Osteoarthritis Pain in Old Mice Aggravates Neuroinflammation and Frailty: The Positive Effect of Morphine Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Induction of Osteoarthritis
2.3. Morphine Treatment
2.4. Behavioral Testing
2.4.1. Weight-Bearing Asymmetry: Incapacitance Test
2.4.2. Mechanical Allodynia: Von Frey Test
2.4.3. Thermal Hyperalgesia: Plantar Test
2.4.4. Locomotor Function:
2.4.5. Frailty Index
2.5. Tissue Collection
2.6. RT-qPCR
2.7. Evaluation of Total Protein and ELISA Assay
2.8. Statistical Analysis
3. Results
3.1. Hypersensitivity in Adult and Old OA Animals and Morphine Effect
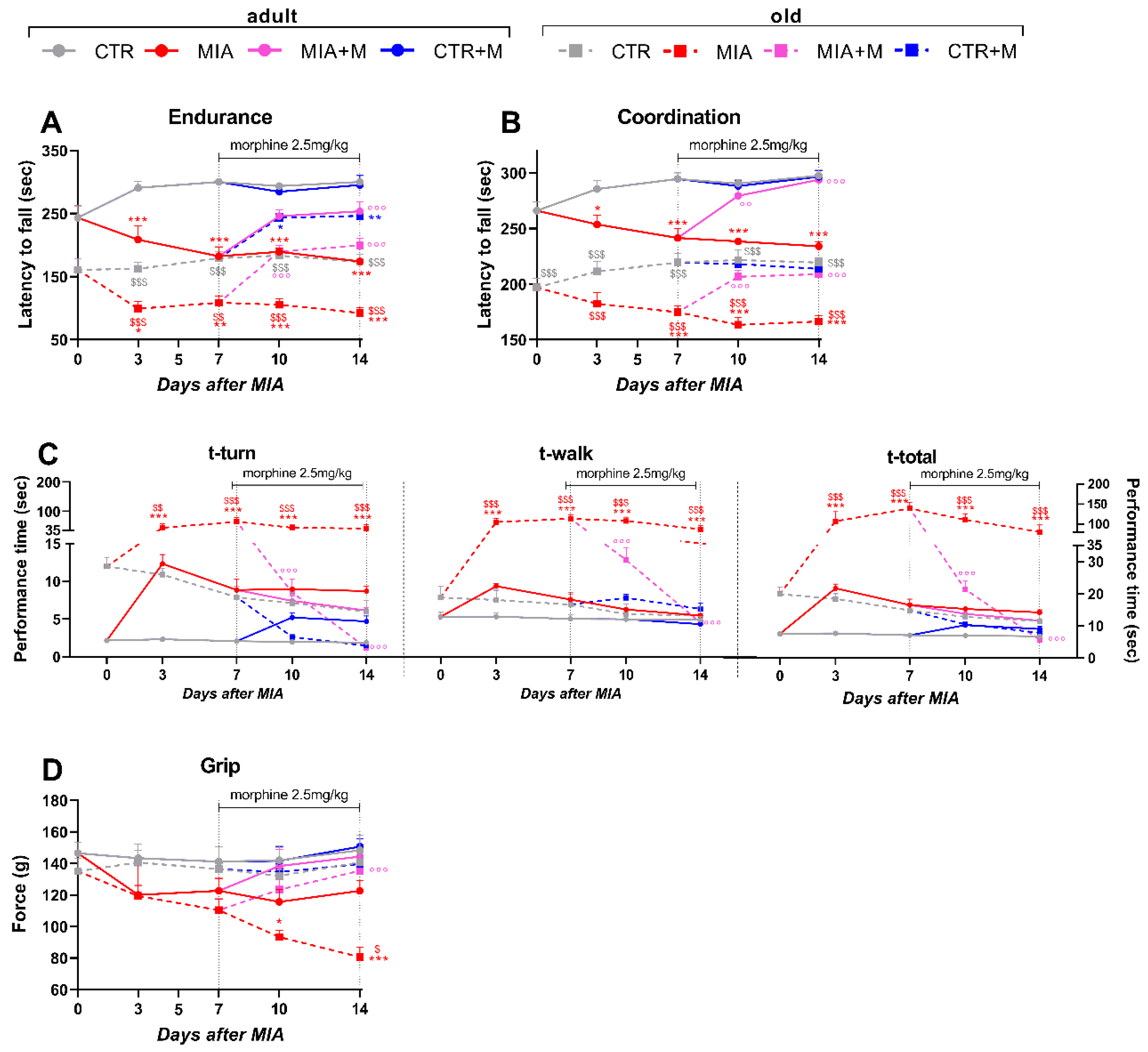
3.2. Functional Activity in Adult and Old OA Mice and Morphine’s Effect
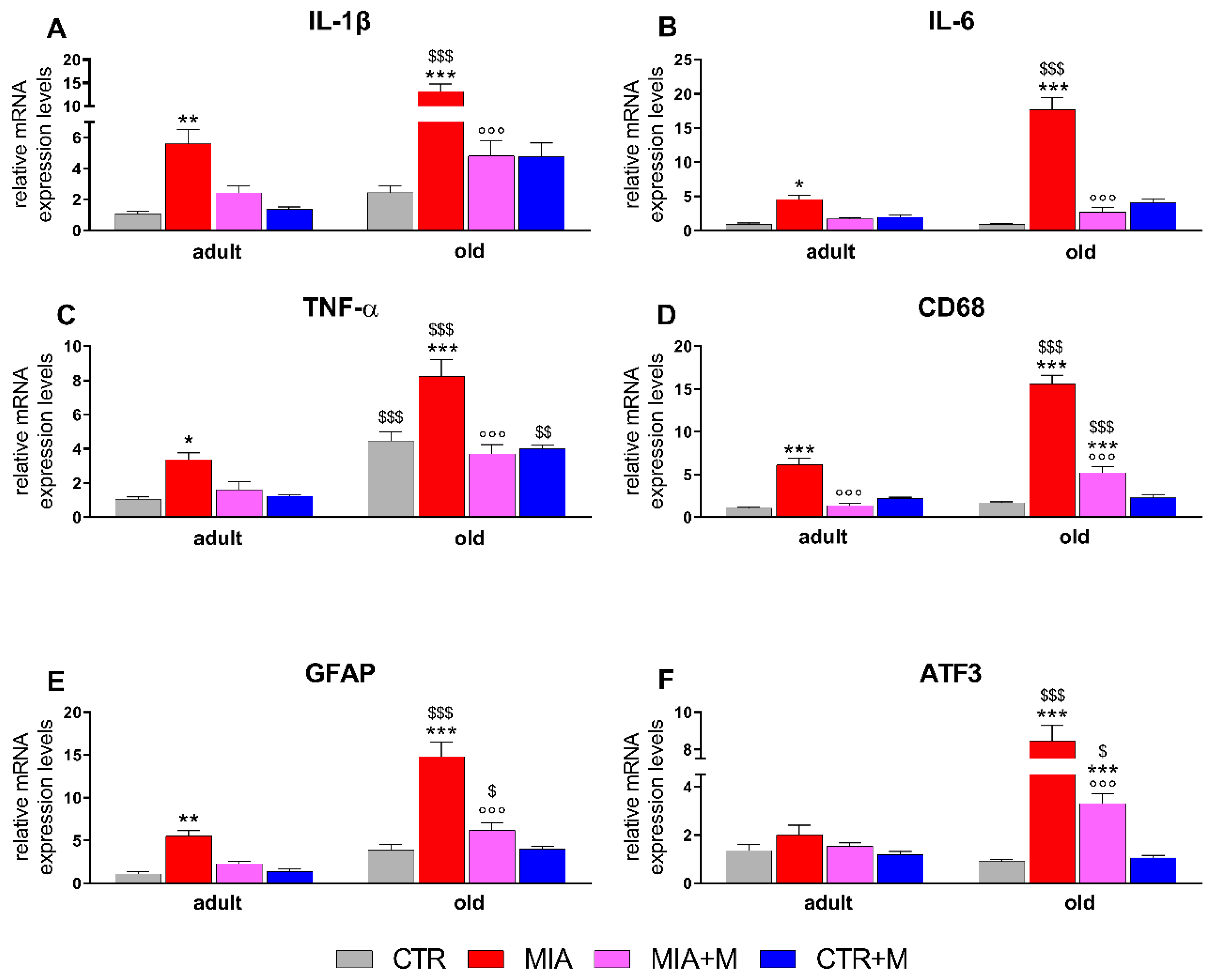
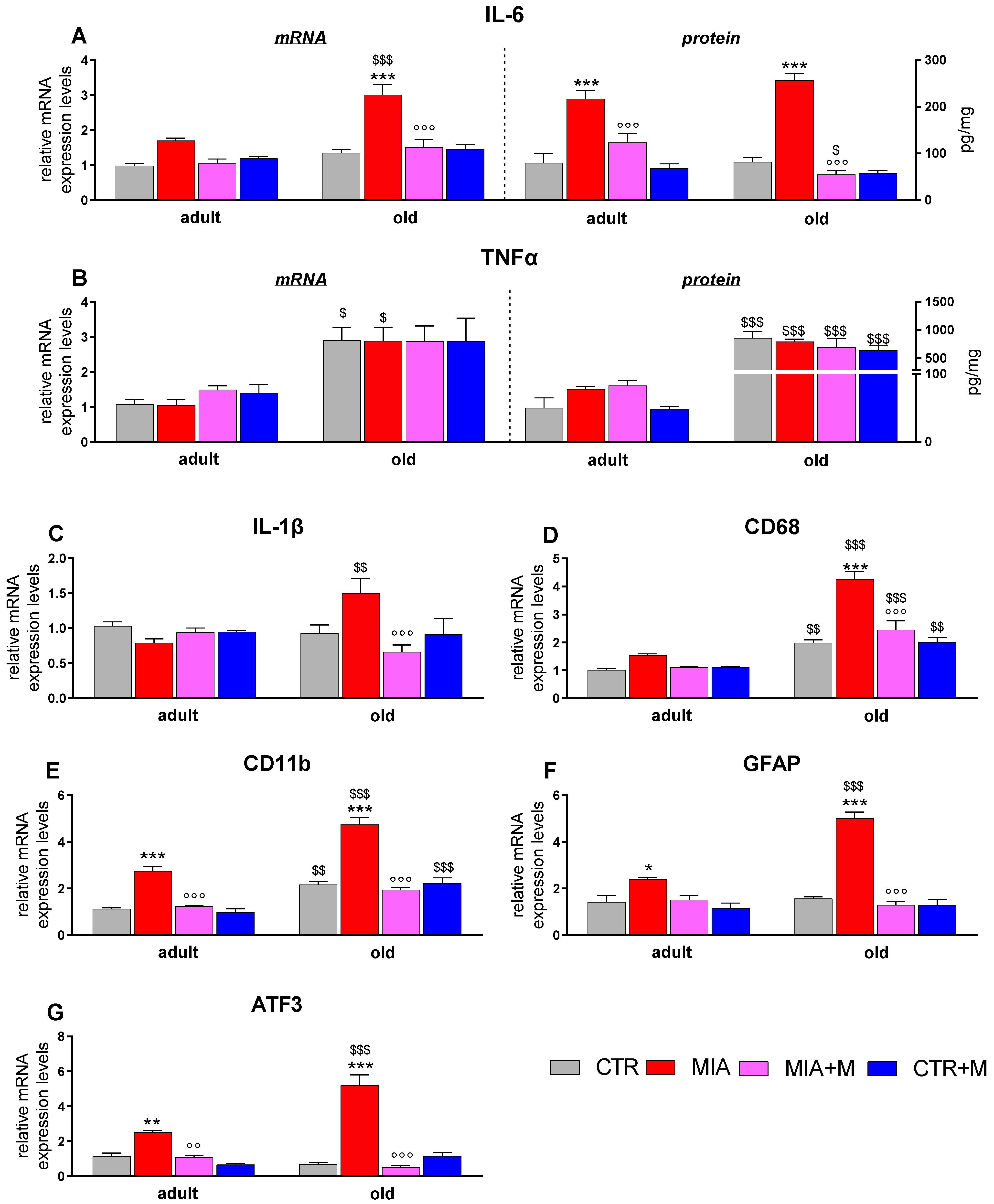
3.3. Inflammatory Markers in Sciatic Nerve, DRGs, and Spinal Cord of Adult and Old OA Mice and Morphine Effect
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hunter, D.J.; Bierma-Zeinstra, S. Osteoarthritis. Lancet 2019, 393, 1745–1759. [Google Scholar] [CrossRef]
- Barr, A.J.; Campbell, T.M.; Hopkinson, D.; Kingsbury, S.R.; Bowes, M.A.; Conaghan, P.G. A systematic review of the relationship between subchondral bone features, pain and structural pathology in peripheral joint osteoarthritis. Arthritis Res. Ther. 2015, 17, 228. [Google Scholar] [CrossRef] [PubMed]
- Felson, D.T. Osteoarthritis as a disease of mechanics. Osteoarthr. Cartil. 2013, 21, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Hayami, T.; Pickarski, M.; Zhuo, Y.; Wesolowski, G.A.; Rodan, G.A.; Duong, L.T. Characterization of articular cartilage and subchondral bone changes in the rat anterior cruciate ligament transection and meniscectomized models of osteoarthritis. Bone 2006, 38, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Shane Anderson, A.; Loeser, R.F. Why is osteoarthritis an age-related disease? Best Pract. Res. Clin. Rheumatol. 2010, 24, 15–26. [Google Scholar] [CrossRef]
- Rainbow, R.; Ren, W.; Zeng, L. Inflammation and Joint Tissue Interactions in OA: Implications for Potential Therapeutic Approaches. Arthritis 2012, 2012, 741582. [Google Scholar] [CrossRef]
- Arendt-Nielsen, L.; Madsen, H.; Jarrell, J.; Gregersen, H.; Drewes, A.M. Pain evoked by distension of the uterine cervix in women with dysmenorrhea: Evidence for central sensitization. Acta Obstet. Et Gynecol. Scand. 2014, 93, 741–748. [Google Scholar] [CrossRef] [PubMed]
