A Deep Convolutional Neural Network for the Early Detection of Heart Disease
Abstract
1. Introduction
- Early detection of heart disease by utilizing a deep-learning network.
- Comparison of the proposed work with existing state-of-the-art approaches.
- Offering a real-time application of the proposed methodology.
- The proposed method is evaluated using different performance metrics like accuracy, precision, recall, and F1-score.
2. Literature Review
3. Proposed Methodology
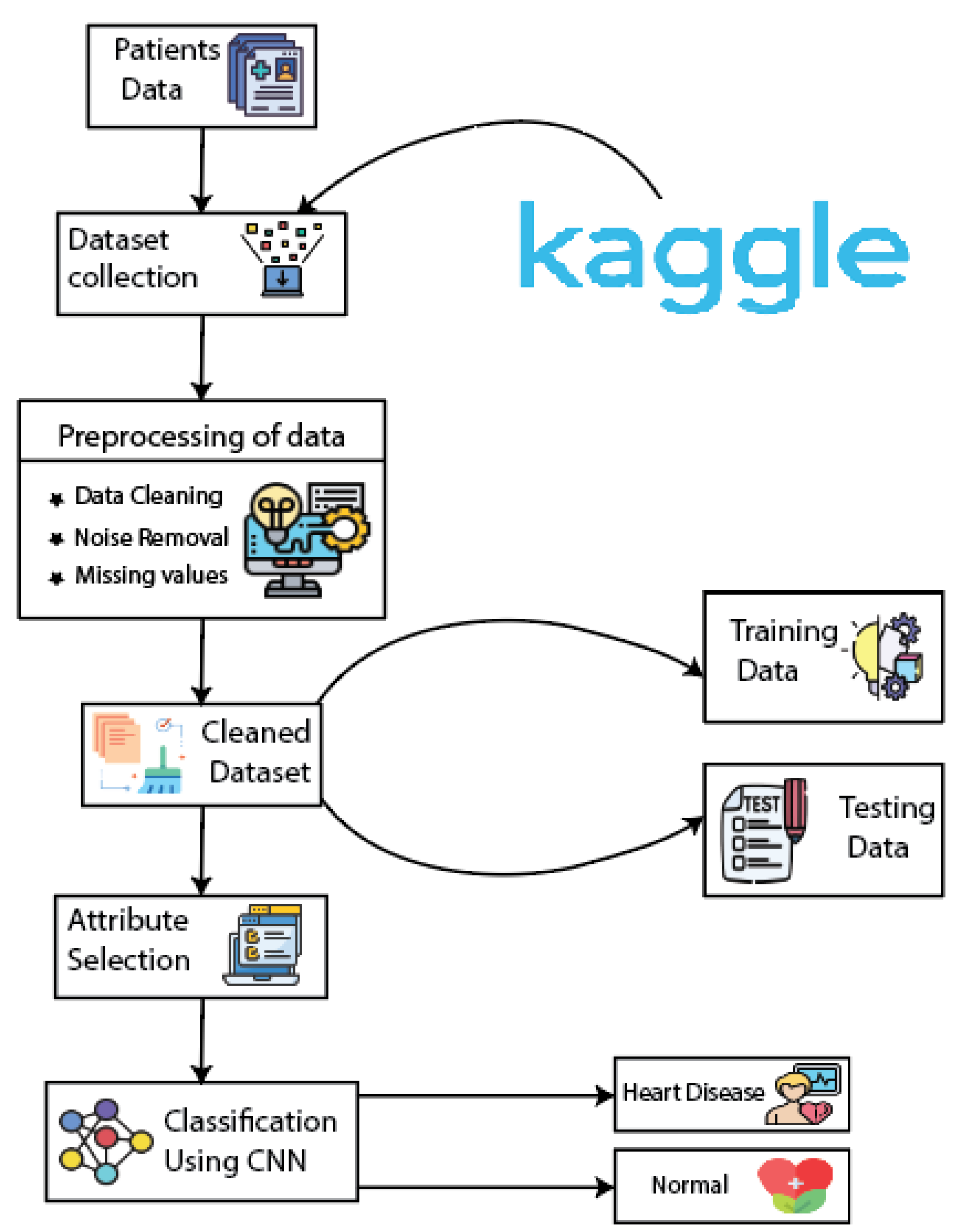
3.1. Dataset
3.2. Data Preprocessing
3.3. Deep Convolutional Neural Network
3.4. Sigmoid Function
3.5. Nadam Optimization Algorithm
4. Results and Discussion
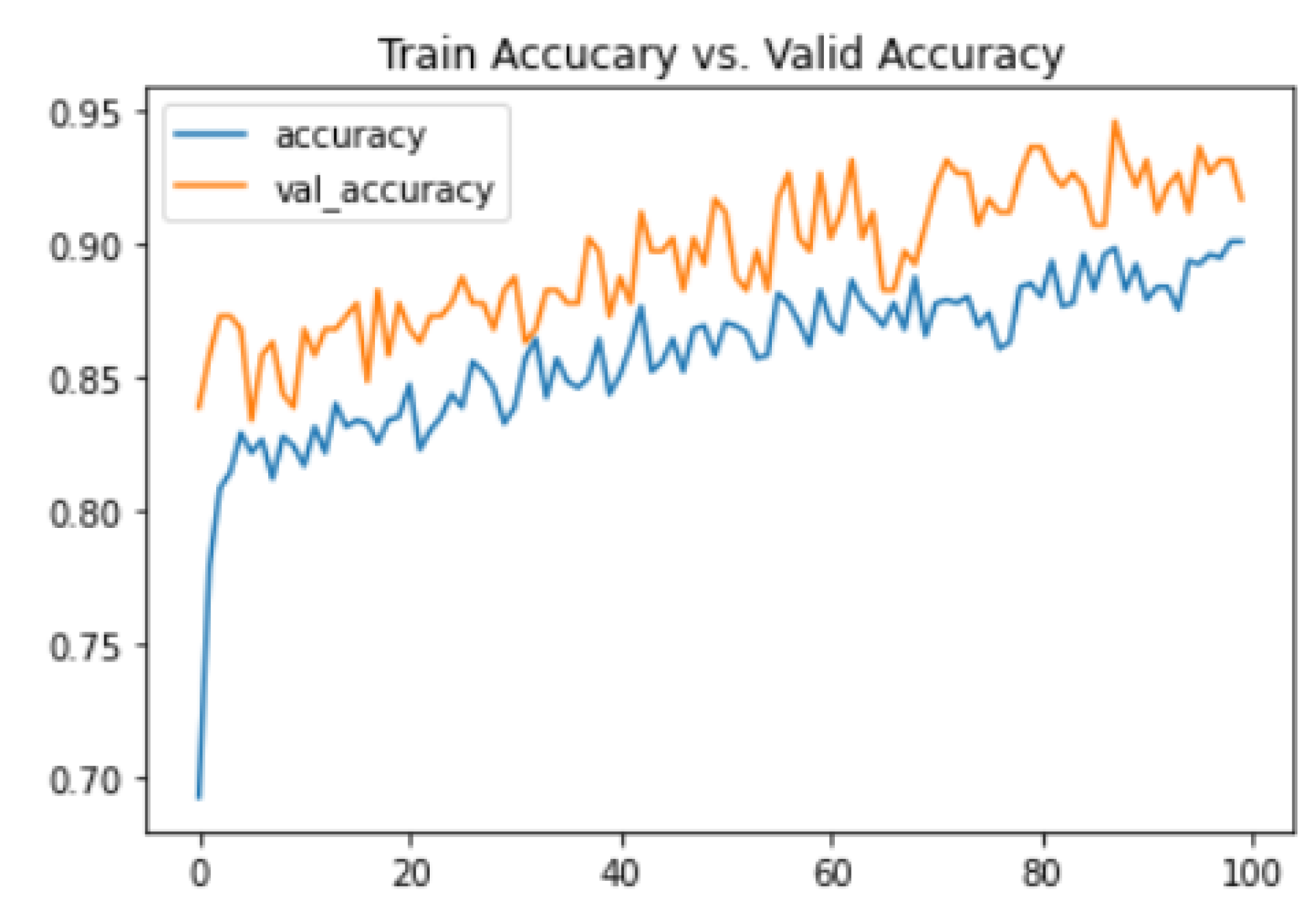
4.1. Experimental Setup
4.2. Performance Metrics
4.2.1. Accuracy
4.2.2. Precision
4.2.3. Recall
4.2.4. F1-Score
4.3. Classification Using CNN
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haenlein, M.; Kaplan, A. A brief history of artificial intelligence: On the past, present, and future of artificial intelligence. Calif. Manag. Rev. 2019, 61, 5–14. [Google Scholar] [CrossRef]
- Nichifor, E.; Trifan, A.; Nechifor, E.M. Artificial intelligence in electronic commerce: Basic chatbots and the consumer journey. Amfiteatru Econ. 2021, 23, 87–101. [Google Scholar] [CrossRef]
- Gould, R.R.; Tahmasebian, K. The Routledge Handbook of Translation and Activism; Routledge: Abingdon, VA, USA, 2020. [Google Scholar]
- Li, Z.; Li, S.; Luo, X. An overview of calibration technology of industrial robots. IEEE/CAA J. Autom. Sin. 2021, 8, 23–36. [Google Scholar] [CrossRef]
- Zhang, C.; Lu, Y. Study on artificial intelligence: The state of the art and future prospects. J. Ind. Inf. Integr. 2021, 23, 100224. [Google Scholar] [CrossRef]
- Al-Shabi, M.; Abuhamdah, A. Using deep learning to detecting abnormal behavior in internet of things. Int. J. Electr. Comput. Eng. 2022, 12, 2108. [Google Scholar] [CrossRef]
