The Relationship between the Plasma Concentration of Electrolytes and Intensity of Sleep Bruxism and Blood Pressure Variability among Sleep Bruxers
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Polysomnographic Examination
2.3. 24-h BP Examination
2.4. Statistical Analysis of the Results
3. Results
4. Discussion
5. Conclusions
- 1.
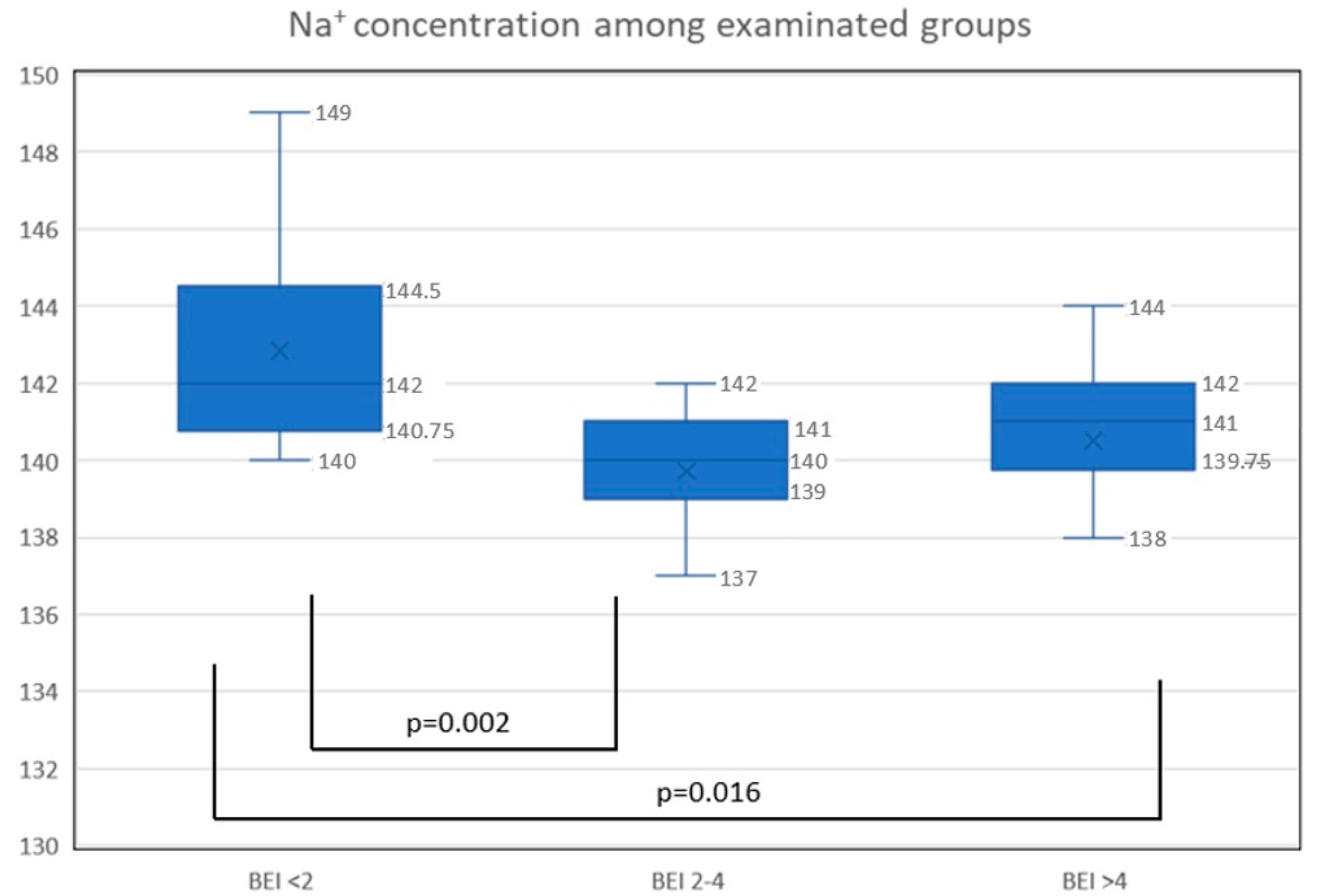
- The plasma concentration of sodium was decreased in bruxers compared to nonbruxers.