- Schaible, H.G. Mechanisms of chronic pain in osteoarthritis. Curr. Rheumatol. Rep. 2012, 14, 549–556. [Google Scholar] [CrossRef]
- Orita, S.; Koshi, T.; Mitsuka, T.; Miyagi, M.; Inoue, G.; Arai, G.; Ishikawa, T.; Hanaoka, E.; Yamashita, K.; Yamashita, M.; et al. Associations between proinflammatory cytokines in the synovial fluid and radiographic grading and pain-related scores in 47 consecutive patients with osteoarthritis of the knee. BMC Musculoskelet. Disord. 2011, 12, 144. [Google Scholar] [CrossRef]
- Ushiyama, T.; Chano, T.; Inoue, K.; Matsusue, Y. Cytokine production in the infrapatellar fat pad: Another source of cytokines in knee synovial fluids. Ann. Rheum. Dis. 2003, 62, 108–112. [Google Scholar] [CrossRef]
- Witoński, D.; Wągrowska-Danilewicz, M.; Kęska, R.; Raczyńska-Witońska, G.; Stasikowska-Kanicka, O. Increased interleukin 6 and tumour necrosis factor α expression in the infrapatellar fat pad of the knee joint with the anterior knee pain syndrome: A preliminary report. Pol. J. Pathol. 2010, 61, 213–218. [Google Scholar] [PubMed]
- Schaible, H.G.; Grubb, B.D. Afferent and spinal mechanisms of joint pain. Pain 1993, 55, 5–54. [Google Scholar] [CrossRef]
- Nascimento, G.C.; Rizzi, E.; Gerlach, R.F.; Leite-Panissi, C.R. Expression of MMP-2 and MMP-9 in the rat trigeminal ganglion during the development of temporomandibular joint inflammation. Braz. J. Med. Biol. Res. 2013, 46, 956–967. [Google Scholar] [CrossRef]
- Arendt-Nielsen, L.; Nie, H.; Laursen, M.B.; Laursen, B.S.; Madeleine, P.; Simonsen, O.H.; Graven-Nielsen, T. Sensitization in patients with painful knee osteoarthritis. Pain 2010, 149, 573–581. [Google Scholar] [CrossRef] [PubMed]
- de Sousa Valente, J. The pharmacology of pain associated with the monoiodoacetate model of osteoarthritis. Front. Pharmacol. 2019, 10, 974. [Google Scholar] [CrossRef] [PubMed]
- Fusco, M.; Skaper, S.D.; Coaccioli, S.; Varrassi, G.; Paladini, A. Degenerative Joint Diseases and Neuroinflammation. Pain Pr. 2017, 17, 522–532. [Google Scholar] [CrossRef]
- Zhang, R.X.; Ren, K.; Dubner, R. Osteoarthritis pain mechanisms: Basic studies in animal models. Osteoarthr. Cartil. 2013, 21, 1308–1315. [Google Scholar] [CrossRef]
- Haywood, A.R.; Hathway, G.J.; Chapman, V. Differential contributions of peripheral and central mechanisms to pain in a rodent model of osteoarthritis. Sci. Rep. 2018, 8, 7122. [Google Scholar] [CrossRef]
