Acute Administration of Ethanol and of a D1-Receptor Antagonist Affects the Behavior and Neurochemistry of Adult Zebrafish
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Housing
2.2. Experimental Design, Drug Treatment Procedure
2.3. Behavioral Test and Procedure
2.4. Evaluation of Levels of Neurochemicals Using HPLC
2.5. Data Calculation and Statistical Analysis
3. Results
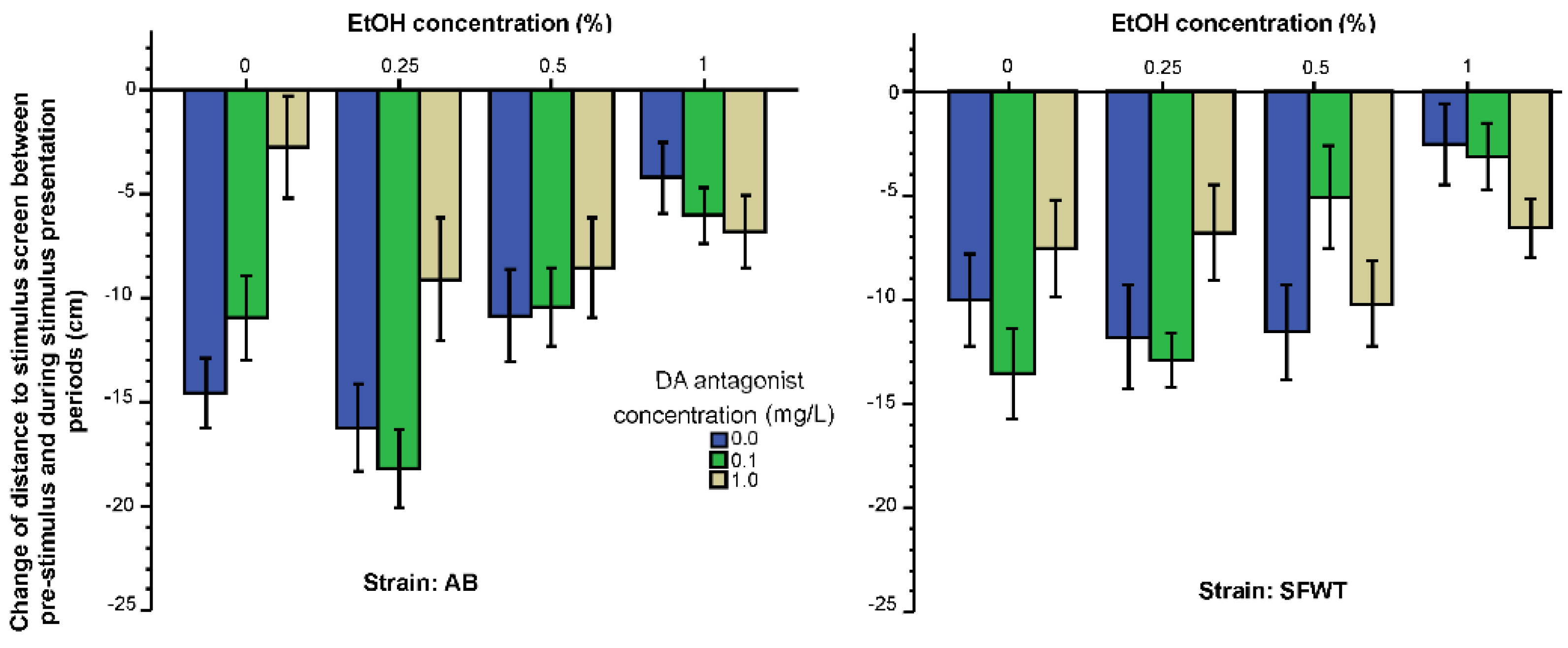
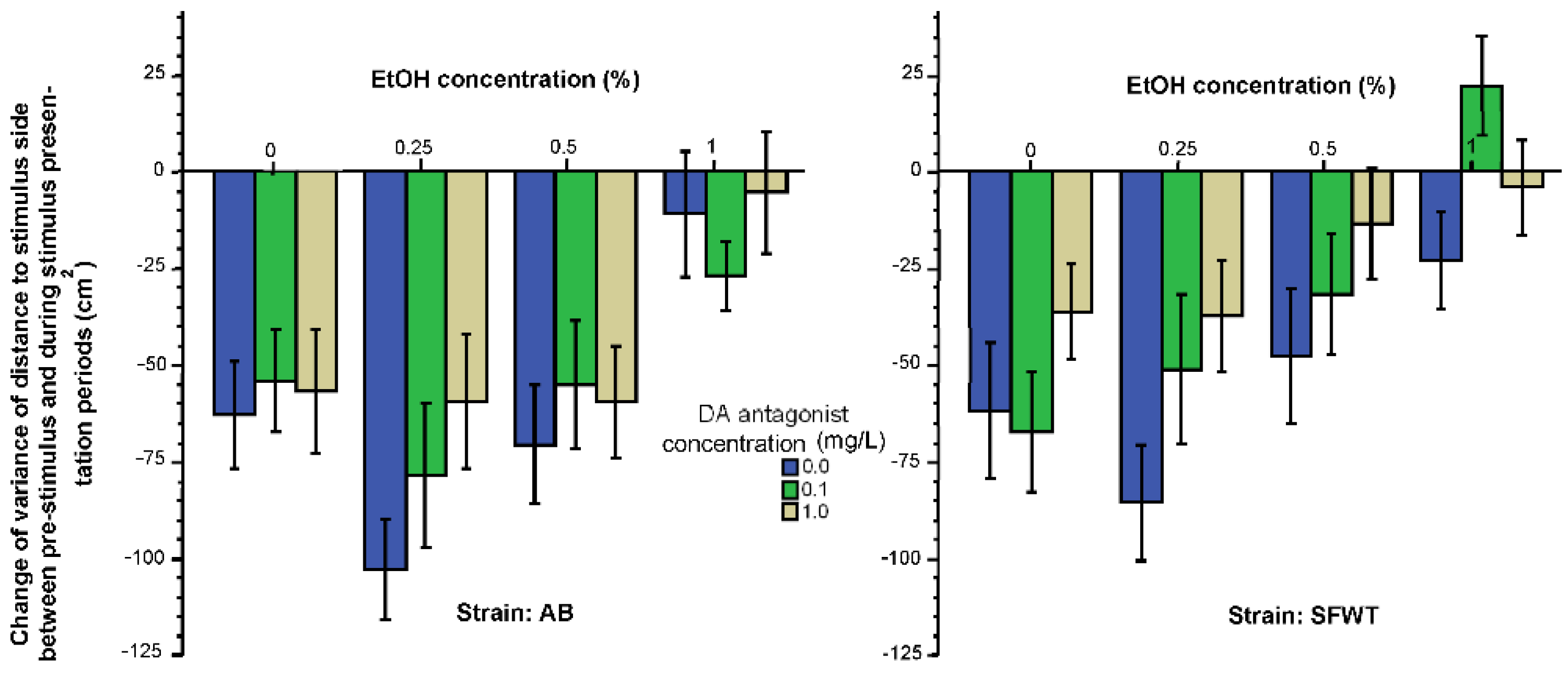
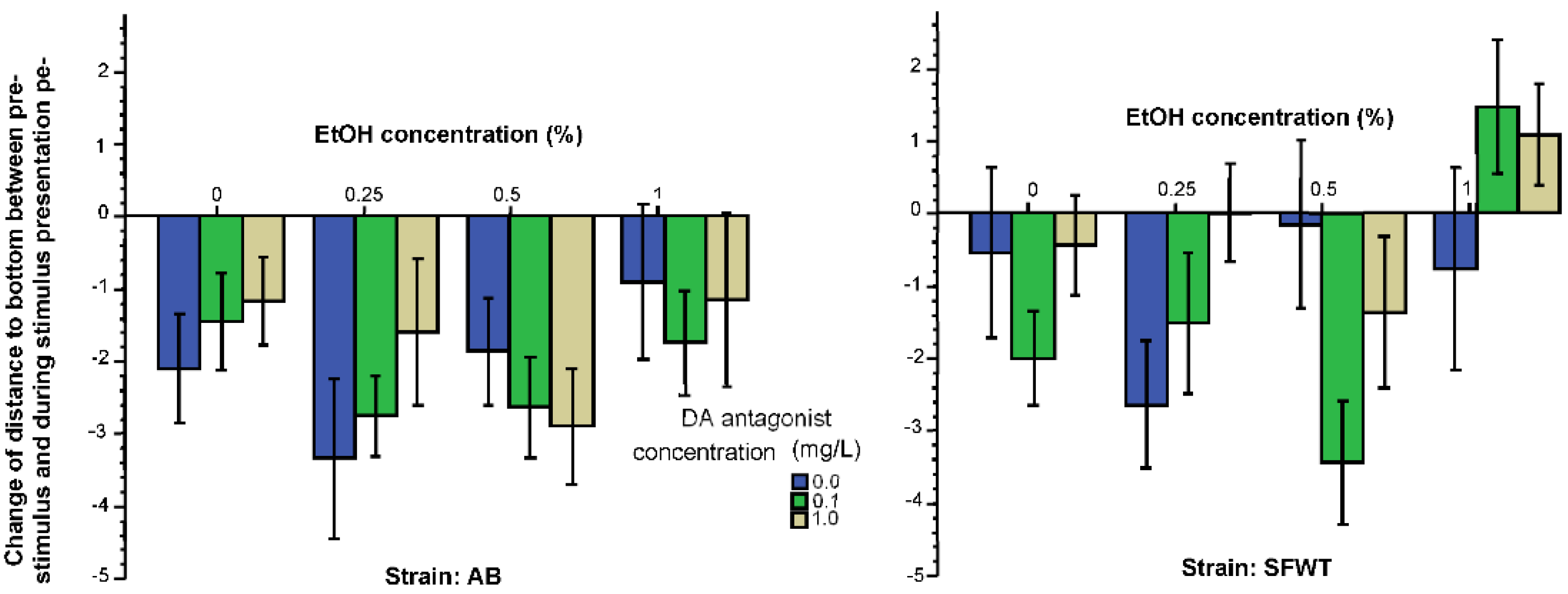
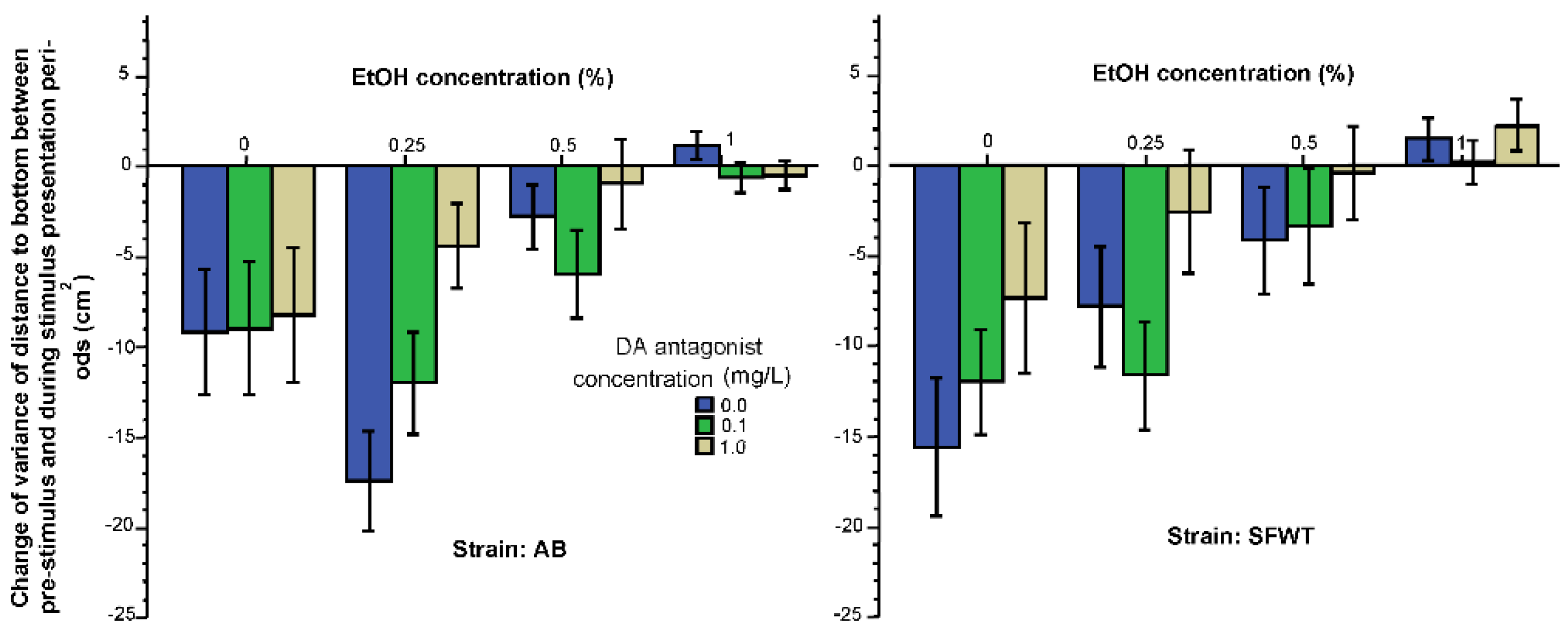
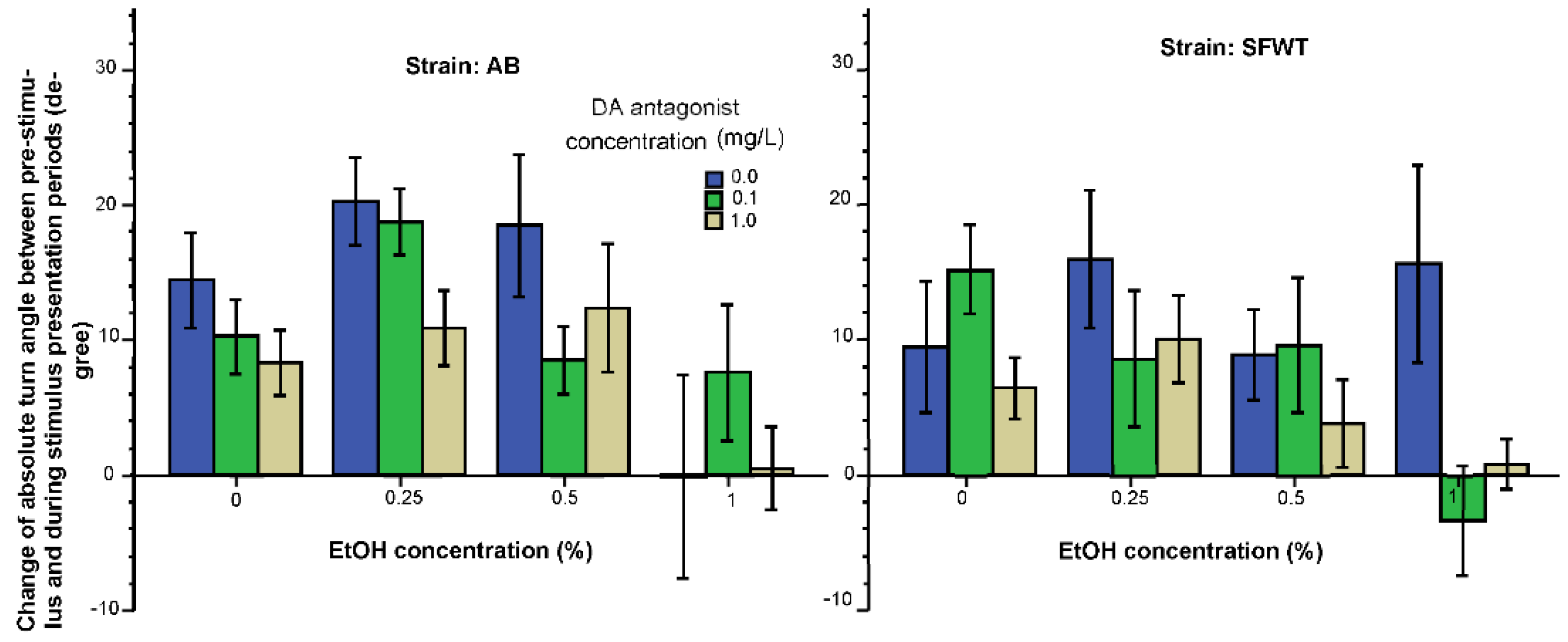
3.1. Behavioral Parameters
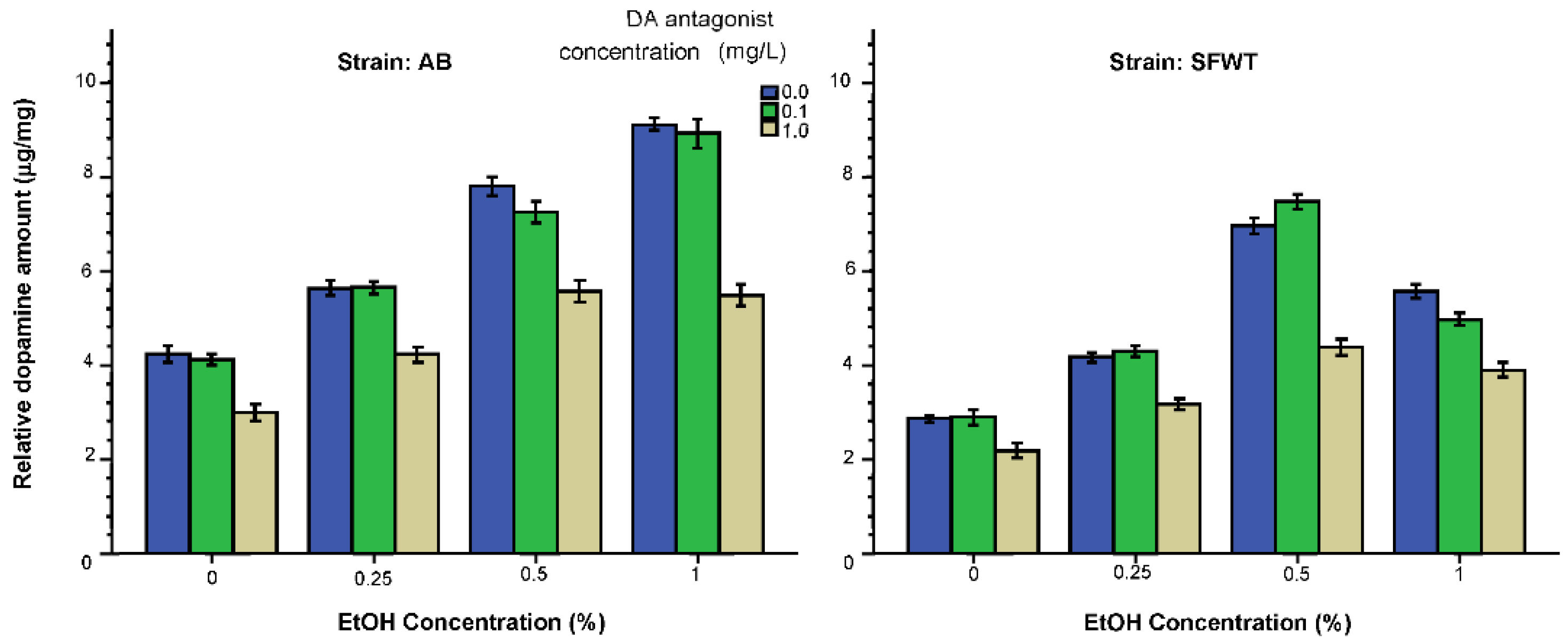
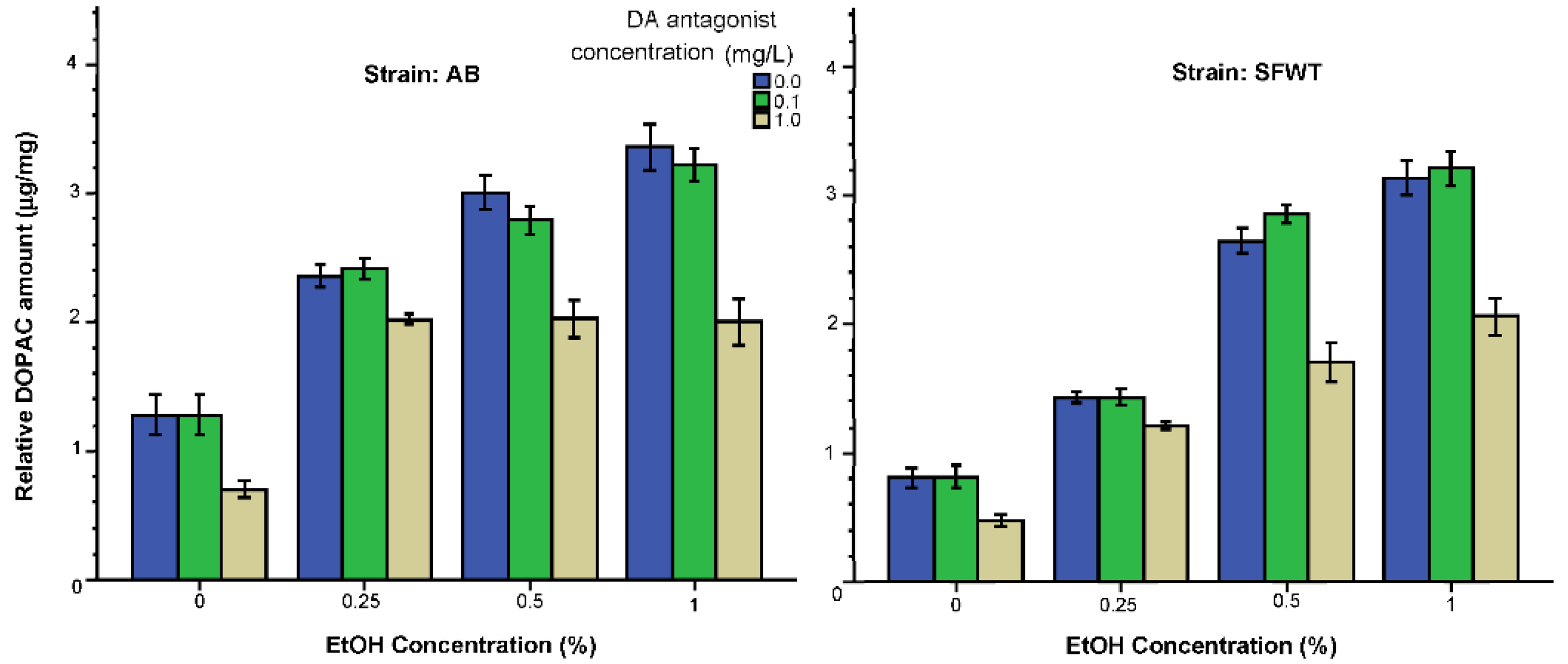
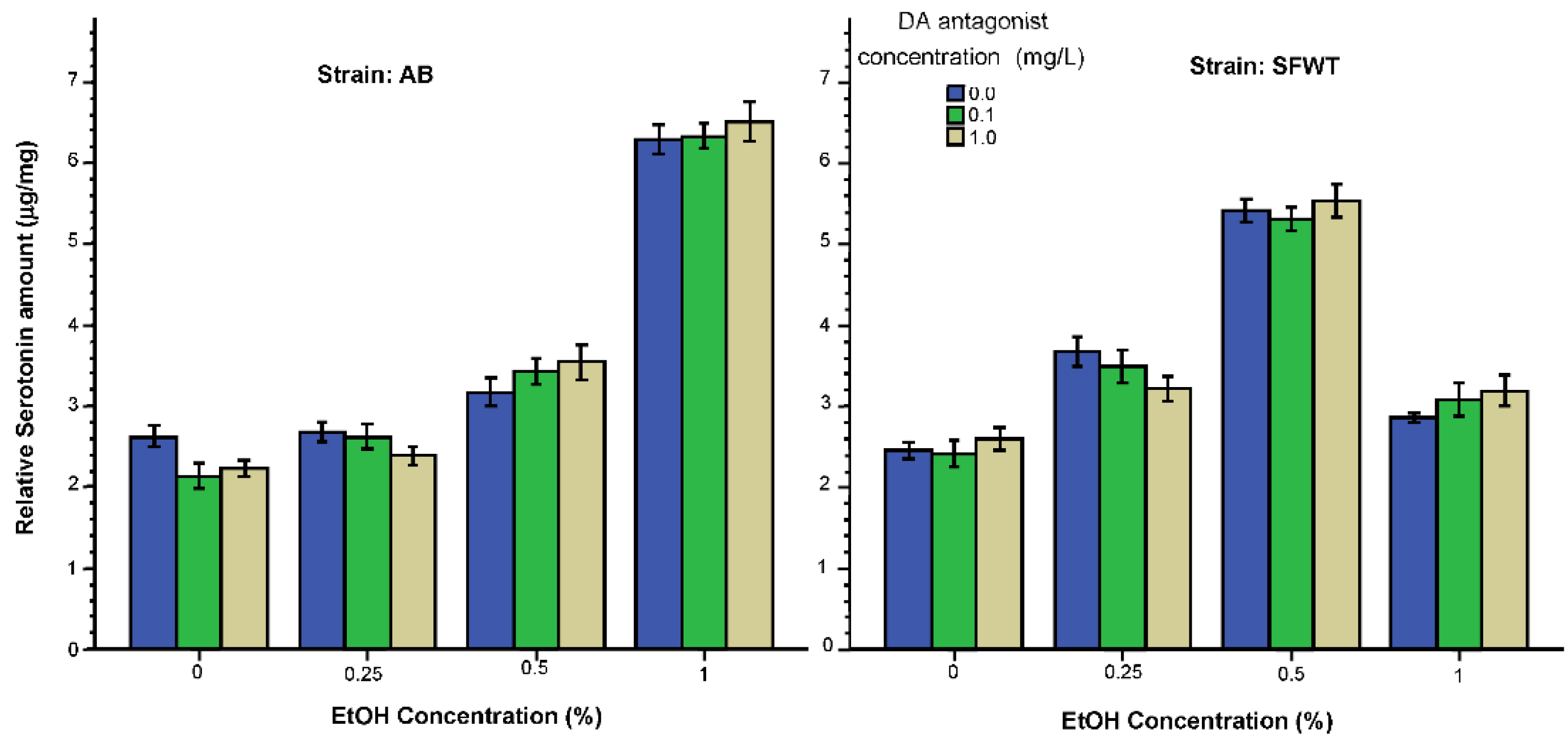
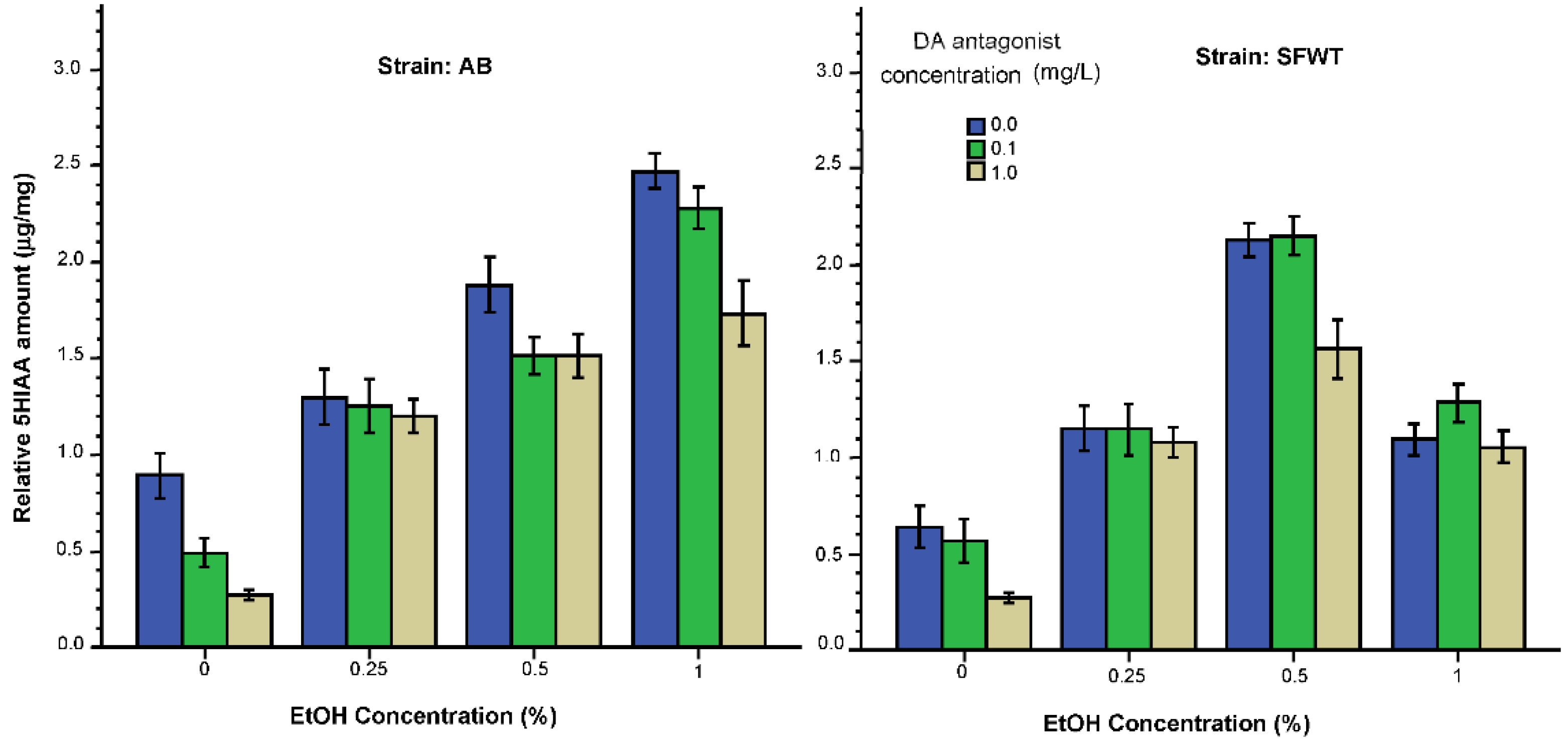
3.2. Neurochemical Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Park, S.H.; Kim, D.J. Global and regional impacts of alcohol use on public health: Emphasis on alcohol policies. Clin. Mol. Hepatol. 2020, 26, 652–661. [Google Scholar] [CrossRef]
- Laramée, P.; Kusel, J.; Leonard, S.; Aubin, H.J.; François, C.; Daeppen, J.B. The economic burden of alcohol dependence in Europe. Alcohol Alcohol. 2013, 48, 259–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Navarro, H.J.; Doran, C.M.; Shakeshaft, A.P. Measuring costs of alcohol harm to others: A review of the literature. Drug Alcohol Depend. 2011, 114, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Anton, R.F.; Kranzler, H.R.; Meyer, R.E. Neurobehavioral aspects of the pharmacotherapy of alcohol dependence. Clin. Neurosci. 1995, 3, 145–154. [Google Scholar]
- Ray, L.A.; Bujarski, S.; Grodin, E.; Hartwell, E.; Green, R.; Venegas, A.; Lim, A.C.; Gillis, A.; Miotto, K. State-of-the-art behavioral and pharmacological treatments for alcohol use disorder. Am. J. Drug Alcohol Abuse 2019, 45, 124–140. [Google Scholar] [CrossRef]
- Morgane, G.L.; Delphine, L.G.; Guillaume, K.; Yves, L.J. The Place of Pharmacotherapy in Alcohol Use Disorder Management in Family Practice-A Systematic Review. Curr. Pharm. Des. 2021, 27, 2737–2745. [Google Scholar] [CrossRef] [PubMed]
- Samson, H.H.; Harris, R.A. Neurobiology of alcohol abuse. Trends Pharmacol. Sci. 1992, 13, 206–211. [Google Scholar] [CrossRef]
- Nevo, I.; Hamon, M. Neurotransmitter and neuromodulatory mechanisms involved in alcohol abuse and alcoholism. Neurochem. Int. 1995, 26, 305–336. [Google Scholar] [CrossRef]