- 2.
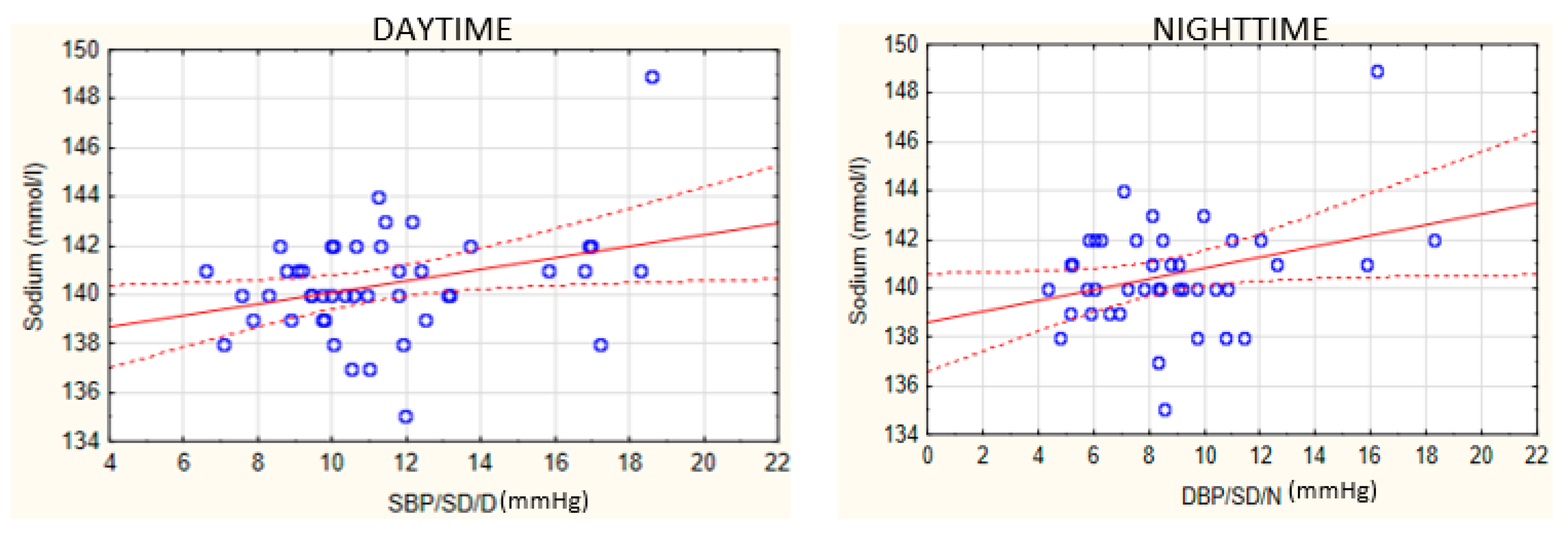
- A relationship exists between plasma sodium concentration and BP variability among patients with SB.
- 3.
