A Highly Sensitive Immunoassay for Determination of Immune Response to SARS-CoV-2 in Capillary Blood Samples
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Samples
2.2. Capillary Blood Collection
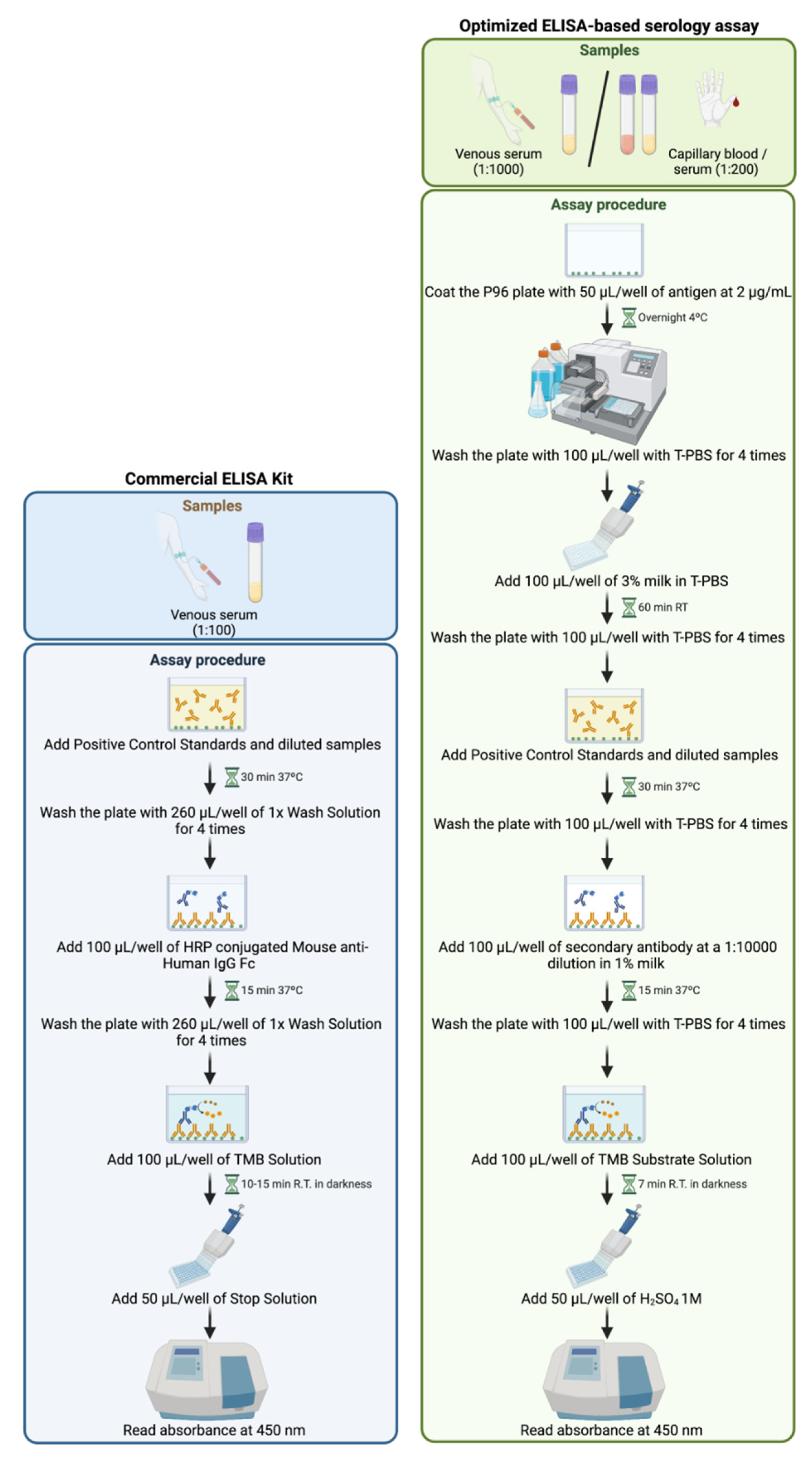
2.3. Commercially Available ELISA Serologic Assay
2.4. Optimized ELISA-Based Serology Assay
2.4.1. Materials
2.4.2. Reagents
- Wash reagent: Tween 0.1% in phosphate-buffered saline (T-PBS).
- Standard and antigen dilution reagent: carbonic acid (H2CO3) buffer 0.2 M at pH 9.4.
- Sample diluent reagent: T-PBS.
- Blocking reagent: milk 3% in T-PBS.
- Stop solution: sulfuric acid (H2SO4) 1M.
2.4.3. Sample Preparation
- Venous serum: samples are diluted to 1:1000 in T-PBS.
- Capillary blood or capillary serum: samples are diluted to 1:200 in T-PBS.