- Bas, D.B.; Su, J.; Sandor, K.; Agalave, N.M.; Lundberg, J.; Codeluppi, S.; Baharpoor, A.; Nandakumar, K.S.; Holmdahl, R.; Svensson, C.I. Collagen antibody-induced arthritis evokes persistent pain with spinal glial involvement and transient prostaglandin dependency. Arthritis Rheum. 2012, 64, 3886–3896. [Google Scholar] [CrossRef]
- Amodeo, G.; Niada, S.; Moschetti, G.; Franchi, S.; Savadori, P.; Brini, A.T.; Sacerdote, P. Secretome of human adipose-derived mesenchymal stem cell relieves pain and neuroinflammation independently of the route of administration in experimental osteoarthritis. Brain Behav. Immun. 2021, 94, 29–40. [Google Scholar] [CrossRef]
- Ro, J.Y.; Zhang, Y.; Tricou, C.; Yang, D.; da Silva, J.T.; Zhang, R. Age and sex differences in acute and osteoarthritis-like pain responses in rats. J. Gerontol. Ser. A 2020, 75, 1465–1472. [Google Scholar] [CrossRef] [PubMed]
- Domenichiello, A.F.; Ramsden, C.E. The silent epidemic of chronic pain in older adults. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2019, 93, 284–290. [Google Scholar] [CrossRef] [PubMed]
- D’Agnelli, S.; Amodeo, G.; Franchi, S.; Verduci, B.; Baciarello, M.; Panerai, A.E.; Bignami, E.G.; Sacerdote, P. Frailty and pain, human studies and animal models. Ageing Res. Rev. 2022, 73, 101515. [Google Scholar] [CrossRef]
- Ferreira-Gomes, J.; Adães, S.; Sousa, R.M.; Mendonça, M.; Castro-Lopes, J.M. Dose-dependent expression of neuronal injury markers during experimental osteoarthritis induced by monoiodoacetate in the rat. Mol. Pain 2012, 8, 50. [Google Scholar] [CrossRef]
- Ogbonna, A.C.; Clark, A.K.; Gentry, C.; Hobbs, C.; Malcangio, M. Pain-like behaviour and spinal changes in the monosodium iodoacetate model of osteoarthritis in C57Bl/6 mice. Eur. J. Pain 2013, 17, 514–526. [Google Scholar] [CrossRef] [PubMed]
- Lockwood, S.M.; Lopes, D.M.; McMahon, S.B.; Dickenson, A.H. Characterisation of peripheral and central components of the rat monoiodoacetate model of osteoarthritis. Osteoarthr. Cartil. 2019, 27, 712–722. [Google Scholar] [CrossRef]
- Flurkey, K.; Currer, J.M.; Harrison, D.E. The Mouse in Aging Research. The Mouse in Biomedical Research, American College Laboratory Animal Medicine, 2nd ed.; Elsevier: Burlington, MA, USA, 2007; pp. 637–672. [Google Scholar] [CrossRef]
- Moschetti, G.; Amodeo, G.; Paladini, M.S.; Molteni, R.; Balboni, G.; Panerai, A.; Sacerdote, P.; Franchi, S. Prokineticin 2 promotes and sustains neuroinflammation in vincristine treated mice: Focus on pain and emotional like behavior. Brain Behav. Immun. 2019, 82, 422–431. [Google Scholar] [CrossRef]