- Ward, R.J.; Lallemand, F.; de Witte, P. Biochemical and neurotransmitter changes implicated in alcohol-induced brain damage in chronic or ‘binge drinking’ alcohol abuse. Alcohol Alcohol. 2009, 44, 128–135. [Google Scholar] [CrossRef] [Green Version]
- Abrahao, K.P.; Salinas, A.G.; Lovinger, D.M. Alcohol and the Brain: Neuronal Molecular Targets, Synapses, and Circuits. Neuron 2017, 96, 1223–1238. [Google Scholar] [CrossRef] [Green Version]
- de Wit, H.; Phillips, T.J. Do initial responses to drugs predict future use or abuse? Neurosci. Biobehav. Rev. 2012, 36, 1565–1576. [Google Scholar] [CrossRef] [PubMed]
- Hinckers, A.S.; Laucht, M.; Schmidt, M.H.; Mann, K.F.; Schumann, G.; Schuckit, M.A.; Heinz, A. Low level of response to alcohol as associated with serotonin transporter genotype and high alcohol intake in adolescents. Biol. Psychiatry 2006, 60, 282–287. [Google Scholar] [CrossRef]
- Elvig, S.K.; McGinn, M.A.; Smith, C.; Arends, M.A.; Koob, G.F.; Vendruscolo, L.F. Tolerance to alcohol: A critical yet understudied factor in alcohol addiction. Pharmacol. Biochem. Behav. 2021, 204, 173155. [Google Scholar] [CrossRef] [PubMed]
- Parker, C.C.; Lusk, R.; Saba, L.M. Alcohol Sensitivity as an Endophenotype of Alcohol Use Disorder: Exploring Its Translational Utility between Rodents and Humans. Brain Sci. 2020, 10, 725. [Google Scholar] [CrossRef]
- Crabbe, J.C. Genetic animal models in the study of alcoholism. Alcohol. Clin. Exp. Res. 1989, 13, 120–127. [Google Scholar] [CrossRef]
- Crabbe, J.C. Use of animal models of alcohol-related behavior. Handb. Clin. Neurol. 2014, 125, 71–86. [Google Scholar] [CrossRef] [PubMed]
- Lovinger, D.M.; Crabbe, J.C. Laboratory models of alcoholism: Treatment target identification and insight into mechanisms. Nat. Neurosci. 2005, 8, 1471–1480. [Google Scholar] [CrossRef]
- Gerlai, R. Embryonic alcohol exposure: Towards the development of a zebrafish model of fetal alcohol spectrum disorders. Dev. Psychobiol. 2015, 57, 787–798. [Google Scholar] [CrossRef]
- Tran, S.; Gerlai, R. Recent advances with a novel model organism: Alcohol tolerance and sensitization in zebrafish (Danio rerio). Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2014, 55, 87–93. [Google Scholar] [CrossRef] [Green Version]