- Mahesh, B. Machine learning algorithms-a review. Int. J. Sci. Res. (IJSR) 2020, 9, 381–386. [Google Scholar]
- Meng, T.; Jing, X.; Yan, Z.; Pedrycz, W. A survey on machine learning for data fusion. Inf. Fusion 2020, 57, 115–129. [Google Scholar] [CrossRef]
- Janiesch, C.; Zschech, P.; Heinrich, K. Machine learning and deep learning. Electron. Mark. 2021, 31, 685–695. [Google Scholar] [CrossRef]
- Cai, L.; Gao, J.; Zhao, D. A review of the application of deep learning in medical image classification and segmentation. Ann. Transl. Med. 2020, 8, 713. [Google Scholar] [CrossRef]
- Ras, G.; Xie, N.; van Gerven, M.; Doran, D. Explainable deep learning: A field guide for the uninitiated. J. Artif. Intell. Res. 2022, 73, 329–397. [Google Scholar] [CrossRef]
- LeCun, Y.; Bengio, Y.; Hinton, G. Deep learning. Nature 2015, 521, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Skandha, S.S.; Gupta, S.K.; Saba, L.; Koppula, V.K.; Johri, A.M.; Khanna, N.N.; Mavrogeni, S.; Laird, J.R.; Pareek, G.; Miner, M.; et al. 3-D optimized classification and characterization artificial intelligence paradigm for cardiovascular/stroke risk stratification using carotid ultrasound-based delineated plaque: Atheromatic™ 2.0. Comput. Biol. Med. 2020, 125, 103958. [Google Scholar]
- Kingma, D.P.; Ba, J. Adam: A method for stochastic optimization. arXiv 2014, arXiv:1412.6980. [Google Scholar]
- Khan, M.R.A.; Muhib, M.; Khan, M.M.A.; Baig, M.M. Classification of Various Diseases Using Machine Learning And Deep Learning Algorithms. J. Sci. Technol. 2021, 6, 34–40. [Google Scholar]
- Jena, B.; Saxena, S.; Nayak, G.K.; Saba, L.; Sharma, N.; Suri, J.S. Artificial intelligence-based hybrid deep learning models for image classification: The first narrative review. Comput. Biol. Med. 2021, 137, 104803. [Google Scholar] [CrossRef]