- Franchi, S.; Amodeo, G.; Gandolla, M.; Moschetti, G.; Panerai, A.E.; Sacerdote, P. Effect of Tapentadol on Splenic Cytokine Production in Mice. Anesth. Analg. 2017, 124, 986–995. [Google Scholar] [CrossRef] [PubMed]
- Hargreaves, K.; Dubner, R.; Brown, F.; Flores, C.; Joris, J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain 1988, 32, 77–88. [Google Scholar] [CrossRef]
- Franchi, S.; Valsecchi, A.E.; Borsani, E.; Procacci, P.; Ferrari, D.; Zaffa, C.; Sartori, P.; Rodella, L.F.; Vescovi, A.; Maione, S.; et al. Intravenous neural stem cells abolish nociceptive hypersensitivity and trigger nerve regeneration in experimental neuropathy. Pain 2012, 153, 850–861. [Google Scholar] [CrossRef]
- Osmon, K.J.; Vyas, M.; Woodley, E.; Thompson, P.; Walia, J.S. Battery of behavioral tests assessing general locomotion, muscular strength, and coordination in mice. J. Vis. Exp. 2018, 131, 55491. [Google Scholar] [CrossRef] [PubMed]
- Deacon, R.M. Measuring motor coordination in mice. J. Vis. Exp. 2013, 75, e2609. [Google Scholar] [CrossRef] [PubMed]
- Amodeo, G.; Verduci, B.; Sartori, P.; Procacci, P.; Conte, V.; Balboni, G.; Sacerdote, P.; Franchi, S. The antagonism of the prokineticin system counteracts bortezomib induced side effects: Focus on mood alterations. Int. J. Mol. Sci. 2021, 22, 10256. [Google Scholar] [CrossRef]
- Montilla-García, Á.; Tejada, M.Á.; Perazzoli, G.; Entrena, J.M.; Portillo-Salido, E.; Fernández-Segura, E.; Cañizares, F.J.; Cobos, E.J. Grip strength in mice with joint inflammation: A rheumatology function test sensitive to pain and analgesia. Neuropharmacology 2017, 125, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, J.C.; Hildebrand, B.A.; Sun, M.; Rockwood, M.R.; Rose, R.A.; Rockwood, K.; Howlett, S.E. A clinical frailty index in aging mice: Comparisons with frailty index data in humans. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2014, 69, 621–632. [Google Scholar] [CrossRef]
- Brini, A.T.; Amodeo, G.; Ferreira, L.M.; Milani, A.; Niada, S.; Moschetti, G.; Franchi, S.; Borsani, E.; Rodella, L.F.; Panerai, A.E.; et al. Therapeutic effect of human adipose-derived stem cells and their secretome in experimental diabetic pain. Sci. Rep. 2017, 7, 9904. [Google Scholar] [CrossRef]
- Thakur, M.; Rahman, W.; Hobbs, C.; Dickenson, A.H.; Bennett, D.L. Characterisation of a peripheral neuropathic component of the rat monoiodoacetate model of osteoarthritis. PLoS ONE 2012, 7, e33730. [Google Scholar] [CrossRef]