- Tran, S.; Facciol, A.; Gerlai, R. The zebrafish, a novel model organism for screening compounds affecting acute and chronic ethanol induced effects. Int. Rev. Neurobiol. 2016, 126, 467–484. [Google Scholar] [CrossRef]
- Howe, K.; Clark, M.D.; Torroja, C.F.; Torrance, J.; Berthelot, C.; Muffato, M.; Collins, J.E.; Humphray, S.; McLaren, K.; Matthews, L.; et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature 2013, 496, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Reimers, M.J.; Hahn, M.E.; Tanguay, R.L. Two zebrafish alcohol dehydrogenases share common ancestry with mammalian class I, II, IV, and V alcohol dehydrogenase genes but have distinct functional characteristics. J. Biol. Chem. 2004, 279, 38303–38312. [Google Scholar] [CrossRef] [Green Version]
- Postlethwait, J.H. The zebrafish genome: A review and msx gene case study. Genome Dyn. 2006, 2, 183–197. [Google Scholar] [CrossRef]
- Chatterjee, D.; Gerlai, R. High Precision Liquid Chromatography Analysis of Dopaminergic and Serotoninergic Responses to Acute Alcohol Exposure in Zebrafish. Behav. Brain Res. 2009, 200, 208–213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalueff, A.V.; Stewart, A.M.; Gerlai, R. Zebrafish as an emerging model for studying complex brain disorders. Trends Pharmacol. Sci. 2014, 35, 63–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerlai, R. Zebrafish Phenomics: Behavioral Screens and Phenotyping of Mutagenized Fish. Curr. Opin. Behav. Sci. 2014, 2, 21–27. [Google Scholar] [CrossRef]
- Gerlai, R. Using Zebrafish to Unravel the Genetics of Complex Brain Disorders. Curr. Top. Behav. Neurosci. 2012, 12, 3–24. [Google Scholar] [CrossRef] [PubMed]
- Gerlai, R. High-throughput Behavioral Screens: The First Step towards Finding Genes Involved in Vertebrate Brain Function Using Zebrafish. Molecules 2010, 15, 2609–2622. [Google Scholar] [CrossRef]
- Gerlai, R. Evolutionary conservation, translational relevance and cognitive function: The future of zebrafish in behavioral neuroscience. Neurosci. Biobehav. Rev. 2020, 116, 426–435. [Google Scholar] [CrossRef]
- Bailey, C.D.C.; Gerlai, R.; Cameron, N.M.; Marcolin, M.L.; McCormick, C.M. Preclinical methodological approaches investigating the effects of alcohol on perinatal and adolescent neurodevelopment. Neurosci. Biobehav. Rev. 2020, 116, 436–451. [Google Scholar] [CrossRef]