- As the results are present in a preliminary study, there is a need for further studies on the pathomechanisms of SB and their relationship with electrolyte plasma concentration as well as the inflammation factors.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- He, F.J.; Markandu, N.D.; Sagnella, G.A.; de Wardener, H.E.; MacGregor, G.A. Plasma sodium. Hypertension 2005, 45, 98–102. [Google Scholar] [CrossRef] [PubMed]
- He, F.J.; MacGregor, G.A. Plasma sodium and hypertension. Kidney Int. 2004, 66, 2454–2466. [Google Scholar] [CrossRef]
- Chrysant, S.G. Effects of High Salt Intake on Blood Pressure and Cardiovascular Disease: The Role of COX Inhibitors. Clin. Cardiol. 2016, 39, 240–242. [Google Scholar] [CrossRef] [PubMed]
- Grillo, A.; Salvi, L.; Coruzzi, P.; Salvi, P.; Parati, G. Sodium intake and hypertension. Nutrients 2019, 11, 1970. [Google Scholar] [CrossRef] [PubMed]
- de Wardener, H.E.; MacGregor, G.A. Harmful effects of dietary salt in addition to hypertension. J. Hum. Hypertens. 2002, 16, 213–223. [Google Scholar] [CrossRef]
- Ellison, D.H. Treatment of disorders of sodium balance in chronic kidney disease. Adv. Chronic Kidney Dis. 2017, 24, 332–341. [Google Scholar] [CrossRef]
- Bae, E.; Lee, T.W.; Jang, H.N.; Cho, H.S.; Jung, S.; Lee, S.; Chang, S.-H.; Park, D.J. Lower serum sodium levels predict poor clinical outcomes in patients with insomnia. BMC Nephrol. 2020, 21, 386. [Google Scholar] [CrossRef]
- Lavigne, G.J.; Khoury, S.; Abe, S.; Yamaguchi, T.; Raphael, K. Bruxism physiology and pathology: An overview for clinicians. J. Oral Rehabil. 2008, 35, 476–494. [Google Scholar] [CrossRef]
- Smardz, J.; Martynowicz, H.; Michalek-Zrabkowska, M.; Wojakowska, A.; Mazur, G.; Winocur, E.; Wieckiewicz, M. Sleep bruxism and occurrence of temporomandibular disorders-related pain: A polysomnographic study. Front. Neurol. 2019, 10, 168. [Google Scholar] [CrossRef]
- Lavigne, G.J.; Huynh, N.; Kato, T.; Okura, K.; Adachi, K.; Yao, D.; Sessle, B. Genesis of sleep bruxism: Motor and autonomic-cardiac interactions. Arch. Oral Biol. 2007, 52, 381–384. [Google Scholar] [CrossRef]
- Huynh, N.; Lavigne, G.J.; Lanfranchi, P.A.; Montplaisir, J.Y.; de Champlain, J. The effect of 2 sympatholytic medications—Propranolol and clonidine—On sleep bruxism: Experimental randomized controlled studies. Sleep 2006, 29, 307–316. [Google Scholar] [CrossRef]
- Huynh, N.; Kato, T.; Rompre, P.H.; Okura, K.; Saber, M.; Lanfranchi, P.A.; Montplaisir, J.Y.; Lavigne, G.J. Sleep bruxism is associated to micro-arousals and an increase in cardiac sympathetic activity. J. Sleep Res. 2006, 15, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Michalek-Zrabkowska, M.; Wieckiewicz, M.; Gac, P.; Smardz, J.; Poreba, R.; Wojakowska, A.; Goslawska, K.; Mazur, G.; Martynowicz, H. Effect of sleep bruxism intensity on blood pressure in normotensives. J. Clin. Med. 2021, 10, 1304. [Google Scholar] [CrossRef]