- Lan, H.; Jiang, D.; Yang, C.; Gao, F.; Gao, F. Y-Net: Hybrid deep learning image reconstruction for photoacoustic tomography in vivo. Photoacoustics 2020, 20, 100197. [Google Scholar] [CrossRef]
- Ranganathan, G. A study to find facts behind preprocessing on deep learning algorithms. J. Innov. Image Process. (JIIP) 2021, 3, 66–74. [Google Scholar]
- Martel, A.L.; Abolmaesumi, P.; Stoyanov, D.; Mateus, D.; Zuluaga, M.A.; Zhou, S.K.; Racoceanu, D.; Joskowicz, L. Medical Image Computing and Computer Assisted Intervention–MICCAI 2020. In Proceedings of the 23rd International Conference, Lima, Peru, 4–8 October 2020; Springer Nature: Berlin/Heidelberg, Germany, 2020; Volume 12261. [Google Scholar]
- Willemink, M.J.; Koszek, W.A.; Hardell, C.; Wu, J.; Fleischmann, D.; Harvey, H.; Folio, L.R.; Summers, R.M.; Rubin, D.L.; Lungren, M.P. Preparing medical imaging data for machine learning. Radiology 2020, 295, 4–15. [Google Scholar]
- Panayides, A.S.; Amini, A.; Filipovic, N.D.; Sharma, A.; Tsaftaris, S.A.; Young, A.; Foran, D.; Do, N.; Golemati, S.; Kurc, T.; et al. AI in medical imaging informatics: Current challenges and future directions. IEEE J. Biomed. Health Inform. 2020, 24, 1837–1857. [Google Scholar] [CrossRef]
- Briganti, G.; Le Moine, O. Artificial intelligence in medicine: Today and tomorrow. Front. Med. 2020, 7, 27. [Google Scholar]
- Vijai, C.; Wisetsri, W. Rise of artificial intelligence in healthcare startups in India. Adv. Manag. 2021, 14, 48–52. [Google Scholar]
- Yoon, S.N.; Lee, D. Artificial intelligence and robots in healthcare: What are the success factors for technology-based service encounters? Int. J. Healthc. Manag. 2018, 12, 218–225. [Google Scholar] [CrossRef]
- World Health Organization. Cardiovascular Diseases (CVDS). 1999. Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 11 June 2021).
- Shah, D.; Patel, S.; Bharti, S.K. Heart Disease Prediction using Machine Learning Techniques. SN Comput. Sci. 2020, 1, 1–6. [Google Scholar] [CrossRef]