- Gerlai, R.; Chatterjee, D.; Pereira, T.; Sawashima, T.; Krishnannair, R. Acute and Chronic alcohol dose: Population differences in behavior and neurochemistry of zebrafish. Genes Brain Behav. 2009, 8, 586–599. [Google Scholar] [CrossRef] [PubMed]
- Love, D.R.; Pichler, F.B.; Dodd, A.; Copp, B.R.; Greenwood, D.R. Technology for high-throughput screens: The present and future using zebrafish. Curr. Opin. Biotechnol. 2004, 15, 564–571. [Google Scholar] [CrossRef] [PubMed]
- Bugel, S.M.; Tanguay, R.L.; Planchart, A. Zebrafish: A marvel of high-throughput biology for 21st century toxicology. Curr. Environ. Health Rep. 2014, 1, 341–352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerlai, R. Phenomics: Fiction or the Future? Trends Neurosci. 2002, 25, 506–509. [Google Scholar] [CrossRef]
- Attwood, A.S.; Munafò, M.R. Effects of acute alcohol consumption and processing of emotion in faces: Implications for understanding alcohol-related aggression. J. Psychopharmacol. 2014, 28, 719–732. [Google Scholar] [CrossRef] [Green Version]
- Gerlai, R.; Prajapati, S.; Ahmad, F. Differences in acute alcohol induced behavioral responses among zebrafish populations. Alcohol. Clin. Exp. Res. 2008, 32, 1763–1773. [Google Scholar] [CrossRef]
- Gerlai, R.; Lahav, M.; Guo, S.; Rosenthal, A. Drinks like a fish: Zebra fish (Danio rerio) as a behavior genetic model to study alcohol effects. Pharmacol. Biochem. Behav. 2000, 67, 773–782. [Google Scholar] [CrossRef]
- Tran, S.; Gerlai, R. Time-course of behavioural changes induced by ethanol in zebrafish (Danio rerio). Behav. Brain Res. 2013, 252, 204–213. [Google Scholar] [CrossRef] [Green Version]
- Hendler, R.A.; Ramchandani, V.A.; Gilman, J.; Hommer, D.W. Stimulant and sedative effects of alcohol. Curr. Top. Behav. Neurosci. 2013, 13, 489–509. [Google Scholar] [CrossRef]
- Charlet, K.; Beck, A.; Heinz, A. The dopamine system in mediating alcohol effects in humans. Curr. Top. Behav. Neurosci. 2013, 13, 461–488. [Google Scholar] [CrossRef]
- Wise, R.A.; Rompre, P.P. Brain dopamine and reward. Annu. Rev. Psychol. 1989, 40, 191–225. [Google Scholar] [CrossRef]