- Gagliese, L.; Melzack, R. Age differences in nociception and pain behaviours in the rat. Neurosci. Biobehav. Rev. 2000, 24, 843–854. [Google Scholar] [CrossRef]
- Gibson, S.J.; Farrell, M. A review of age differences in the neurophysiology of nociception and the perceptual experience of pain. Clin. J. Pain 2004, 20, 227–239. [Google Scholar] [CrossRef]
- Yezierski, R.P. The effects of age on pain sensitivity: Preclinical studies. Pain Med. 2012, 13 (Suppl. 2), S27–S36. [Google Scholar] [CrossRef]
- Miller, R.E.; Malfait, A.M. Osteoarthritis pain: What are we learning from animal models? Best Pract. Res. Clin. Rheumatol. 2017, 31, 676–687. [Google Scholar] [CrossRef] [PubMed]
- Ogbonna, A.C.; Clark, A.K.; Malcangio, M. Development of monosodium acetate-induced osteoarthritis and inflammatory pain in ageing mice. Age 2015, 37, 9792. [Google Scholar] [CrossRef] [PubMed]
- Alsalem, M.; Haddad, M.; Altarifi, A.; Aldossary, S.A.; Kalbouneh, H.; Abojaradeh, A.M.; El-Salem, K. Impairment in locomotor activity as an objective measure of pain and analgesia in a rat model of osteoarthritis. Exp. Ther. Med. 2020, 20, 165. [Google Scholar] [CrossRef]
- Ishikawa, G.; Nagakura, Y.; Takeshita, N.; Shimizu, Y. Efficacy of drugs with different mechanisms of action in relieving spontaneous pain at rest and during movement in a rat model of osteoarthritis. Eur. J. Pharmacol. 2014, 738, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Schaible, H.G. Osteoarthritis pain. Recent advances and controversies. Curr. Opin. Support. Palliat. Care 2018, 12, 148–153. [Google Scholar] [CrossRef]
- Fu, K.; Robbins, S.R.; McDougall, J.J. Osteoarthritis: The genesis of pain. Rheumatology 2018, 57 (Suppl. 4), iv43–iv50. [Google Scholar] [CrossRef] [PubMed]
- Han, F.Y.; Brockman, D.A.; Nicholson, J.R.; Corradini, L.; Smith, M.T. Gait analysis as a robust pain behavioural endpoint in the chronic phase of the monoiodoacetate-induced knee joint pain in the rat. Behav. Pharmacol. 2022, 33, 23–31. [Google Scholar] [CrossRef]
- MacSorley, R.; White, J.; Conerly, V.H.; Walker, J.T.; Lofton, S.; Ragland, G.; Davey, D.; Robertson, A. Pain assessment and management strategies for elderly patients. Home Healthc. Now 2014, 32, 272–285. [Google Scholar] [CrossRef]
- Kee, C.C.; Epps, C.D. Pain management practices of nurses caring for older patients with osteoarthritis. West. J. Nurs. Res. 2001, 23, 195–210. [Google Scholar] [CrossRef]
