |
| Namitome et al. [25] (2020) | Retrospective study | 909 men | Aged ≧ 50 years underwent pressure-flow studies | Older Small prostate volume Less urgency Weak streaming Low Qmax Nomogram | |
| Donkelaar et al. [26] (2017) | Retrospective study | 1222 men | Aged ≧ 50 years underwent pressure-flow studies (Comparison LinPURR, BCI, WFmax) | LinPURR nomogram: simple and easy, no complex calculation BCI WFmax: more complex calculation Three methods have high correlation agreement | |
| Guo et al. [28] (2017) | Retrospective study | 67 men | Urinary retention men underwent UDS | Piso < 50 cmH2O: mechanical stop test BCI < 100 | |
| Tanabe et al. [29] (2011) | Retrospective study | 288 men | Surgical indications underwent pressure-flow studies (Comparing operate and not operated with weak detrusor) | Schafer’s nomogram |
5. Management of DU with BOO
6. The Efficacy of BOO Surgery for DU
7. Predictors for a Successful BOO Surgery for DU
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Faraj, K.; Doo, F.; Boura, J.; Vereecke, A.; Chancellor, M.B. A cross-sectional study in the USA of the epidemiology and quality of life of underactive bladder symptoms. Int. Urol. Nephrol. 2016, 48, 1797–1802. [Google Scholar] [CrossRef]
- Abrams, P.; Cardozo, L.; Fall, M.; Griffiths, D.; Rosier, P.; Ulmsten, U.; Van Kerrebroeck, P.; Victor, A.; Wein, A. The standardisation of terminology of lower urinary tract function: Report from the Standardisation Sub-committee of the International Continence Society. Neurourol. Urodyn. 2002, 21, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Chapple, C.R.; Osman, N.I.; Birder, L.; van Koeveringe, G.A.; Oelke, M.; Nitti, V.W.; Drake, M.J.; Yamaguchi, O.; Abrams, P.; Smith, P.P. The underactive bladder: A new clinical concept? Eur. Urol. 2015, 68, 351–353. [Google Scholar] [CrossRef] [PubMed]
- Aldamanhori, R.; Chapple, C.R. Underactive bladder, detrusor underactivity, definition, symptoms, epidemiology, etiopathogenesis, and risk factors. Curr. Opin. Urol. 2017, 27, 293–299. [Google Scholar] [CrossRef]
- Schäfer, W.; Abrams, P.; Liao, L.; Mattiasson, A.; Pesce, F.; Spangberg, A.; Sterling, A.M.; Zinner, N.R.; van Kerrebroeck, P. Good urodynamic practices: Uroflowmetry, filling cystometry, and pressure-flow studies. Neurourol. Urodyn. 2002, 21, 261–274. [Google Scholar] [CrossRef] [PubMed]
- Porru, D.; Madeddu, G.; Campus, G.; Montisci, I.; Scarpa, R.M.; Usai, E. Evaluation of morbidity of multi-channel pressure-flow studies. Neurourol. Urodyn. 1999, 18, 647–652. [Google Scholar] [CrossRef]
- Rollema, H.J.; Van Mastrigt, R. Improved indication and follow-up in transurethral resection of the prostate using the computer program CLIM; a prospective study. J. Urol. 1992, 148, 111–115. [Google Scholar] [CrossRef]
- Van Mastrigt, R.; Rollema, H.J. The prognostic value of bladder contractility in transurethral resection of the prostate. J. Urol. 1992, 148, 1856–1860. [Google Scholar] [CrossRef]
- Rademakers, K.L.; van Koeveringe, G.A.; Oelke, M. Detrusor underactivity in men with lower urinary tract symptoms/benign prostatic obstruction: Characterization and potential impact on indications for surgical treatment of the prostate. Curr. Opin. Urol. 2016, 26, 3–10. [Google Scholar] [CrossRef]
- Rademakers, K.; Drake, M.J.; Gammie, A.; Djurhuus, J.C.; Rosier, P.F.W.M.; Abrams, P.; Abrams, P.; Harding, C. Male bladder outlet obstruction: Time to re-evaluate the definition and reconsider our diagnostic pathway? ICI-RS 2015. Neurourol. Urodyn. 2017, 36, 894–901. [Google Scholar] [CrossRef]