2.4.4. Assay Procedure
- Coat the P96 plate with 50 µL/well of 2 µg/mL recombinant RBD of the SARS-CoV-2 S1 spike protein in dilution reagent.
- Cover the P96 plate and incubate at 4 °C overnight.
- Remove the lid and wash the plate with 100 µL/well of T-PBS 4 times.
- Tap the plate on a paper towel to remove any remaining buffer from washes.
- Add 100 µL of 3% milk in T-PBS.
- Cover the plate and incubate for 1 h at room temperature.
- Remove the lid and wash the plate with 100 µL/well of T-PBS 4 times.
- Tap the plate on a paper towel to remove any remaining buffer from washes.
- Add 100 µL of Positive Control Standards or the diluted human samples per well, cover the plate, and incubate for 30 min at 37 °C.
- Remove the lid and wash the plate with 100 µL of T-PBS 4 times.
- Tap the plate on a paper towel to remove any remaining buffer from washes.
- Add 100 µL of secondary antibody at a 1:10,000 dilution in 1% milk and incubate for 15 min at 37 °C.
- Remove the lid and wash the plate with 100 µL of T-PBS 4 times.
- Tap the plate on a paper towel to remove any remaining buffer from washes.
- Add 100 µL of TMB Substrate Solution per well and incubate the plate in the dark for 7 min at room temperature.
- Add 50 µL per well of H2SO4 1M.
- Read absorbance immediately on a spectrophotometer at 450 nm.
2.5. Statistical Analysis