- Goodwin, J.L.; Kraemer, J.J.; Bajwa, Z.H. The use of opioids in the treatment of osteoarthritis: When, why, and how? Curr. Rheumatol. Rep. 2009, 11, 5–14. [Google Scholar] [CrossRef]
- Dolati, S.; Tarighat, F.; Pashazadeh, F.; Shahsavarinia, K.; Gholipouri, S.; Soleimanpour, H. The role of opioids in pain management in elderly patients with chronic kidney disease: A review article. Anesthesiol. Pain Med. 2020, 10, e105754. [Google Scholar] [CrossRef] [PubMed]
- Han, F.Y.; Brockman, D.A.; Nicholson, J.R.; Corradini, L.; Smith, M.T. Pharmacological characterization of the chronic phase of the monoiodoacetate-induced rat model of osteoarthritis pain in the knee joint. Clin. Exp. Pharmacol. Physiol. 2021, 48, 1515–1522. [Google Scholar] [CrossRef] [PubMed]
- Rashid, M.H.; Theberge, Y.; Elmes, S.J.; Perkins, M.N.; McIntosh, F. Pharmacological validation of early and late phase of rat mono-iodoacetate model using the Tekscan system. Eur. J. Pain 2013, 17, 210–222. [Google Scholar] [CrossRef]
- DiSabato, D.J.; Quan, N.; Godbout, J.P. Neuroinflammation: The devil is in the details. J. Neurochem. 2016, 139 (Suppl. 2), 136–153. [Google Scholar] [CrossRef] [PubMed]
- Mody, P.H.; Dos Santos, N.L.; Barron, L.R.; Price, T.J.; Burton, M.D. eIF4E phosphorylation modulates pain and neuroinflammation in the aged. Geroscience 2020, 42, 1663–1674. [Google Scholar] [CrossRef]
- Gavini, C.K.; Elshareif, N.; Aubert, G.; Germanwala, A.V.; Calcutt, N.A.; Mansuy-Aubert, V. LXR agonist improves peripheral neuropathy and modifies PNS immune cells in aged mice. J. Neuroinflammation 2022, 19, 57. [Google Scholar] [CrossRef]
- Nikodemova, M.; Small, A.L.; Kimyon, R.S.; Watters, J.J. Age-dependent differences in microglial responses to systemic inflammation are evident as early as middle age. Physiol. Genom. 2016, 48, 336–344. [Google Scholar] [CrossRef]
- Haight, E.S.; Forman, T.E.; Cordonnier, S.A.; James, M.L.; Tawfik, V.L. Microglial modulation as a target for chronic pain: From the bench to the bedside and back. Anesthesia Analg. 2019, 128, 737–746. [Google Scholar] [CrossRef]
- Yuan, X.; Klein, D.; Kerscher, S.; West, B.L.; Weis, J.; Katona, I.; Martini, R. Macrophage depletion ameliorates peripheral neuropathy in aging mice. J. Neurosci. 2018, 38, 4610–4620. [Google Scholar] [CrossRef]
- Muley, M.M.; Krustev, E.; Reid, A.R.; McDougall, J.J. Prophylactic inhibition of neutrophil elastase prevents the development of chronic neuropathic pain in osteoarthritic mice. J. Neuroinflammation 2017, 14, 168. [Google Scholar] [CrossRef]