- Tanaka, Y.; Masumori, N.; Itoh, N.; Furuya, S.; Ogura, H.; Tsukamoto, T. Is the short-term outcome of transurethral resection of the prostate affected by preoperative degree of bladder outlet obstruction, status of detrusor contractility or detrusor overactivity? Int. J. Urol. 2006, 13, 1398–1404. [Google Scholar] [CrossRef]
- Han, D.H.; Jeong, Y.S.; Choo, M.S.; Lee, K.S. The efficacy of transurethral resection of the prostate in the patients with weak bladder contractility index. Urology 2008, 71, 657–661. [Google Scholar] [CrossRef] [PubMed]
- Aizawa, N.; Igawa, Y. Pathophysiology of the underactive bladder. Investig. Clin. Urol. 2017, 58 (Suppl. 2), S82–S89. [Google Scholar] [CrossRef]
- Smith, P.P. Pathophysiology of the Underactive Bladder: Evolving New Concepts. Curr. Bladder Dysfunct. Rep. 2017, 12, 35–41. [Google Scholar] [CrossRef][Green Version]
- Gosling, J. The structure of the bladder and urethra in relation to function. Urol. Clin. N. Am. 1979, 6, 31–38. [Google Scholar] [CrossRef]
- Levin, R.M.; Longhurst, P.A.; Barasha, B.; McGuire, E.J.; Elbadawi, A.; Wein, A.J. Studies on experimental bladder outlet obstruction in the cat: Long-term functional effects. J. Urol. 1992, 148, 939–943. [Google Scholar] [CrossRef]
- Saito, M.; Wein, A.J.; Levin, R.M. Effect of partial outlet obstruction on contractility: Comparison between severe and mild obstruction. Neurourol. Urodyn. 1993, 12, 573–583. [Google Scholar] [CrossRef]
- Osman, N.I.; Chapple, C.R. Contemporary concepts in the aetiopathogenesis of detrusor underactivity. Nat. Rev. Urol. 2014, 11, 639–648. [Google Scholar] [CrossRef] [PubMed]
- Chancellor, M.B. The overactive bladder progression to underactive bladder hypothesis. Int. Urol. Nephrol. 2014, 46 (Suppl. 1), S23–S27. [Google Scholar] [CrossRef] [PubMed]
- Kato, K.; Monson, F.C.; Longhurst, P.A.; Wein, A.J.; Haugaard, N.; Levin, R.M. The functional effects of long-term outlet obstruction on the rabbit urinary bladder. J. Urol. 1990, 143, 600–606. [Google Scholar] [CrossRef]
- Cho, K.J.; Koh, J.S.; Choi, J.; Kim, J.C. Changes in Adenosine Triphosphate and Nitric Oxide in the Urothelium of Patients with Benign Prostatic Hyperplasia and Detrusor Underactivity. J. Urol. 2017, 198, 1392–1396. [Google Scholar] [CrossRef]
- Jiang, Y.H.; Kuo, H.C. Urothelial Barrier Deficits, Suburothelial Inflammation and Altered Sensory Protein Expression in Detrusor Underactivity. J. Urol. 2017, 197, 197–203. [Google Scholar] [CrossRef]
- Igawa, Y.; Aizawa, N.; Homma, Y. Beta3-adrenoceptor agonists: Possible role in the treatment of overactive bladder. Korean J. Urol. 2010, 51, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Gheinani, A.H.; Kiss, B.; Moltzahn, F.; Keller, I.; Bruggmann, R.; Rehrauer, H.; Fournier, C.A.; Burkhard, F.C.; Monastyrskaya, K. Characterization of miRNA-regulated networks, hubs of signaling, and biomarkers in obstruction-induced bladder dysfunction. JCI Insight 2017, 2, e89560. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Miyata, Y.; Matsuo, T.; Mitsunari, K.; Asai, A.; Ohba, K.; Sakai, H. A Review of Oxidative Stress and Urinary Dysfunction Caused by Bladder Outlet Obstruction and Treatments Using Antioxidants. Antioxidants 2019, 8, 132. [Google Scholar] [CrossRef] [PubMed]
- Fusco, F.; Creta, M.; De Nunzio, C.; Iacovelli, V.; Mangiapia, F.; Li Marzi, V.; Agrò, E.F. Progressive bladder remodeling due to bladder outlet obstruction: A systematic review of morphological and molecular evidences in humans. BMC Urol. 2018, 18, 15. [Google Scholar] [CrossRef]
- Cockayne, D.A.; Hamilton, S.G.; Zhu, Q.M.; Dunn, P.M.; Zhong, Y.; Novakovic, S.; Malmberg, A.B.; Cain, G.; Berson, A.; Kassotakis, L.; et al. Urinary bladder hyporeflexia and reduced pain-related behaviour in P2 × 3-deficient mice. Nature 2000, 407, 1011–1015. [Google Scholar] [CrossRef]