3. Results
3.1. Participant Characteristics
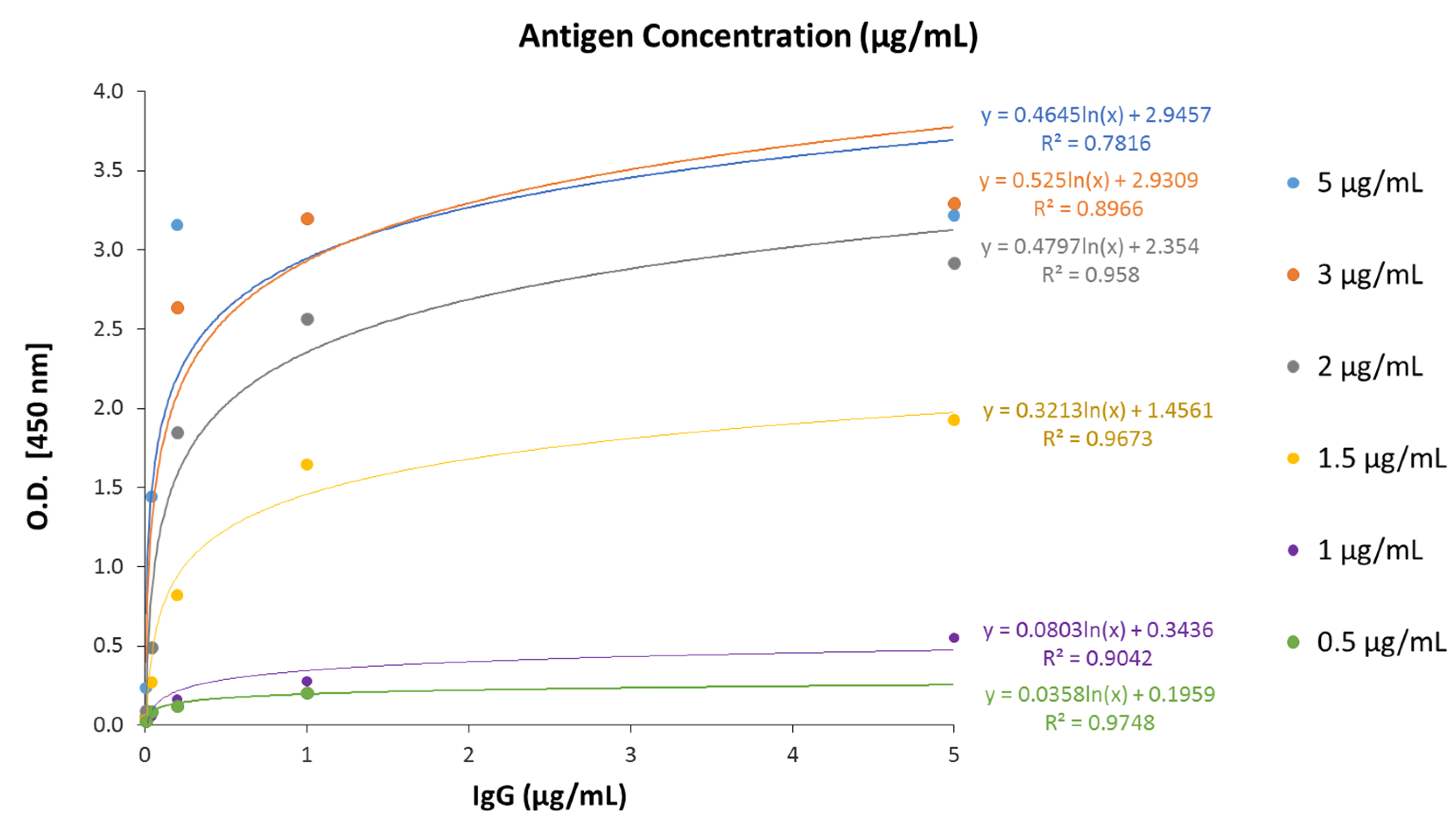
3.2. Analytical Characteristics of the Optimized ELISA-Based Serology Assay
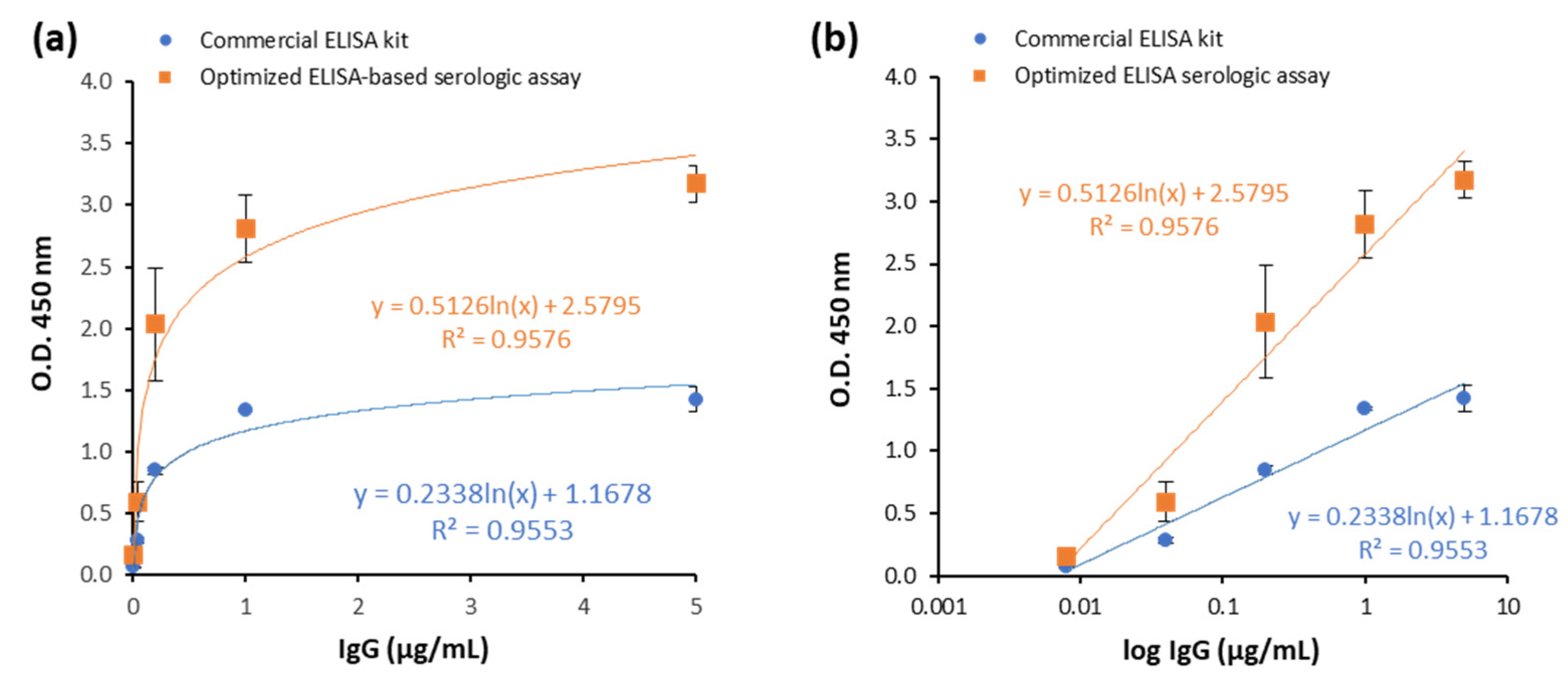
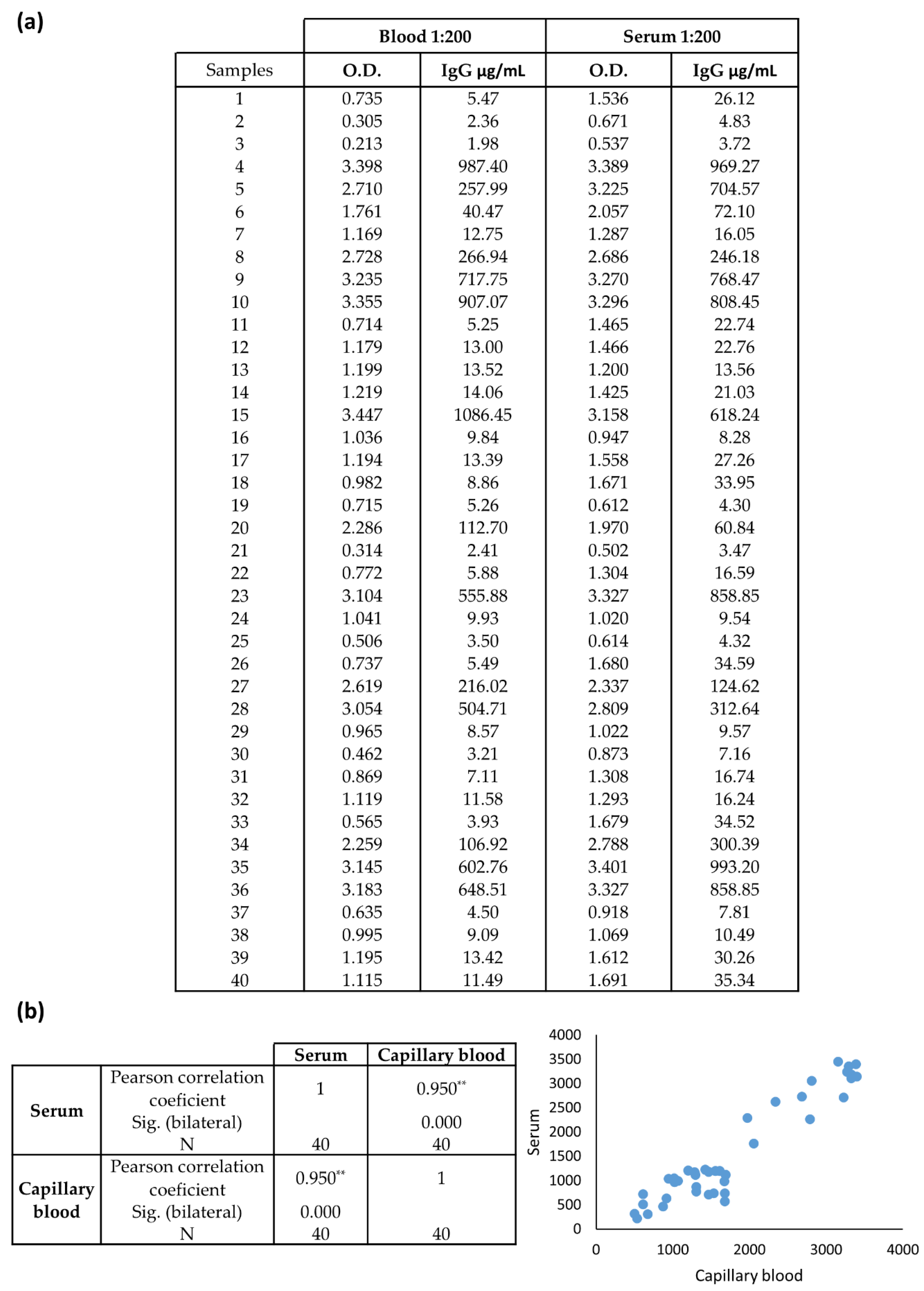
3.3. Assay Performance of the Optimized ELISA Serologic Assay Compared to Commercial ELISA Kit
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kiryanov, S.A.; Levina, T.A.; Kadochnikova, V.V.; Konopleva, M.V.; Suslov, A.P.; Trofimov, D.Y. Clinical Evaluation of Nasopharyngeal, Oropharyngeal, Nasal Swabs, and Saliva for the Detection of SARS-CoV-2 by Direct RT-PCR. Diagnostics 2022, 12, 1091. [Google Scholar] [CrossRef] [PubMed]
- da Silva, R.C.M.; Marinho, L.C.N.; Silva, D.N.D.A.; de Lima, K.C.; Pirih, F.Q.; Martins, A.R.L.D.A. Saliva as a possible tool for the SARS-CoV-2 detection: A review. Travel Med. Infect. Dis. 2020, 38, 101920. [Google Scholar] [CrossRef] [PubMed]
- Tee, M.L.; Abrilla, A.A.; Tee, C.A.; Dalmacio, L.M.M.; Villaflor, V.J.P.; Abubakar, A.-Z.A.; Tagayuna, P.Y.; Aquino, S.S.C.; Bernardo, V.A.L.; Matias, R.R. Saliva as alternative to naso-oropharyngeal swab for SARS-CoV-2 detection by RT-qPCR: A multicenter cross-sectional diagnostic validation study. Sci. Rep. 2022, 12, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, M.; Sudrajat, D.G.; Muzellina, V.N.; Kurniawan, J.; Rizka, A.; Utari, A.P.; Pribadi, R.R.; Idrus, M.F.; Yusra, Y.; Meilany, S.; et al. The value of anal swab RT-PCR for COVID-19 diagnosis in adult Indonesian patients. BMJ Open Gastroenterol. 2021, 8, e000590. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, X.; Wang, F.; Geng, J.; Liu, B.; Han, F. Value of anal swabs for SARS-COV-2 detection: A literature review. Int. J. Med. Sci. 2021, 18, 2389–2393. [Google Scholar] [CrossRef]
- Fernández-Rojas, M.A.; Esparza, M.A.L.-R.; Campos-Romero, A.; Calva-Espinosa, D.Y.; Moreno-Camacho, J.L.; Mendlovic, F.; Plett-Torres, T.; Alcántar-Fernández, J. Seroconversion dynamic and SARS-CoV-2 seropositivity in unvaccinated population during the first and second outbreaks in Mexico. Sci. Rep. 2022, 12, 1–10. [Google Scholar] [CrossRef]
- Louis, R.; Pu, R.; Logan, T.D.; Trimmer-Smith, L.; Chamblain, R.; Gallagher, A.; De Rochars, V.M.B.; Nelson, E.; Cummings, D.A.T.; Long, M.T.; et al. SARS-CoV-2 infections in infants in Haiti 2020–2021; evidence from a seroepidemiological cohort. PLoS ONE 2022, 17, e0273482. [Google Scholar] [CrossRef]
- Szewczyk-Dąbrowska, A.; Budziar, W.; Baniecki, K.; Pikies, A.; Harhala, M.; Jędruchniewicz, N.; Kaźmierczak, Z.; Gembara, K.; Klimek, T.; Witkiewicz, W.; et al. Dynamics of anti-SARS-CoV-2 seroconversion in individual patients and at the population level. PLoS ONE 2022, 17, e0274095. [Google Scholar] [CrossRef]