- Reddy, G.T.; Reddy, M.; Lakshmanna, K.; Rajput, D.S.; Kaluri, R.; Srivastava, G. Hybrid genetic algorithm and a fuzzy logic classifier for heart disease diagnosis. Evol. Intell. 2020, 13, 185–196. [Google Scholar] [CrossRef]
- Ansari, W.M.; Humphries, S.E.; Naveed, A.K.; Khan, O.J.; Khan, D.A.; Khattak, E.H. Effect of Coronary Artery Disease risk SNPs on serum cytokine levels and cytokine imbalance in Premature Coronary Artery Disease. Cytokine 2019, 122, 154060. [Google Scholar] [CrossRef]
- Paquette, M.; Chong, M.; Thériault, S.; Dufour, R.; Paré, G.; Baass, A. Polygenic risk score predicts prevalence of cardiovascular disease in patients with familial hypercholesterolemia. J. Clin. Lipidol. 2017, 11, 725–732. [Google Scholar] [CrossRef]
- Zhao, J.; Feng, Q.; Wu, P.; Lupu, R.A.; Wilke, R.A.; Wells, Q.S.; Denny, J.C.; Wei, W.Q. Learning from longitudinal data in electronic health record and genetic data to improve cardiovascular event prediction. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef]
- Otoom, A.F.; Abdallah, E.E.; Kilani, Y.; Kefaye, A.; Ashour, M. Effective diagnosis and monitoring of heart disease. Int. J. Softw. Eng. Its Appl. 2015, 9, 143–156. [Google Scholar]
- Vembandasamy, K.; Sasipriya, R.; Deepa, E. Heart diseases detection using Naive Bayes algorithm. Int. J. Innov. Sci. Eng. Technol. 2015, 2, 441–444. [Google Scholar]
- Chaurasia, V.; Pal, S. Data mining approach to detect heart diseases. Int. J. Adv. Comput. Sci. Inf. Technol. 2014, 2, 56–66. [Google Scholar]
- Parthiban, G.; Srivatsa, S. Applying machine learning methods in diagnosing heart disease for diabetic patients. Int. J. Appl. Inf. Syst. 2012, 3, 25–30. [Google Scholar] [CrossRef]
- Deepika, K.; Seema, S. Predictive analytics to prevent and control chronic diseases. In Proceedings of the 2016 2nd International Conference on Applied and Theoretical Computing and Communication Technology (iCATccT), Bengaluru, India, 21–23 July 2016; pp. 381–386. [Google Scholar]