- Vale, L.; Jesus, F.; Marcelissen, T.; Rieken, M.; Geavlete, B.; Rahnama’i, M.S.; Martens, F.; Cruz, F.; Antunes-Lopes, T.; on behalf of the EAU Young Academic Urologists Functional Urology Working Group. Pathophysiological mechanisms in detrusor underactivity: Novel experimental findings. Low. Urin. Tract Symptoms 2019, 11, 92–98. [Google Scholar] [CrossRef]
- Yamaguchi, O.; Nomiya, M.; Andersson, K.E. Functional consequences of chronic bladder ischemia. Neurourol. Urodyn. 2014, 33, 54–58. [Google Scholar] [CrossRef]
- Jiang, Y.H.; Kuo, H.C. High percentage of neurologic deficits in the electrophysiology study of the lower urinary tract in patients with detrusor underactivity and chronic urinary retention. Neurourol. Urodyn. 2021, 40, 883–890. [Google Scholar] [CrossRef]
- Kalil, J.; D’Ancona, C.A.L. Detrusor underactivity versus bladder outlet obstruction clinical and urodynamic factors. Int. Braz. J. Urol. 2020, 46, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Wada, N.; Watanabe, M.; Ishikawa, M.; Takeuchi, K.; Miyauchi, K.; Abe, N.; Banjo, H.; Kita, M.; Kakizaki, H. Uroflowmetry pattern in detrusor underactivity and bladder outlet obstruction in male patients with lower urinary tract symptoms. Low. Urin. Tract Symptoms 2021, 13, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Matsukawa, Y.; Yoshida, M.; Yamaguchi, O.; Takai, S.; Majima, T.; Funahashi, Y.; Yono, M.; Sekido, N.; Gotoh, M. Clinical characteristics and useful signs to differentiate detrusor underactivity from bladder outlet obstruction in men with non-neurogenic lower urinary tract symptoms. Int. J. Urol. 2020, 27, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Luo, F.; Sun, H.H.; Su, Y.H.; Zhang, Z.H.; Wang, Y.S.; Zhao, Z.; Li, J. Assessment of noninvasive predictors of bladder detrusor underactivity in BPH/LUTs patients. Int. Urol. Nephrol. 2017, 49, 787–792. [Google Scholar] [CrossRef]
- De Nunzio, C.; Lombardo, R.; Cicione, A.; Trucchi, A.; Carter, S.; Tema, G.; Nacchia, A.; Vicentini, C.; Tubaro, A. The role of bladder wall thickness in the evaluation of detrusor underactivity: Development of a clinical nomogram. Neurourol. Urodyn. 2020, 39, 1115–1123. [Google Scholar] [CrossRef]
- Manieri, C.; Carter, S.S.; Romano, G.; Trucchi, A.; Valenti, M.; Tubaro, A. The diagnosis of bladder outlet obstruction in men by ultrasound measurement of bladder wall thickness. J. Urol. 1998, 159, 761–765. [Google Scholar] [CrossRef]
- Rademakers, K.L.; van Koeveringe, G.A.; Oelke, M.; FORCE Research Group, Maastricht and Hannover. Ultrasound detrusor wall thickness measurement in combination with bladder capacity can safely detect detrusor underactivity in adult men. World J. Urol. 2017, 35, 153–159. [Google Scholar] [CrossRef]
- Gammie, A.; Kaper, M.; Steup, A.; Yoshida, S.; Dorrepaal, C.; Kos, T.; Abrams, P. Signs and symptoms that distinguish detrusor underactivity from mixed detrusor underactivity and bladder outlet obstruction in male patients. Neurourol. Urodyn. 2018, 37, 1501–1505. [Google Scholar] [CrossRef] [PubMed]
- Oelke, M.; Rademakers, K.L.; van Koeveringe, G.A.; FORCE Research Group, Maastricht & Hannover. Unravelling detrusor underactivity: Development of a bladder outlet resistance-Bladder contractility nomogram for adult male patients with lower urinary tract symptoms. Neurourol. Urodyn. 2016, 35, 980–986. [Google Scholar] [CrossRef]
- Oelke, M.; Rademakers, K.L.; van Koeveringe, G.A. Detrusor contraction power parameters (BCI and W max) rise with increasing bladder outlet obstruction grade in men with lower urinary tract symptoms: Results from a urodynamic database analysis. World J. Urol. 2014, 32, 1177–1183. [Google Scholar] [CrossRef][Green Version]