- Gangwisch, J.E. A review of evidence for the link between sleep duration and hypertension. Am. J. Hypertens. 2014, 27, 1235–1242. [Google Scholar] [CrossRef] [PubMed]
- Jarrin, D.C.; Alvaro, P.K.; Bouchard, M.-A.; Jarrin, S.D.; Drake, C.L.; Morin, C.M. Insomnia and hypertension: A systematic review. Sleep Med. Rev. 2018, 41, 3–38. [Google Scholar] [CrossRef] [PubMed]
- van den Eeden, S.K.; Albers, K.B.; Davidson, J.E.; Kushida, C.A.; Leimpeter, A.D.; Nelson, L.M.; Popat, R.; Tanner, C.M.; Bibeau, K.; Quesenberry, C.P. Risk of cardiovascular disease associated with a restless legs syndrome diagnosis in a retrospective cohort study from Kaiser permanente Northern California. Sleep 2015, 38, 1009–1015. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.; Zhao, Y.; Yu, W.; Dong, H.; Xue, X.; Ding, J.; Xing, W.; Wang, W. Association of obstructive sleep apnea with hypertension: A systematic review and meta-analysis. J. Glob. Health 2018, 8, 010405. [Google Scholar] [CrossRef]
- Atilgan, Z.; Buyukkaya, R.; Yaman, F.; Tekbas, G.; Atilgan, S.; Gunay, A.; Palanci, Y.; Guven, S. Bruxism: Is it a new sign of the cardiovascular diseases? Eur. Rev. Med. Pharmacol. Sci. 2011, 15, 1369–1374. [Google Scholar]
- Michalek-Zrabkowska, M.; Wieckiewicz, M.; Macek, P.; Gac, P.; Smardz, J.; Wojakowska, A.; Poreba, R.; Mazur, G.; Martynowicz, H. The relationship between simple snoring and sleep bruxism: A polysomnographic study. Int. J. Environ. Res. Public Health 2020, 17, 8960. [Google Scholar] [CrossRef]
- Lobbezoo, F.; Ahlberg, J.; Raphael, K.G.; Wetselaar, P.; Glaros, A.G.; Kato, T.; Santiago, V.; Winocur, E.; de Laat, A.; de Leeuw, R.; et al. International consensus on the assessment of bruxism: Report of a work in progress. J. Oral Rehabil. 2018, 45, 837–844. [Google Scholar] [CrossRef]
- O’Brien, E.; Parati, G.; Stergiou, G.; Asmar, R.; Beilin, L.; Bilo, G.; Clement, D.; de la Sierra, A.; de Leeuw, P.; Dolan, E.; et al. European society of hypertension position paper on ambulatory blood pressure monitoring. J. Hypertens. 2013, 31, 1731–1768. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.; Mancia, G.; Spiering, W.; Rosei, E.A.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH guidelines for the management of arterial hypertension. Kardiol. Pol. 2019, 77, 71–159. [Google Scholar] [CrossRef] [PubMed]
- Michalek-Zrabkowska, M.; Wieckiewicz, M.; Smardz, J.; Gac, P.; Poreba, R.; Wojakowska, A.; Mazur, G.; Martynowicz, H. Determination of inflammatory markers, hormonal disturbances, and sleepiness associated with sleep bruxism among adults. Nat. Sci. Sleep 2020, 12, 969–979. [Google Scholar] [CrossRef]