- Miyamoto, S.; Nakamura, J.; Ohtori, S.; Orita, S.; Nakajima, T.; Omae, T.; Hagiwara, S.; Takazawa, M.; Suzuki, M.; Suzuki, T.; et al. Pain-related behavior and the characteristics of dorsal-root ganglia in a rat model of hip osteoarthritis induced by mono-iodoacetate. J. Orthop. Res. 2017, 35, 1424–1430. [Google Scholar] [CrossRef]
- Bourassa, V.; Deamond, H.; Yousefpour, N.; Fitzcharles, M.A.; Ribeiro-da-Silva, A. Pain-related behavior is associated with increased joint innervation, ipsilateral dorsal horn gliosis, and dorsal root ganglia activating transcription factor 3 expression in a rat ankle joint model of osteoarthritis. Pain Rep. 2020, 5, e846. [Google Scholar] [CrossRef]
- Ferreira-Gomes, J.; Garcia, M.M.; Nascimento, D.; Almeida, L.; Quesada, E.; Castro-Lopes, J.M.; Pascual, D.; Goicoechea, C.; Neto, F.L. TLR4 Antagonism Reduces Movement-Induced Nociception and ATF-3 Expression in Experimental Osteoarthritis. J. Pain Res. 2021, 14, 2615–2627. [Google Scholar] [CrossRef]
- Lin, Y.; Liu, L.; Jiang, H.; Zhou, J.; Tang, Y. Inhibition of interleukin-6 function attenuates the central sensitization and pain behavior induced by osteoarthritis. Eur. J. Pharmacol. 2017, 811, 260–267. [Google Scholar] [CrossRef]
- Moschetti, G.; Amodeo, G.; Maftei, D.; Lattanzi, R.; Procacci, P.; Sartori, P.; Balboni, G.; Onnis, V.; Conte, V.; Panerai, A.; et al. Targeting prokineticin system counteracts hypersensitivity, neuroinflammation, and tissue damage in a mouse model of bortezomib-induced peripheral neuropathy. J. Neuroinflammation 2019, 16, 89. [Google Scholar] [CrossRef]
- Castelli, M.; Amodeo, G.; Negri, L.; Lattanzi, R.; Maftei, D.; Gotti, C.; Pistillo, F.; Onnis, V.; Congu, C.; Panerai, A.E.; et al. Antagonism of the Prokineticin System Prevents and Reverses Allodynia and Inflammation in a Mouse Model of Diabetes. PLoS ONE 2016, 11, e0146259. [Google Scholar] [CrossRef]
- Sagar, D.R.; Burston, J.J.; Hathway, G.J.; Woodhams, S.G.; Pearson, R.G.; Bennett, A.J.; Kendall, D.A.; Scammell, B.E.; Chapman, V. The contribution of spinal glial cells to chronic pain behaviour in the monosodium iodoacetate model of osteoarthritic pain. Mol. Pain 2011, 7, 88. [Google Scholar] [CrossRef]
- Jiang, H.; Xu, L.; Liu, W.; Xiao, M.; Ke, J.; Long, X. Chronic Pain Causes Peripheral and Central Responses in MIA-Induced TMJOA Rats. Cell. Mol. Neurobiol. 2022, 42, 1441–1451. [Google Scholar] [CrossRef]