- Abraha, I.; Eusebi, P.; Germani, A.; Pasquarelli, E.; Pascolini, S.; Antonietti, R.; Argenti, S.; Fioravanti, A.; Martini, E.; Aristei, L.; et al. Temporal trends and differences of SARS-CoV-2-specific antibody responses in symptomatic and asymptomatic subjects: A longitudinal study from Umbria in Italy. BMJ Open 2022, 12, e056370. [Google Scholar] [CrossRef]
- Friedman-Klabanoff, D.J.; Tjaden, A.H.; Santacatterina, M.; Munawar, I.; Sanders, J.W.; Herrington, D.M.; Wierzba, T.F.; Berry, A.A. Vaccine-induced seroconversion in participants in the North Carolina COVID-19 community Research Partnership. Vaccine 2022, 40, 6133–6140. [Google Scholar] [CrossRef]
- Huergo, L.F.; Paula, N.M.; Gonçalves, A.C.A.; Kluge, C.H.S.; Marins, P.H.S.A.; Camargo, H.S.C.; Sant’Ana, T.P.; Farias, L.R.P.; Aldrighi, J.D.; Lima, S.; et al. SARS-CoV-2 Seroconversion in Response to Infection and Vaccination: A Time Series Local Study in Brazil. Microbiol. Spectr. 2022, 10, e01026-22. [Google Scholar] [CrossRef] [PubMed]
- Pettersson, H.; Manley, B.; Hernandez, S.; McPhillps, D. Tracking COVID-19 Vaccinations Worldwide. Available online: https://edition.cnn.com/interactive/2021/health/global-covid-vaccinations/ (accessed on 30 September 2022).