- Namitome, R.; Takei, M.; Takahashi, R.; Kikutake, C.; Yokomizo, A.; Yamaguchi, O.; Eto, M. A Prediction Model of Detrusor Underactivity Based on Symptoms and Noninvasive Test Parameters in Men with Lower Urinary Tract Symptoms: An Analysis of a Large Group of Patients undergoing Pressure-Flow Studies. J. Urol. 2020, 203, 779–785. [Google Scholar] [CrossRef]
- Donkelaar, S.C.T.; Rosier, P.; de Kort, L. Comparison of three methods to analyze detrusor contraction during micturition in men over 50 years of age. Neurourol. Urodyn. 2017, 36, 2153–2159. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.P.; Comiter, C.V.; Elliott, C.S. Urodynamics of men with urinary retention. Int. J. Urol. 2017, 24, 703–707. [Google Scholar] [CrossRef]
- Tanabe, T.; Ishizuka, O.; Ichino, M.; Ogawa, T.; Imamura, T.; Kurizaki, Y.; Iijima, K.; Nishizawa, O. Analysis of the Pressure-Flow Study in Weak Detrusor Patients with Benign Prostatic Hypertrophy. Low. Urin. Tract Symptoms 2011, 3, 109–112. [Google Scholar] [CrossRef] [PubMed]
- Osman, N.I.; Chapple, C.R. Are There Pharmacotherapeutic Options for Underactive Bladder? Eur. Urol. Focus. 2018, 4, 6–7. [Google Scholar] [CrossRef]
- Barendrecht, M.M.; Oelke, M.; Laguna, M.P.; Michel, M.C. Is the use of parasympathomimetics for treating an underactive urinary bladder evidence-based? BJU Int. 2007, 99, 749–752. [Google Scholar] [CrossRef]
- Yamanishi, T.; Yasuda, K.; Kamai, T.; Tsujii, T.; Sakakibara, R.; Uchiyama, T.; Yoshida, K.-I. Combination of a cholinergic drug and an alpha-blocker is more effective than monotherapy for the treatment of voiding difficulty in patients with underactive detrusor. Int. J. Urol. 2004, 11, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Finkbeiner, A.E. Is bethanechol chloride clinically effective in promoting bladder emptying? A literature review. J. Urol. 1985, 134, 443–449. [Google Scholar] [CrossRef]
- Janssen, D.A.W. Struggle with Pharmacotherapy for Underactive Bladder: Could Patients with Detrusor Underactivity Benefit from Transient Receptor Potential Vanilloid 4 Agonists? Eur Urol. 2018, 74, 346–347. [Google Scholar] [CrossRef]
- Deruyver, Y.; Weyne, E.; Dewulf, K.; Rietjens, R.; Pinto, S.; Van Ranst, N.; Franken, J.; Vanneste, M.; Albersen, M.; Gevaert, T.; et al. Intravesical Activation of the Cation Channel TRPV4 Improves Bladder Function in a Rat Model for Detrusor Underactivity. Eur. Urol. 2018, 74, 336–345. [Google Scholar] [CrossRef]
- Matsukawa, Y.; Majima, T.; Funahashi, Y.; Fujita, T.; Ishida, S.; Kato, M.; Gotoh, M. Effects of tadalafil versus silodosin on voiding function in male patients with non-neurogenic detrusor underactivity: A comparative study using propensity score matching. Int. J. Urol. 2021, 28, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.P.; Chancellor, M.B. Emerging role of botulinum toxin in the management of voiding dysfunction. J. Urol. 2004, 171 Pt 1, 2128–2137. [Google Scholar] [CrossRef] [PubMed]
- Kuo, H.C. Botulinum A toxin urethral injection for the treatment of lower urinary tract dysfunction. J. Urol. 2003, 170, 1908–1912. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Liao, L.; Zhang, F. Efficacy and safety of botulinum toxin a injection into urethral sphincter for underactive bladder. BMC Urol. 2019, 19, 60. [Google Scholar] [CrossRef] [PubMed]
- Dobberfuhl, A.D.; Chen, A.; Alkaram, A.F.; De, E.J.B. Spontaneous voiding is surprisingly recoverable via outlet procedure in men with underactive bladder and documented detrusor underactivity on urodynamics. Neurourol. Urodyn. 2019, 38, 2224–2232. [Google Scholar] [CrossRef] [PubMed]