- Dwivedi, A.K. Performance evaluation of different machine learning techniques for prediction of heart disease. Neural Comput. Appl. 2018, 29, 685–693. [Google Scholar] [CrossRef]
- Kausar, N.; Ghous, H. A Comparative Analysis On Cleveland And Statlog Heart Disease Datasets Using Data Mining Techniques. LC Int. J. STEM 2020, 1, 24–43. [Google Scholar]
- Ayon, S.I.; Islam, M.M.; Hossain, M.R. Coronary artery heart disease prediction: A comparative study of computational intelligence techniques. IETE J. Res. 2020, 68, 2488–2507. [Google Scholar] [CrossRef]
- Son, L.H. Generalized picture distance measure and applications to picture fuzzy clustering. Appl. Soft Comput. 2016, 46, 284–295. [Google Scholar] [CrossRef]
- Shi, G.; Wang, J.; Qiang, Y.; Yang, X.; Zhao, J.; Hao, R.; Yang, W.; Du, Q.; Kazihise, N.G.F. Knowledge-guided synthetic medical image adversarial augmentation for ultrasonography thyroid nodule classification. Comput. Methods Programs Biomed. 2020, 196, 105611. [Google Scholar] [CrossRef]
- Ullah, A.; Anwar, S.M.; Bilal, M.; Mehmood, R.M. Classification of arrhythmia by using deep learning with 2-D ECG spectral image representation. Remote Sens. 2020, 12, 1685. [Google Scholar] [CrossRef]
- Chee, K.J.; Ramli, D.A. Electrocardiogram Biometrics Using Transformer’s Self-Attention Mechanism for Sequence Pair Feature Extractor and Flexible Enrollment Scope Identification. Sensors 2022, 22, 3446. [Google Scholar] [CrossRef]
- Roy, S.S.; Ahmed, M.; Akhand, M.A.H. Noisy image classification using hybrid deep learning methods. J. Inf. Commun. Technol. 2018, 17, 233–269. [Google Scholar]
- Balamurugan, R.; Ratheesh, S.; Venila, Y.M. Classification of heart disease using adaptive Harris hawk optimization-based clustering algorithm and enhanced deep genetic algorithm. Soft Comput. 2022, 26, 2357–2373. [Google Scholar] [CrossRef]