- Marasco, V.; Carniti, C.; Guidetti, A.; Farina, L.; Magni, M.; Miceli, R.; Calabretta, L.; Verderio, P.; Ljevar, S.; Serpenti, F.; et al. T-cell immune response after mRNA SARS-CoV-2 vaccines is frequently detected also in the absence of seroconversion in patients with lymphoid malignancies. Br. J. Haematol. 2021, 196, 548–558. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Li, Y.; Guo, E.; He, L.; Liu, J.; Bin Yang, B.; Li, F.; Wang, Z.; Li, Y.; Xiao, R.; et al. Dynamics and Correlation Among Viral Positivity, Seroconversion, and Disease Severity in COVID-19. Ann. Intern. Med. 2021, 174, 453–461. [Google Scholar] [CrossRef]
- Warrington, J.S.; Crothers, J.W.; Goodwin, A.; Coulombe, L.; Hong, T.; Bryan, L.; Wojewoda, C.; Fung, M.; Warrington, G.; Clark, V.; et al. All Hands-On Deck and All Decks on Hand: Surmounting Supply Chain Limitations During the COVID-19 Pandemic. Acad. Pathol. 2021, 8, 23742895211011928. [Google Scholar] [CrossRef]
- Vo, L.H.; Le, T. COVID-19 test-kit trade and trade policy: Implications for developing countries. World Econ. 2022, 45, 3246–3268. [Google Scholar] [CrossRef] [PubMed]
- Biswal, J.K.; Ranjan, R.; Dahiya, S.S.; Mallick, S.; Mohapatra, J.K. Regenerated silica-based RNA purification columns to address the short supply of RNA purification kits for COVID-19 diagnosis. Mol. Biol. Rep. 2021, 48, 6871–6877. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Diaz, D.J.; Sakthivel, D.; Nguyen, H.H.T.; Farrokzhad, K.; Hopper, W.; Narh, C.A.; Richards, J.S. Strategies That Facilitate Extraction-Free SARS-CoV-2 Nucleic Acid Amplification Tests. Viruses 2022, 14, 1311. [Google Scholar] [CrossRef] [PubMed]
- Ndayishimiye, C.; Sowada, C.; Dyjach, P.; Stasiak, A.; Middleton, J.; Lopes, H.; Dubas-Jakóbczyk, K. Associations between the COVID-19 Pandemic and Hospital Infrastructure Adaptation and Planning—A Scoping Review. Int. J. Environ. Res. Public Heal. 2022, 19, 8195. [Google Scholar] [CrossRef]
- Dinnes, J.; Sharma, P.; Berhane, S.; van Wyk, S.S.; Nyaaba, N.; Domen, J.; Taylor, M.; Cunningham, J.; Davenport, C.; Dittrich, S.; et al. Rapid, point-of-care antigen tests for diagnosis of SARS-CoV-2 infection. Cochrane Database Syst. Rev. 2022, 7, CD013705. [Google Scholar] [CrossRef]
- Meyers, E.; Heytens, S.; Formukong, A.; Vercruysse, H.; De Sutter, A.; Geens, T.; Hofkens, K.; Janssens, H.; Nys, E.; Padalko, E.; et al. Comparison of Dried Blood Spots and Venous Blood for the Detection of SARS-CoV-2 Antibodies in a Population of Nursing Home Residents. Microbiol. Spectr. 2021, 9, e00178-21. [Google Scholar] [CrossRef]
- Krleza, J.L.; Dorotic, A.; Grzunov, A.; Maradin, M. Capillary blood sampling: National recommendations on behalf of the Croatian Society of Medical Biochemistry and Laboratory Medicine. Biochem. Med. 2015, 25, 335–358. [Google Scholar] [CrossRef] [PubMed]
- Alkhatib, M.; Bellocchi, M.C.; Marchegiani, G.; Grelli, S.; Micheli, V.; Stella, D.; Zerillo, B.; Carioti, L.; Svicher, V.; Rogliani, P.; et al. First Case of a COVID-19 Patient Infected by Delta AY.4 with a Rare Deletion Leading to a N Gene Target Failure by a Specific Real Time PCR Assay: Novel Omicron VOC Might Be Doing Similar Scenario? Microorganisms 2022, 10, 268. [Google Scholar] [CrossRef] [PubMed]
- Čretnik, T.; Retelj, M.; Janežič, S.; Mahnič, A.; Tesovnik, T.; Šket, R.; Bizjan, B.J.; Jeverica, S.; Ravnik, M.; Rak, M.; et al. Accuracy of Allplex SARS-CoV-2 assay amplification curve analysis for the detection of SARS-CoV-2 variant Alpha. Futur. Microbiol. 2022, 17, 1125–1131. [Google Scholar] [CrossRef]