- Blaivas, J.G.; Forde, J.C.; Davila, J.L.; Policastro, L.; Tyler, M.; Aizen, J.; Badri, A.; Purohit, R.S.; Weiss, J.P. Surgical treatment of detrusor underactivity: A short term proof of concept study. Int. Braz. J. Urol. 2017, 43, 540–548. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, T.; Huard, J.; Pruchnic, R.; Yoshimura, N.; Qu, Z.; Cao, B.; de Groat, W.C.; Kumon, H.; Chancellor, M.B. Muscle-derived cell transplantation and differentiation into lower urinary tract smooth muscle. Urology 2001, 57, 826–831. [Google Scholar] [CrossRef]
- Levanovich, P.E.; Diokno, A.; Hasenau, D.L.; Lajiness, M.; Pruchnic, R.; Chancellor, M.B. Intradetrusor injection of adult musclederived cells for the treatment of underactive bladder: Pilot study. Int. Urol. Nephrol. 2015, 47, 465–467. [Google Scholar] [CrossRef]
- Tirney, S.; Mattes, C.E.; Yoshimura, N.; Yokayama, T.; Ozawa, H.; Tzeng, E.; Birder, L.A.; Kanai, A.J.; Huard, J.; De Groat, W.C.; et al. Nitric oxide synthase gene therapy for erectile dysfunction: Comparison of plasmid, adenovirus, and adenovirustransduced myoblast vectors. Mol. Urol. 2001, 5, 37–43. [Google Scholar] [CrossRef]
- Chai, T.C.; Kudze, T. New therapeutic directions to treat underactive bladder. Investig. Clin. Urol. 2017, 58 (Suppl. 2), S99–S106. [Google Scholar] [CrossRef]
- Woo, M.J.; Ha, Y.S.; Lee, J.N.; Kim, B.S.; Kim, H.T.; Kim, T.-H.; Yoo, E.S. Comparison of Surgical Outcomes Between Holmium Laser Enucleation and Transurethral Resection of the Prostate in Patients with Detrusor Underactivity. Int. Neurourol. J. 2017, 21, 46–52. [Google Scholar] [CrossRef]
- Choi, S.W.; Choi, Y.S.; Bae, W.J.; Kim, S.J.; Cho, H.J.; Hong, S.H.; Lee, J.Y.; Hwang, T.K.; Kim, S.W. 120 W Greenlight HPS Laser Photoselective Vaporization of the Prostate for Treatment of Benign Prostatic Hyperplasia in Men with Detrusor Underactivity. Korean J. Urol. 2011, 52, 824–828. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lomas, D.J.; Krambeck, A.E. Long-term Efficacy of Holmium Laser Enucleation of the Prostate in Patients with Detrusor Underactivity or Acontractility. Urology 2016, 97, 208–211. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, C.R.; Mynderse, L.A.; Lightner, D.J.; Husmann, D.A.; Krambeck, A.E. Efficacy of holmium laser enucleation of the prostate in patients with non-neurogenic impaired bladder contractility: Results of a prospective trial. Urology 2014, 83, 428–432. [Google Scholar] [CrossRef]
- Abdelhakim, M.A.; Rammah, A.; Abozamel, A.H.; El-Sheikh, M.G.; Abdelazeem, M.S.; Abdallah, S.M.; Abdelaziz, A.Y. Does detrusor underactivity affect the results of transurethral resection of prostate? Int. Urol. Nephrol. 2021, 53, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Pyun, J.H.; Kang, S.G.; Kang, S.H.; Cheon, J.; Kim, J.J.; Lee, J.G. Efficacy of holmium laser enucleation of the prostate (HoLEP) in men with bladder outlet obstruction (BOO) and non-neurogenic bladder dysfunction. Kaohsiung J. Med. Sci. 2017, 33, 458–463. [Google Scholar] [CrossRef]
- Yu, Z.; Li, J.; Li, Z.; Hou, R. Photoselective Vaporization of the Prostate and Simultaneous Suprapubic Cystostomy for the Treatment of Benign Prostatic Hyperplasia in Patients with Mild to Severe Detrusor Underactivity. Urol. Int. 2015, 95, 269–275. [Google Scholar] [CrossRef]
- Thomas, A.W.; Cannon, A.; Bartlett, E.; Ellis-Jones, J.; Abrams, P. The natural history of lower urinary tract dysfunction in men: The influence of detrusor underactivity on the outcome after transurethral resection of the prostate with a minimum 10-year urodynamic follow-up. BJU Int. 2004, 93, 745–750. [Google Scholar] [CrossRef]