- Mehmood, A.; Iqbal, M.; Mehmood, Z.; Irtaza, A.; Nawaz, M.; Nazir, T.; Masood, M. Prediction of heart disease using deep convolutional neural networks. Arab. J. Sci. Eng. 2021, 46, 3409–3422. [Google Scholar] [CrossRef]
- Rabbi, M.F.; Uddin, M.P.; Ali, M.A.; Kibria, M.F.; Afjal, M.I.; Islam, M.S.; Nitu, A.M. Performance evaluation of data mining classification techniques for heart disease prediction. Am. J. Eng. Res. 2018, 7, 278–283. [Google Scholar]
- Manimurugan, S.; Almutairi, S.; Aborokbah, M.M.; Narmatha, C.; Ganesan, S.; Chilamkurti, N.; Alzaheb, R.A.; Almoamari, H. Two-Stage Classification Model for the Prediction of Heart Disease Using IoMT and Artificial Intelligence. Sensors 2022, 22, 476. [Google Scholar] [CrossRef] [PubMed]
- Du, N.; Cao, Q.; Yu, L.; Liu, N.; Zhong, E.; Liu, Z.; Shen, Y.; Chen, K. FM-ECG: A fine-grained multi-label framework for ECG image classification. Inf. Sci. 2021, 549, 164–177. [Google Scholar] [CrossRef]
- Yin, X.; Goudriaan, J.; Lantinga, E.A.; Vos, J.; Spiertz, H.J. A flexible sigmoid function of determinate growth. Ann. Bot. 2003, 91, 361–371. [Google Scholar] [CrossRef]
- More, K.; Raihan, M.; More, A.; Padule, S.; Mondal, S. A12176 Smart phone based “heart attack” risk prediction; innovation of clinical and social approach for preventive cardiac health. J. Hypertens. 2018, 36, e321. [Google Scholar] [CrossRef]
- Qrenawi, M.I.; Al Sarraj, W. Identification of cardiovascular diseases risk factors among diabetes patients using ontological data mining techniques. In Proceedings of the 2018 International Conference on Promising Electronic Technologies (ICPET), Deir El-Balah, Palestine, 3–4 October 2018; pp. 129–134. [Google Scholar]
- Xu, W.; Yu, K.; Ye, J.; Li, H.; Chen, J.; Yin, F.; Xu, J.; Zhu, J.; Li, D.; Shu, Q. Automatic pediatric congenital heart disease classification based on heart sound signal. Artif. Intell. Med. 2022, 126, 102257. [Google Scholar]