- Kawarai, Y.; Orita, S.; Nakamura, J.; Miyamoto, S.; Suzuki, M.; Inage, K.; Hagiwara, S.; Suzuki, T.; Nakajima, T.; Akazawa, T.; et al. Changes in proinflammatory cytokines, neuropeptides, and microglia in an animal model of monosodium iodoacetate-induced hip osteoarthritis. J. Orthop. Res. 2018, 36, 2978–2986. [Google Scholar] [CrossRef]
- Wang, X.; Loram, L.C.; Ramos, K.; de Jesus, A.J.; Thomas, J.; Cheng, K.; Reddy, A.; Somogyi, A.A.; Hutchinson, M.R.; Watkins, L.R.; et al. Morphine activates neuroinflammation in a manner parallel to endotoxin. Proc. Natl. Acad. Sci. USA 2012, 109, 6325–6330. [Google Scholar] [CrossRef]
- Zhou, J.; Ma, R.; Jin, Y.; Fang, J.; Du, J.; Shao, X.; Liang, Y.; Fang, J. Molecular mechanisms of opioid tolerance: From opioid receptors to inflammatory mediators (Review). Exp. Ther. Med. 2021, 22, 1004. [Google Scholar] [CrossRef]
- Carcolé, M.; Kummer, S.; Gonçalves, L.; Zamanillo, D.; Merlos, M.; Dickenson, A.H.; Fernández-Pastor, B.; Cabañero, D.; Maldonado, R. Sigma-1 receptor modulates neuroinflammation associated with mechanical hypersensitivity and opioid tolerance in a mouse model of osteoarthritis pain. Br. J. Pharmacol. 2019, 176, 3939–3955. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amodeo, G.; Franchi, S.; Galimberti, G.; Comi, L.; D’Agnelli, S.; Baciarello, M.; Bignami, E.G.; Sacerdote, P. Osteoarthritis Pain in Old Mice Aggravates Neuroinflammation and Frailty: The Positive Effect of Morphine Treatment. Biomedicines 2022, 10, 2847. https://doi.org/10.3390/biomedicines10112847
Amodeo G, Franchi S, Galimberti G, Comi L, D’Agnelli S, Baciarello M, Bignami EG, Sacerdote P. Osteoarthritis Pain in Old Mice Aggravates Neuroinflammation and Frailty: The Positive Effect of Morphine Treatment. Biomedicines. 2022; 10(11):2847. https://doi.org/10.3390/biomedicines10112847
Chicago/Turabian StyleAmodeo, Giada, Silvia Franchi, Giulia Galimberti, Laura Comi, Simona D’Agnelli, Marco Baciarello, Elena Giovanna Bignami, and Paola Sacerdote. 2022. "Osteoarthritis Pain in Old Mice Aggravates Neuroinflammation and Frailty: The Positive Effect of Morphine Treatment" Biomedicines 10, no. 11: 2847. https://doi.org/10.3390/biomedicines10112847
APA StyleAmodeo, G., Franchi, S., Galimberti, G., Comi, L., D’Agnelli, S., Baciarello, M., Bignami, E. G., & Sacerdote, P. (2022). Osteoarthritis Pain in Old Mice Aggravates Neuroinflammation and Frailty: The Positive Effect of Morphine Treatment. Biomedicines, 10(11), 2847. https://doi.org/10.3390/biomedicines10112847