- Yang, A.C.; Tsai, S.-J.; Yang, C.-H.; Kuo, C.-H.; Chen, T.-J.; Hong, C.-J. Reduced physiologic complexity is associated with poor sleep in patients with major depression and primary insomnia. J. Affect. Disord. 2011, 131, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Barthélémy, J.-C.; Pichot, V.; Dauphinot, V.; Celle, S.; Laurent, B.; Garcin, A.; Maudoux, D.; Kerleroux, J.; Lacour, J.-R.; Kossovsky, M.; et al. Autonomic nervous system activity and decline as prognostic indicators of cardiovascular and cerebrovascular events: The ‘PROOF’ study. Neuroepidemiology 2007, 29, 18–28. [Google Scholar] [CrossRef]
- Bell-Reuss, E.; Trevino, D.L.; Gottschalk, C.W. Effect of renal sympathetic nerve stimulation on proximal water and sodium reabsorption. J. Clin. Investig. 1976, 57, 1104–1107. [Google Scholar] [CrossRef] [PubMed]
- Nukazawa, S.; Yoshimi, H.; Sato, S. Autonomic nervous activities associated with bruxism events during sleep. CRANIO® 2018, 36, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Stockand, J.D. Vasopressin regulation of renal sodium excretion. Kidney Int. 2010, 78, 849–856. [Google Scholar] [CrossRef]
- Cuzzo, B.; Padala, S.A.; Lappin, S.L. Physiology, Vasopressin; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Watts, S.W.; Morrison, S.F.; Davis, R.P.; Barman, S.M. Serotonin and blood pressure regulation. Pharmacol. Rev. 2012, 64, 359–388. [Google Scholar] [CrossRef]
- Ni, W.; Geddes, T.J.; Priestley, J.R.C.; Szasz, T.; Kuhn, D.M.; Watts, S.W. The Existence of a local 5-hydroxytryptaminergic system in peripheral arteries. Br. J. Pharmacol. 2008, 154, 663–674. [Google Scholar] [CrossRef]
- Ni, W.; Thompson, J.M.; Northcott, C.A.; Lookingland, K.; Watts, S.W. The serotonin transporter is present and functional in peripheral arterial smooth muscle. J. Cardiovasc. Pharmacol. 2004, 43, 770–781. [Google Scholar] [CrossRef] [PubMed]
- Rubin, A.N.; Espiridion, E.D.; Kattan, M.; Desmarais, E.C. Serotonin syndrome with atypical hypernatremia. Cureus 2018, 10, e3616. [Google Scholar] [CrossRef] [PubMed]
- Volpi-Abadie, J.; Kaye, A.M.; Kaye, A.D. Serotonin syndrome. Ochsner J. 2013, 13, 533–540. [Google Scholar] [PubMed]
- Campello, C.P.; Moraes, S.L.D.; Vasconcelos, B.C.D.E.; Lima, E.L.S.D.; Pellizzer, E.P.; Lemos, C.A.A.; Muniz, M.T.C. Polymorphisms of the serotonin receptors genes in patients with bruxism: A systematic review. J. Appl. Oral Sci. 2021, 29. [Google Scholar] [CrossRef] [PubMed]
- Isa Kara, M.; Ertaş, E.T.; Ozen, E.; Atıcı, M.; Aksoy, S.; Erdogan, M.S.; Kelebek, S. BiteStrip analysis of the effect of fluoxetine and paroxetine on sleep bruxism. Arch. Oral Biol. 2017, 80, 69–74. [Google Scholar] [CrossRef]
- Ploceniak, C. Bruxism and Magnesium, My Clinical Experiences since 1980. Rev. Stomatol. Chir. Maxillofac. 1990, 91 (Suppl. S1), 127. [Google Scholar] [PubMed]
- Alkhatatbeh, M.J.; Hmoud, Z.L.; Abdul-Razzak, K.K.; Alem, E.M. Self-reported sleep bruxism is associated with vitamin D deficiency and low dietary calcium intake: A case-control study. BMC Oral Health 2021, 21, 21. [Google Scholar] [CrossRef]