| Ref | Objective | Techniques | Accuracy % | Precision % | Recall % | F1 Score % |
|---|---|---|---|---|---|---|
| [26] | The early detection of cardiovascular disease in patients. | NB, DT, DF, and K-NN classifiers | KNN:90.7, DT: 80.2, RF: 84.2, NB: 88.15 | N/A | N/A | N/A |
| [34] | A comparative study of intelligent computational techniques | SVM, NB LR, DNN, DT, RF, and K-NN. | SVM:97.41, NB: 91.38, LR: 96.29, DNN: 98.15, DT: 96.42, RF: 90.46, KNN: 96.42 | N/A | N/A | N/A |
| [37] | Medical image classification | AOC-CapsNet | 93.1 | 92 | 90.3 | 91.9 |
| [38] | Handling imbalanced medical images | CNN framework | N/A | N/A | N/A | N/A |
| [39] | Ultrasonography thyroid nodule image synthesis | KACGAN-based model | 91.46 | N/A | N/A | N/A |
| [40] | Classification of arrhythmia | 2-D CNN | 99.11% | 98.58 | N/A | 98 |
| [42] | Classification of noisy images | Five hybrid CNN models | DVAE- CNN: 62.8, DVAE-CDAE-CNN: 53.91 | N/A | N/A | N/A |
| [43] | Heart-disease prediction | AHHO and deep genetic algorithm | 97.3 | 95.6 | N/A | N/A |
| [44] | Heart-disease prediction | CNN | 97 | N/A | N/A | N/A |
| [45] | Heart-disease prediction | ANN, SVM, and KNN | SVM: 85.18, KNN: 80.74, ANN: 73.33 | N/A | N/A | N/A |
| [46] | AI and image-classification-based heart-disease prediction | HLDA-MALO and hybrid R-CNN with SE-ResNet-101 model | 99.15 | 98.06 | 99.15 | 99.02 |
| [47] | Detection of abnormalities in ECG images. | FM-ECG framework | CECG: N/A, DECG: N/A | 79.23, 90.42 | 69.10, 83.59 | 73.88, 86.87 |
| Sr No. | Attributes | Representation | Description | Type |
|---|---|---|---|---|
| 1 | Age | age | Age in years | Integer |
| 2 | Gender | sex | Male and female | Binary(1 for male and 0 for female) |
| 3 | Chest pain | cp | Four types of chest pain | Categorical |