- De Smet, D.; Vanhee, M.; Maes, B.; Swaerts, K.; De Jaeger, P.; Maelegheer, K.; Van Hoecke, F.; Martens, G.A. Cycle Threshold Probability Score for Immediate and Sensitive Detection of B.1.351 SARS-CoV-2 Lineage. Am. J. Clin. Pathol. 2021, 157, 731–741. [Google Scholar] [CrossRef]
- Ibba, G.; Sau, R.; Angioj, F.; Abbondio, M.; Rubino, S.; Uzzau, S. A straightforward molecular strategy to retrospectively investigate the spread of SARS-CoV-2 VOC202012/01 B.1.1.7 variant. J. Infect. Dev. Ctries. 2021, 15, 242–246. [Google Scholar] [CrossRef] [PubMed]
- So, M.-K.; Park, S.; Lee, K.; Kim, S.-K.; Chung, H.-S.; Lee, M. Variant Prediction by Analyzing RdRp/S Gene Double or Low Amplification Pattern in Allplex SARS-CoV-2. Assay. Diagnostics 2021, 11, 1854. [Google Scholar] [CrossRef]
- Andreasson, U.; Perret-Liaudet, A.; van Waalwijk van Doorn, L.J.C.; Blennow, K.; Chiasserini, D.; Engelborghs, S.; Fladby, T.; Genc, S.; Kruse, N.; Kuiperij, H.B.; et al. A Practical Guide to Immunoassay Method Validation. Front. Neurol. 2015, 6, 179. [Google Scholar] [CrossRef] [PubMed]
- Miesse, P.K.; Collier, B.B.; Grant, R.P. Monitoring of SARS-CoV-2 antibodies using dried blood spot for at-home collection. Sci. Rep. 2022, 12, 1–10. [Google Scholar] [CrossRef]
- Lim, S.M.; Cheng, H.L.; Jia, H.; Kongsuphol, P.; Shunmuganathan, B.D.; Chen, M.W.; Ng, S.Y.; Gao, X.; Turaga, S.P.; Heussler, S.P.; et al. Finger stick blood test to assess postvaccination SARS-CoV-2 neutralizing antibody response against variants. Bioeng. Transl. Med. 2022, 7, e10293. [Google Scholar] [CrossRef]
- Baggio, S.; Togni, G.; Eckerle, I.; Vuillemier, N.; Kaiser, L.; Gétaz, L. Feasibility of home-based ELISA capillary blood self-testing for anti-SARS-CoV-2 antibodies. Pr. Lab. Med. 2022, 31, e00290. [Google Scholar] [CrossRef]
- Brown, L.; Byrne, R.L.; Fraser, A.; Owen, S.I.; Cubas-Atienzar, A.I.; Williams, C.T.; Kay, G.A.; Cuevas, L.E.; Fitchett, J.R.A.; Fletcher, T.; et al. Self-sampling of capillary blood for SARS-CoV-2 serology. Sci. Rep. 2021, 11, 1–6. [Google Scholar] [CrossRef]
- Mohammed, T.; Brewer, J.V.; Pyatt, M.; Whitbourne, S.B.; Gaziano, J.M.; Edson, C.; Holodniy, M. Evaluation of Independent Self-Collected Blood Specimens for COVID-19 Antibody Detection among the US Veteran Population. Diagn. Microbiol. Infect. Dis. 2022, 104, 115770. [Google Scholar] [CrossRef]
- Anderson, M.; Holzmayer, V.; Vallari, A.; Taylor, R.; Moy, J.; Cloherty, G. Expanding access to SARS-CoV-2 IgG and IgM serologic testing using fingerstick whole blood, plasma, and rapid lateral flow assays. J. Clin. Virol. 2021, 141, 104855. [Google Scholar] [CrossRef] [PubMed]
- Moeller, M.E.; Engsig, F.N.; Bade, M.; Fock, J.; Pah, P.; Soerensen, A.L.; Bang, D.; Donolato, M.; Benfield, T. Rapid Quantitative Point-Of-Care Diagnostic Test for Post COVID-19 Vaccination Antibody Monitoring. Microbiol. Spectr. 2022, 10, e00396-22. [Google Scholar] [CrossRef]
- Cutts, T.; Grolla, A.; Jones, S.; Cook, B.W.M.; Qiu, X.; Theriault, S.S. Inactivation of Zaire ebola virus Variant Makona in Human Serum Samples Analyzed by Enzyme-Linked Immunosorbent Assay. J. Infect. Dis. 2016, 214, S218–S221. [Google Scholar] [CrossRef]
- Kariwa, H.; Fujii, N.; Takashima, I. Inactivation of SARS Coronavirus by Means of Povidone-Iodine, Physical Conditions and Chemical Reagents. Dermatology 2006, 212, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Leclercq, I.; Batéjat, C.; Burguière, A.M.; Manuguerra, J. Heat inactivation of the Middle East respiratory syndrome coronavirus. Influ. Other Respir. Viruses 2014, 8, 585–586. [Google Scholar] [CrossRef] [PubMed]