- Gholamrezaei, A.; Bonakdar, Z.S.; Mirbagher, L.; Hosseini, N. Sleep disorders in systemic lupus erythematosus. Does vitamin D play a role? Lupus 2014, 23, 1054–1058. [Google Scholar] [CrossRef]
| Mean | Minimum | Maximum | SD | |
|---|---|---|---|---|
| AHI (n/h) | 5.21 | 0.00 | 41.20 | 9.41 |
| ODI (n/h) | 4.68 | 0.00 | 39.10 | 8.01 |
| BEI (n/h) | 4.94 | 0.40 | 12.30 | 3.54 |
| Snore (%) | 6.68 | 0.00 | 68.8 | 14.89 |
| TST (min) | 418.3 | 153.50 | 523.50 | 68.77 |
| SL (min) | 23.89 | 1.70 | 163.50 | 24.06 |
| WASO (min) | 33.47 | 3.00 | 206.50 | 34.16 |
| N1 (%TST) | 5.10 | 0.30 | 17.90 | 3.98 |
| N2 (%TST) | 50.31 | 34.00 | 73.10 | 8.16 |
| N3 (%TST) | 21.90 | 6.90 | 39.40 | 7.19 |
| REM (%TST) | 22.69 | 6.30 | 35.20 | 5.28 |
| ArI (n/h) | 5.71 | 0.00 | 21.90 | 4.10 |
| Mean SpO2 (%) | 94.80 | 85.30 | 96.50 | 1.84 |
| Minimum SpO2 (%) | 88.88 | 57.00 | 96.00 | 6.53 |
| SpO2 < 90% (%) | 0.87 | 0.00 | 13.40 | 2.63 |
| 2 ≤ BEI < 4 | BEI ≥ 4 | BEI < 2 | Entire Group | p-Value | |
|---|---|---|---|---|---|
| Mg2+ (mg/dL) | 0.89 ± 1.8 | 1.39 ± 2.1 | 1.48 ± 2.3 | 1.25 ± 2.0 | 0.801 |
| K+ (mmol/L) | 4.3 ± 0.6 | 4.2 ± 0.3 | 4.6 ± 0.3 | 4.3 ± 0.4 | 0.445 |
| Ca2+ (mg/dL) | 9.1 | 9.2 | 9.3 | 9.2 ± 0.2 | 0.539 |
| Variable | Entire Group | BEI < 2 | 2 ≤ BEI < 4 | BEI ≥ 4 | ||
|---|---|---|---|---|---|---|
| SBP (mm Hg) | 24-h mean | Average | 109.8 ± 7.3 | 112.6 ± 7.1 | 110.1 ± 7.4 | 108.9 ± 7.4 |
| Variability | 12.5 ± 3.1 | 12.7 ± 4.3 | 12.5 ± 2.9 | 12.6 ± 2.8 | ||
| Minimum | 84.6 ± 7.0 | 89.1 ± 8.2 | 85.6 ± 6.4 | 82.4 ± 6.6 | ||
| Maximum | 141.9 ± 14.5 | 140.0 ± 5.7 | 146.7 ± 17.3 | 138.7 ± 13.3 | ||
| Decline (%) | 10.1 ± 6.3 | 7.1 ± 6.2 | 11.1 ± 8.1 | 10.1 ± 4.6 | ||
| Daytime | Average | 113.0 ± 8.0 | 115.0 ± 7.2 | 113.7 ± 8.60 | 111.9 ± 8.0 | |
| Variability | 11.3 ± 2.8 | 10.5 ± 3.5 | 11.4 ± 2.8 | 11.4 ± 2.9 | ||
| Minimum | 89.8 ± 8.2 | 91.4 ± 8.2 | 90.6 ± 8.5 | 88.6 ± 8.1 | ||
| Maximum | 141.8 ± 14.5 | 140.0 ± 5.7 | 146.7 ± 17.4 | 138.3 ± 13.0 | ||
| Nighttime | Average | 101.3 ± 8.1 | 106.3 ± 8,7 | 100.8 ± 7.3 | 100.2 ± 8.1 | |
| Variability | 9.4 ± 3.2 | 9.7 ± 3.1 | 7.7 ± 2.6 | 8.1 ± 10.6 | ||
| Minimum | 85.4 ± 7.9 | 91.1 ± 9.2 | 87.7 ± 8.0 | 81.8 ± 5.7 | ||
| Maximum | 120.8 ± 12.2 | 128.8 ± 8.3 | 115.8 ± 10.3 | 122.0 ± 12.3 | ||
| DBP (mm Hg) | 24-h mean | Average | 68.2 ± 5.6 | 70.3 ± 7.5 | 67.3 ± 4.9 | 68.4 ± 5.5 |
| Variability | 11.3 ± 2.8 | 10.7 ± 3.2 | 11.7 ± 2.8 | 11.1 ± 2.8 | ||
| Minimum | 46.4 ± 5.7 | 48.4 ± 8.4 | 46.5 ± 5.4 | 45.7 ± 5.1 | ||
| Maximum | 100.2 ± 16.8 | 98.7 ± 11.8 | 105.8 ± 18.9 | 96.3 ± 15.6 | ||
| Decline (%) | 15.4 ± 7.8 | 11.8 ± 9.4 | 18.8 ± 16.7 | 15.3 ± 4.6 | ||
| Daytime | Average | 71.3 ± 6.1 | 72.8 ± 7.4 | 70.5 ± 6.3 | 71.4 ± 5.7 | |
| Variability | 10.3 ± 3.1 | 9.7 ± 3.4 | 10.8 ± 3.1 | 10.1 ± 3.1 | ||