- Thomas, D.; Zorn, K.C.; Zaidi, N.; Chen, S.A.; Zhang, Y.; Te, A.; Chughtai, B. Does urodynamics predict voiding after benign prostatic hyperplasia surgery in patients with detrusor underactivity? Asian J. Urol. 2019, 6, 264–269. [Google Scholar] [CrossRef]
- Lee, K.H.; Kuo, H.C. Recovery of Voiding Efficiency and Bladder Function in Male Patients with Non-neurogenic Detrusor Underactivity After Transurethral Bladder Outlet Surgery. Urology 2019, 123, 235–241. [Google Scholar] [CrossRef]
- Wu, S.Y.; Kuo, H.C. Predictive factors for recovery of voiding function after transurethral prostate surgery in men with small prostate volume and very low detrusor contractility. Low. Urin. Tract Symptoms 2020, 12, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.F.; Peng, C.H.; Kuo, H.C. Will detrusor acontractility recover after medical or surgical treatment? A longitudinal long-term urodynamic follow-up. Neurourol. Urodyn. 2021, 40, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Zhong, P.; Zhao, Y.-R.; Qiao, B.-M.; Yang, F.-J.; Zhu, Y.; Yang, Z.-Q.; Niu, Y.-J. Comparison of Two Numerical Parameters to Assess Detrusor Contractility in Prognosing Short-Term Outcome after Transurethral Resection of the Prostate. Urol. Int. 2020, 104, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Plata, M.; Santander, J.; Trujillo, C.G.; Bravo-Balado, A.; Robledo, D.; Higuera, T.; Caicedo, J.I. Impact of detrusor underactivity on the postoperative outcomes after benign prostatic enlargement surgery. Neurourol. Urodyn. 2021, 40, 868–875. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.C.; Park, J.; Kim, J.K.; Cho, S.Y.; Jeong, H.; Oh, S.J.; Paick, J.-S.; Son, H. Can preoperative detrusor underactivity influence surgical outcomes of 120 W HPS vaporization of the prostate (PVP) or holmium laser enucleation of the prostate (HoLEP)? A serial 3-year follow-up study. Neurourol. Urodyn. 2018, 37, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Masumori, N.; Furuya, R.; Tanaka, Y.; Furuya, S.; Ogura, H.; Tsukamoto, T. The 12-year symptomatic outcome of transurethral resection of the prostate for patients with lower urinary tract symptoms suggestive of benign prostatic obstruction compared to the urodynamic findings before surgery. BJU Int. 2010, 105, 1429–1433. [Google Scholar] [CrossRef]
- Cho, M.C.; Yoo, S.; Park, J.; Cho, S.Y.; Son, H.; Oh, S.J.; Paick, J.-S. Effect of preoperative detrusor underactivity on long-term surgical outcomes of photovaporization and holmium laser enucleation in men with benign prostatic hyperplasia: A lesson from 5-year serial follow-up data. BJU Int. 2019, 123, E34–E42. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhao, Y.R.; Zhong, P.; Qiao, B.M.; Yang, Z.Q.; Niu, Y.J. Detrusor underactivity influences the efficacy of TURP in patients with BPO. Int. Urol. Nephrol. 2021, 53, 835–841. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.-Y.; Wang, C.-S.; Juan, Y.-S. Detrusor Underactivity in Men with Bladder Outlet Obstruction. Biomedicines 2022, 10, 2954. https://doi.org/10.3390/biomedicines10112954
Lee H-Y, Wang C-S, Juan Y-S. Detrusor Underactivity in Men with Bladder Outlet Obstruction. Biomedicines. 2022; 10(11):2954. https://doi.org/10.3390/biomedicines10112954
Chicago/Turabian StyleLee, Hsiang-Ying, Chien-Sheng Wang, and Yung-Shun Juan. 2022. "Detrusor Underactivity in Men with Bladder Outlet Obstruction" Biomedicines 10, no. 11: 2954. https://doi.org/10.3390/biomedicines10112954
APA StyleLee, H.-Y., Wang, C.-S., & Juan, Y.-S. (2022). Detrusor Underactivity in Men with Bladder Outlet Obstruction. Biomedicines, 10(11), 2954. https://doi.org/10.3390/biomedicines10112954