- Tran, S.; Facciol, A.; Nowicki, M.; Chatterjee, D.; Gerlai, R. Acute alcohol exposure increases tyrosine hydroxylase protein expression and dopamine synthesis in zebrafish. Behav. Brain Res. 2017, 317, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Tran, S.; Nowicki, M.; Muraleetharan, A.; Chatterjee, D.; Gerlai, R. Differential effects of acute administration of SCH-23390, a D1-receptor antagonist, and of ethanol on swimming activity, anxiety-related responses, and neurochemistry of zebrafish. Psychopharmacology 2015, 232, 3709–3718. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, D.; Shams, S.; Gerlai, R. Chronic and acute alcohol administration induced neurochemical changes in the brain: Comparison of distinct zebrafish populations. Amino Acids 2014, 46, 921–930. [Google Scholar] [CrossRef] [Green Version]
- Avery, D.H.; Overall, J.E.; Calil, H.M.; Hollister, L.E. Alcohol-induced euphoria: Alcoholics compared to nonalcoholics. Int. J. Addict. 1982, 17, 823–845. [Google Scholar] [CrossRef] [PubMed]
- Miller, N.; Greene, K.; Dydynski, A.; Gerlai, R. Effects of nicotine and alcohol on zebrafish (Danio rerio) shoaling. Behav. Brain Res. 2012, 240, 192–196. [Google Scholar] [CrossRef]
- Ariyasiri, K.; Gerlai, R.; Kim, C.H. Acute ethanol induces behavioral changes and alters c-fos expression in specific brain regions, including the mammillary body, in zebrafish. Prog. Neuropsychopharm. Biol. Psychiatry 2021, 109, 110264. [Google Scholar] [CrossRef]
- Al-Imari, L.; Gerlai, R. Sight of conspecifics as reward in associative learning tasks for zebrafish (Danio rerio). Behav. Brain Res. 2008, 189, 216–219. [Google Scholar] [CrossRef]
- Sison, M.; Gerlai, R. Associative learning performance is impaired in zebrafish (Danio rerio) by the NMDA-R antagonist MK-801. Neurobiol. Learn. Mem. 2011, 96, 230–237. [Google Scholar] [CrossRef] [Green Version]
- Scerbina, T.; Chatterjee, D.; Gerlai, R. Dopamine receptor antagonism disrupts social preference in zebrafish, a strain comparison study. Amino Acids 2012, 43, 2059–2072. [Google Scholar] [CrossRef]
- Gerlai, R. Animated Images in the analysis of zebrafish behaviour. Curr. Zool. 2017, 63, 35–44. [Google Scholar] [CrossRef]
- Saif, M.; Chatterjee, D.; Buske, C.; Gerlai, R. Sight of conspecific images induces changes in neurochemistry in zebrafish. Behav. Brain Res. 2013, 243, 294–299. [Google Scholar] [CrossRef]
- Fernandes, Y.; Rampersad, M.; Gerlai, R. Embryonic alcohol exposure impairs the dopaminergic system and social behavioural responses in adult zebrafish. Int. J. Neuropsychopharmacol. 2015, 18, pyu089. [Google Scholar] [CrossRef] [Green Version]