- Park, S.L.; Huang, Y.-J.S.; Hsu, W.-W.; Hettenbach, S.M.; Higgs, S.; Vanlandingham, D.L. Virus-specific thermostability and heat inactivation profiles of alphaviruses. J. Virol. Methods 2016, 234, 152–155. [Google Scholar] [CrossRef]
- Batéjat, C.; Grassin, Q.; Manuguerra, J.-C.; Leclercq, I. Heat inactivation of the severe acute respiratory syndrome coronavirus 2. J. Biosaf. Biosecur. 2021, 3, 1–3. [Google Scholar] [CrossRef]
- Hu, X.; An, T.; Situ, B.; Hu, Y.; Ou, Z.; Li, Q.; He, X.; Zhang, Y.; Tian, P.; Sun, D.; et al. Heat inactivation of serum interferes with the immunoanalysis of antibodies to SARS-CoV-2. J. Clin. Lab. Anal. 2020, 34, e23411. [Google Scholar] [CrossRef]
| Features | Total |
|---|---|
| Participants (n, %) • SARS-CoV-2 PCR + • SARS-CoV-2 PCR − | 123 (100%) • 98 (80%) • 25 (20%) |
| Sex (n, %) • Male • Female | 123 (100%) • 84 (68%) • 39 (32%) |
| Median age (IQR) (years) • Male • Female | 64 (56–72) • 66 (56–72) • 58 (57–69) |
| Commercial ELISA Kit | Optimized ELISA-Based Serological Assay | |||||
|---|---|---|---|---|---|---|
| Samples | 1:100 | 1:50 | 1:200 | 1:500 | 1:800 | 1:1000 |
| 1 | 1.569 ± 0.047 | 3.324 ± 0.037 | 3.330 ± 0.006 | 3.317 ± 0.006 | 3.281 ± 0.004 | 3.298 ± 0.025 |
| 2 | 0.884 ± 0.009 | 3.396 ± 0.061 | 3.365 ± 0.006 | 3.107 ± 0.030 | 3.165 ± 0.058 | 2.897 ± 0.052 |
| 3 | 1.604 ± 0.003 | 3.357 ± 0.001 | 3.447 ± 0.002 | 3.440 ± 0.047 | 3.321 ± 0.013 | 3.379 ± 0.028 |
| 4 | 0.178 ± 0.005 | 3.194 ± 0.025 | 3.149 ± 0.054 | 1.793 ± 0.031 | 1.016 ± 0.095 | 0.809 ± 0.018 |
| 5 | 0.921 ± 0.023 | 3.471 ± 0.026 | 3.450 ± 0.006 | 3.404 ± 0.018 | 3.302 ± 0.063 | 3.332 ± 0.008 |
| 6 | 0.154 ± 0.000 | 3.178 ±0.006 | 3.228 ± 0.013 | 1.778 ± 0.028 | 1.115 ± 0.122 | 0.791 ± 0.014 |
| 7 | 0.051 ± 0.001 | 1.958 ± 0.018 | 0.895 ± 0.032 | 0.357 ± 0.018 | 0.280 ± 0.011 | 0.269 ± 0.013 |
| 8 | 0.027 ± 0.002 | 1.322 ± 0.066 | 0.903 ± 0.041 | 0.403 ± 0.004 | 0.306 ± 0.011 | 0.306 ± 0.040 |
| Participants | rRT-PCR | Optimized ELISA-Based SEROLOGY Assay | IgG Commercial ELISA Kit |
|---|---|---|---|
| 123 | Positive 98 (80%) | Positive: 74 (76%) Negative: 24 (24%) | Positive: 75 (77%) Negative: 23 (23%) |
| Negative 25 (20%) | Positive: 3 (12%) Negative: 22 (88%) | Positive: 3 (12%) Negative: 22 (88%) | |
| Total | Positive 77 Negative 46 | Positive 78 Negative 45 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez, B.G.; Bort, A.; Mora-Rodríguez, J.M.; Díaz-Yuste, A.; Gasalla, J.M.; Sánchez-Chapado, M.; Sebastián-Martín, A.; Díaz-Laviada, I. A Highly Sensitive Immunoassay for Determination of Immune Response to SARS-CoV-2 in Capillary Blood Samples. Biomedicines 2022, 10, 2897. https://doi.org/10.3390/biomedicines10112897
Sánchez BG, Bort A, Mora-Rodríguez JM, Díaz-Yuste A, Gasalla JM, Sánchez-Chapado M, Sebastián-Martín A, Díaz-Laviada I. A Highly Sensitive Immunoassay for Determination of Immune Response to SARS-CoV-2 in Capillary Blood Samples. Biomedicines. 2022; 10(11):2897. https://doi.org/10.3390/biomedicines10112897
Chicago/Turabian StyleSánchez, Belén G., Alicia Bort, José María Mora-Rodríguez, Alba Díaz-Yuste, José Manuel Gasalla, Manuel Sánchez-Chapado, Alba Sebastián-Martín, and Inés Díaz-Laviada. 2022. "A Highly Sensitive Immunoassay for Determination of Immune Response to SARS-CoV-2 in Capillary Blood Samples" Biomedicines 10, no. 11: 2897. https://doi.org/10.3390/biomedicines10112897
APA StyleSánchez, B. G., Bort, A., Mora-Rodríguez, J. M., Díaz-Yuste, A., Gasalla, J. M., Sánchez-Chapado, M., Sebastián-Martín, A., & Díaz-Laviada, I. (2022). A Highly Sensitive Immunoassay for Determination of Immune Response to SARS-CoV-2 in Capillary Blood Samples. Biomedicines, 10(11), 2897. https://doi.org/10.3390/biomedicines10112897