| Minimum | 50.8 ± 7.1 | 52.4 ± 6.8 | 50.7 ± 6.9 | 50.5 ± 7.5 | ||
| Maximum | 99.5 ± 16.4 | 98.5 ± 11.9 | 105.8 ± 18.8 | 94.9 ± 14.2 | ||
| Nighttime | Average | 60.1 ± 6.4 | 63.8 ± 9.9 | 58.6 ± 5.5 | 60.1 ± 5.6 | |
| Variability | 8.5 ± 3.0 | 9.2 ± 3.4 | 7.4 ± 2.8 | 9.1 ± 2.8 | ||
| Minimum | 46.8 ± 5.5 | 48.5 ± 8.7 | 47.4 ± 5.8 | 45.7 ± 3.7 | ||
| Maximum | 78.4 ± 13.0 | 82.8 ± 11.1 | 75.1 ± 12.6 | 79.5 ± 13.0 | ||
| MAP (mm Hg) | 24-h mean | Average | 82.6 ± 5.6 | 84.7 ± 7.0 | 82.2 ± 4.9 | 82.3 ± 5.7 |
| Variability | 10.8 ± 2.5 | 10.5 ± 2.9 | 11.1 ± 2.5 | 10.7 ± 2.5 | ||
| Minimum | 61.3 ± 5.6 | 62.3 ± 8.1 | 61.7 ± 5.5 | 60.7 ± 5.2 | ||
| Maximum | 113.9 ± 14.7 | 113.6 ± 9.3 | 118.5 ± 16.9 | 110.4 ± 13.8 | ||
| Decline (%) | 12.1 ± 6.4 | 10.0 ± 7.2 | 13.2 ± 8.4 | 11.9 ± 4.2 | ||
| Daytime | Average | 85.4 ± 6.2 | 87.0 ± 6.9 | 85.2 ± 6.3 | 85.1 ± 6.1 | |
| Variability | 9.9 ± 2.7 | 9.4 ± 3.2 | 10.2 ± 2.5 | 9.7 ± 2.7 | ||
| Minimum | 65.4 ± 6.4 | 66.7 ± 6.9 | 65.4 ± 7.0 | 65.0 ± 5.9 | ||
| Maximum | 112.1 ± 8.7 | 118.5 ± 16.9 | 109.0 ± 12.8 | 113.0 ± 14.5 | ||
| Nighttime | Average | 75.0 ± 9.1 | 78.3 ± 9.1 | 74.1 ± 5.5 | 74.7 ± 6.1 | |
| Variability | 8.0 ± 3.0 | 9.2 ± 3.3 | 6.7 ± 2.5 | 8.7 ± 3.0 | ||
| Minimum | 61.8 ± 6.0 | 62.7 ± 8.8 | 63.5 ± 6.3 | 60.2 ± 4.4 | ||
| Maximum | 92.2 ± 13.1 | 98.7 ± 14.1 | 88.5 ± 11.4 | 92.9 ± 13.6 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanclerska, J.; Wieckiewicz, M.; Szymanska-Chabowska, A.; Poreba, R.; Gac, P.; Wojakowska, A.; Mazur, G.; Martynowicz, H. The Relationship between the Plasma Concentration of Electrolytes and Intensity of Sleep Bruxism and Blood Pressure Variability among Sleep Bruxers. Biomedicines 2022, 10, 2804. https://doi.org/10.3390/biomedicines10112804
Kanclerska J, Wieckiewicz M, Szymanska-Chabowska A, Poreba R, Gac P, Wojakowska A, Mazur G, Martynowicz H. The Relationship between the Plasma Concentration of Electrolytes and Intensity of Sleep Bruxism and Blood Pressure Variability among Sleep Bruxers. Biomedicines. 2022; 10(11):2804. https://doi.org/10.3390/biomedicines10112804
Chicago/Turabian StyleKanclerska, Justyna, Mieszko Wieckiewicz, Anna Szymanska-Chabowska, Rafal Poreba, Pawel Gac, Anna Wojakowska, Grzegorz Mazur, and Helena Martynowicz. 2022. "The Relationship between the Plasma Concentration of Electrolytes and Intensity of Sleep Bruxism and Blood Pressure Variability among Sleep Bruxers" Biomedicines 10, no. 11: 2804. https://doi.org/10.3390/biomedicines10112804
APA StyleKanclerska, J., Wieckiewicz, M., Szymanska-Chabowska, A., Poreba, R., Gac, P., Wojakowska, A., Mazur, G., & Martynowicz, H. (2022). The Relationship between the Plasma Concentration of Electrolytes and Intensity of Sleep Bruxism and Blood Pressure Variability among Sleep Bruxers. Biomedicines, 10(11), 2804. https://doi.org/10.3390/biomedicines10112804