- Mahabir, S.; Chatterjee, D.; Buske, C.; Gerlai, R. Maturation of shoaling in two zebrafish strains: A behavioral and neurochemical analysis. Behav. Brain Res. 2013, 247, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahabir, S.; Chatterjee, D.; Gerlai, R. Strain dependent neurochemical changes induced by embryonic alcohol exposure in zebrafish. Neurotoxicol. Teratol. 2014, 41, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Pan, Y.; Chaterjee, D.; Gerlai, R. Strain dependent gene expression and neurochemical levels in the brain of zebrafish: Focus on a few alcohol related targets. Physiol. Behav. 2012, 107, 773–780. [Google Scholar] [CrossRef] [Green Version]
- Pannia, E.; Tran, S.; Rampersad, M.; Gerlai, R. Acute ethanol exposure induces behavioural differences in two zebrafish (Danio rerio) strains: A time course analysis. Behav. Brain Res. 2013, 259, 174–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerlai, R. Reproducibility and replicability in zebrafish behavioral neuroscience research. Pharmacol. Biochem. Behav. 2019, 178, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Cameron, D.L.; Williams, J.T. Dopamine D1 receptors facilitate transmitter release. Nature 1993, 366, 344–347. [Google Scholar] [CrossRef]
- Darbin, O.; Risso, J.J.; Rostain, J.C. Dopaminergic control of striatal 5-HT level at normobaric condition and at pressure. Undersea Hyperb. Med. 2010, 37, 159–166. [Google Scholar]
- Steketee, J.D. Injection of SCH 23390 into the ventral tegmental area blocks the development of neurochemical but not behavioral sensitization to cocaine. Behav. Pharmacol. 1998, 9, 69–76. [Google Scholar]
- Kurata, K.; Shibata, R. Effects of D1 and D2 antagonists on the transient increase of dopamine release by dopamine agonists by means of brain dialysis. Neurosci. Lett. 1991, 133, 77–80. [Google Scholar] [CrossRef]
- Tran, S.; Chatterjee, D.; Gerlai, R. An integrative analysis of ethanol tolerance and withdrawal in zebrafish (Danio rerio). Behav. Brain Res. 2015, 276, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, Y.; Gerlai, R. Long-term behavioral changes in response to early developmental exposure to ethanol in zebrafish. Alcohol. Clin. Exp. Res. 2009, 33, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Saverino, C.; Gerlai, R. The social zebrafish: Behavioral responses to conspecific, heterospecific, and computer animated fish. Behav. Brain Res. 2008, 191, 77–87. [Google Scholar] [CrossRef] [Green Version]
- Egan, R.J.; Bergner, C.L.; Hart, P.C.; Cachat, J.M.; Canavello, P.R.; Elegante, M.F.; Elkhayat, S.I.; Bartels, B.K.; Tien, A.K.; Tien, D.H.; et al. Understanding behavioral and physiological phenotypes of stress and anxiety in zebrafish. Behav. Brain Res. 2009, 205, 38–44. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, O.; Seguin, D.; Gerlai, R. An automated predator avoidance task in zebrafish. Behav. Brain Res. 2011, 216, 166–171. [Google Scholar] [CrossRef] [Green Version]
- Shams, S.; Seguin, D.; Facciol, A.; Chatterjee, D.; Gerlai, R. Effect of Social Isolation on Anxiety-Related Behaviors, Cortisol, and Monoamines in Adult Zebrafish. Behav. Neurosci. 2017, 131, 492–504. [Google Scholar] [CrossRef]
- Soares, M.C.; Gerlai, R.; Maximino, C. The integration of sociality, monoamines, and stress neuroendocrinology in fish models: Applications in the neurosciences. J. Fish Biol. 2018, 93, 170–191. [Google Scholar] [CrossRef]
- Gerlai, R. Fear responses and antipredatory behavior of zebrafish: A translational perspective. In Behavioral and Neural Genetics of Zebrafish, 1st ed.; Gerlai, R., Ed.; Elsevier: Amsterdam, The Netherlands; Academic Press: Cambridge, MA, USA, 2020; pp. 155–173. [Google Scholar]
- Wong, K.; Elegante, M.; Bartels, B.; Elkhayat, S.; Tien, D.; Roy, S.; Goodspeed, J.; Suciu, C.; Tan, J.; Grimes, C.; et al. Analyzing habituation responses to novelty in zebrafish (Danio rerio). Behav. Brain Res. 2010, 208, 450–457. [Google Scholar] [CrossRef]
- Qin, M.; Wong, A.; Seguin, D.; Gerlai, R. Induction of social behaviour in zebrafish: Live versus computer animated fish as stimuli. Zebrafish 2014, 11, 185–197. [Google Scholar] [CrossRef] [Green Version]
- Miller, N.; Gerlai, R. From Schooling to Shoaling: Patterns of Collective Motion in Zebrafish (Danio rerio). PLoS ONE 2012, 7, e48865. [Google Scholar] [CrossRef]
- Buske, C.; Gerlai, R. Shoaling develops with age in Zebrafish (Danio rerio). Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2011, 35, 1409–1415. [Google Scholar] [CrossRef] [PubMed]
- Nabinger, D.D.; Altenhofen, S.; Peixoto, J.V.; da Silva, J.M.K.; Gerlai, R.; Bonan, C.D. Feeding status alters exploratory and anxiety-like behaviors in zebrafish larvae exposed to quinpirole. Prog. Neuro-Psychopharm. Biol. Psychiatry 2020, 108, 110179. [Google Scholar] [CrossRef] [PubMed]
- Gerlai, R.; Crusio, W.E. Organization of motor and posture patterns in paradise fish (Macropodus opercularis): Environmental and genetic components of phenotypical correlation structures. Behav. Genet. 1995, 25, 385–396. [Google Scholar] [CrossRef]
- Blaser, R.; Gerlai, R. Behavioral phenotyping in Zebrafish: Comparison of three behavioral quantification methods. Behav. Res. Methods 2006, 38, 456–469. [Google Scholar] [CrossRef]
- Gerlai, R.; Roder, J. Abnormal exploratory behaviour in transgenic mice carrying multiple copies of the human gene for S100ß. J. Psychiatry Neurosci. 1995, 2, 105–112. [Google Scholar]
- Gerlai, R. Behavioral and Neural Genetics of Zebrafish, 1st ed.; Elsevier: Amsterdam, The Netherlands; Academic Press: Cambridge, MA, USA, 2020; p. 614. [Google Scholar]
- Gerlai, R. Zebrafish antipredatory responses: A future for translational research? Behav. Brain Res. 2010, 207, 223–231. [Google Scholar] [CrossRef] [Green Version]
- Postlethwait, J.H.; Talbot, W.S. Zebrafish genomics: From mutants to genes. Trends Genet. 1997, 13, 183–190. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scerbina, T.; Gerlai, R. Acute Administration of Ethanol and of a D1-Receptor Antagonist Affects the Behavior and Neurochemistry of Adult Zebrafish. Biomedicines 2022, 10, 2878. https://doi.org/10.3390/biomedicines10112878
Scerbina T, Gerlai R. Acute Administration of Ethanol and of a D1-Receptor Antagonist Affects the Behavior and Neurochemistry of Adult Zebrafish. Biomedicines. 2022; 10(11):2878. https://doi.org/10.3390/biomedicines10112878
Chicago/Turabian StyleScerbina, Tanya, and Robert Gerlai. 2022. "Acute Administration of Ethanol and of a D1-Receptor Antagonist Affects the Behavior and Neurochemistry of Adult Zebrafish" Biomedicines 10, no. 11: 2878. https://doi.org/10.3390/biomedicines10112878
APA StyleScerbina, T., & Gerlai, R. (2022). Acute Administration of Ethanol and of a D1-Receptor Antagonist Affects the Behavior and Neurochemistry of Adult Zebrafish. Biomedicines, 10(11), 2878. https://doi.org/10.3390/biomedicines10112878