| 4 | Cholesterol level | Chol | Measure of cholesterol in mg/dl | Integer |
| 5 | Resting blood pressure | trestbps | Blood pressure when the body is in a state of rest | Integer |
| 6 | Fasting blood sugar | fbs | Blood sugar level while fasting | Binary (1 for true and 0 for false) |
| 7 | MaxHR | thalach | Maximal heart rate | Integer |
| 8 | Rest ECG | restecg | Resting electrocardiograph | categorical |
| 9 | Exercise-induced angina | exang | Exercise-induced angina | Binary (1 for yes and 0 for no) |
| 10 | Old peak | oldpeak | ST depression brought by exercise comparative to rest | Continuous |
| 11 | Slope | slope | Slope of exercise peak | Discrete |
| 12 | Vessels | ca | No. of major vessels | Continuous |
| 13 | Thalassemia | thal | Normal, fixed, and reversible defects | discrete |
| 14 | Heart disease | target | Predicted attribute | Binary |
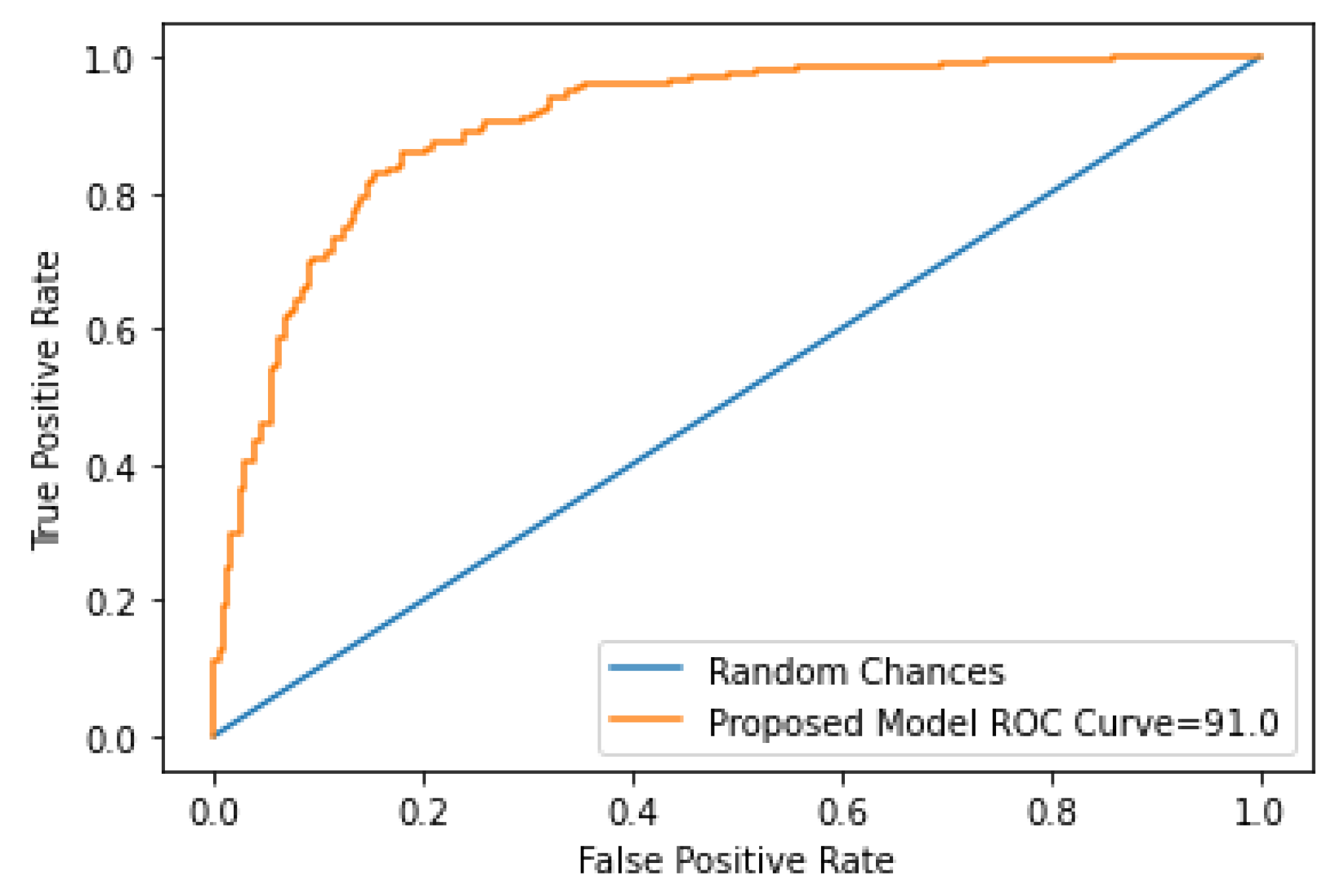
| Performance Metrics | Accuracy (%) |
|---|---|
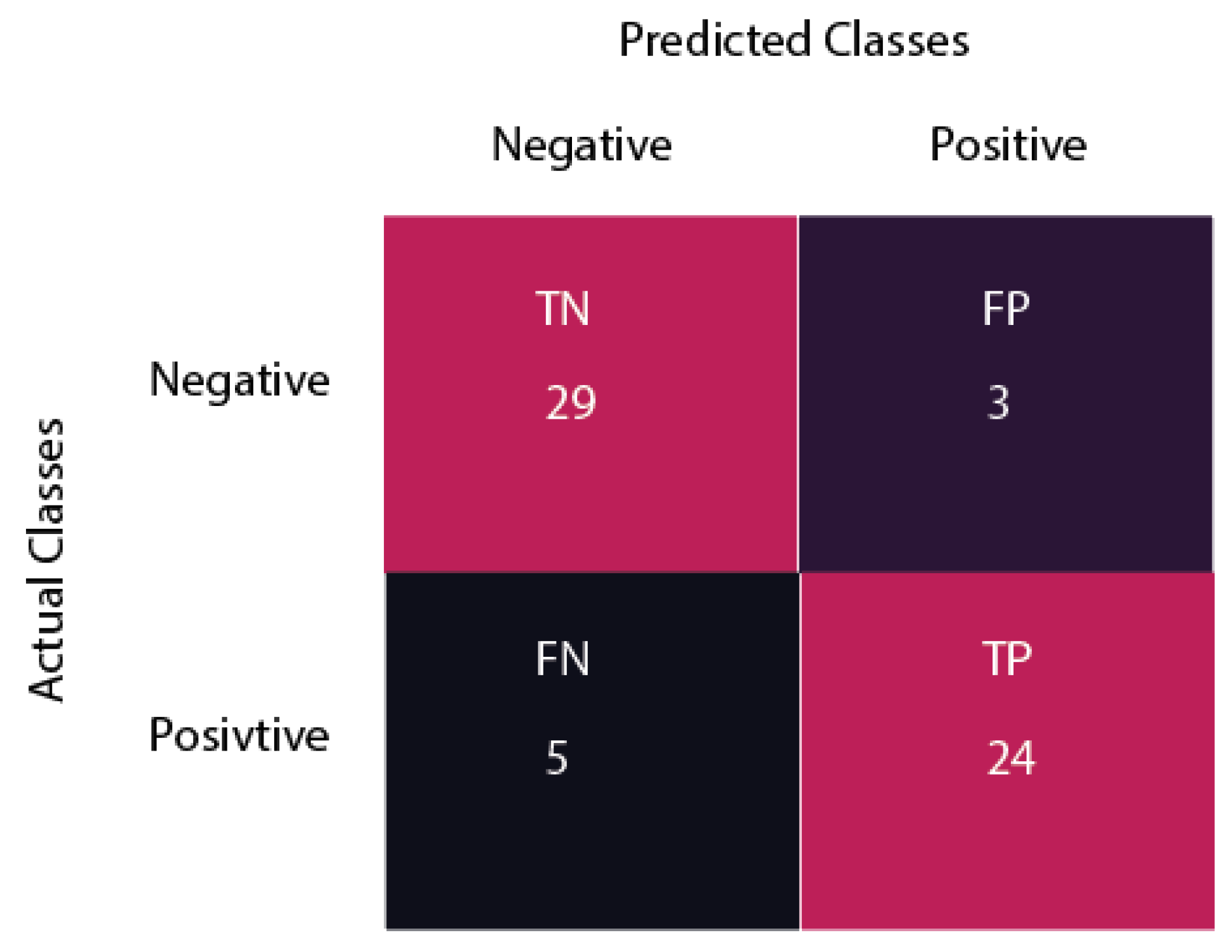
| Accuracy | 91.71 |
| Precision | 88.88 |
| Recall | 82.75 |
| F1 score | 85.70 |
| Author (Technique) | Accuracy (%) |
|---|---|
| Fazle Rabbi et al. [46] (artificial neural network) | 73.3 |
| Qrenawi, Mohammed et al. [51] (Rmonto ontology-driven data mining) | 90.0 |
| Kaanchan More et al. [50] (risk-factor-based approach) | 86.7 |
| Weize Xu et al. [52] (random forest and Adaboost classifier) | 78.4 |
| Proposed approach | 91.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arooj, S.; Rehman, S.u.; Imran, A.; Almuhaimeed, A.; Alzahrani, A.K.; Alzahrani, A. A Deep Convolutional Neural Network for the Early Detection of Heart Disease. Biomedicines 2022, 10, 2796. https://doi.org/10.3390/biomedicines10112796
Arooj S, Rehman Su, Imran A, Almuhaimeed A, Alzahrani AK, Alzahrani A. A Deep Convolutional Neural Network for the Early Detection of Heart Disease. Biomedicines. 2022; 10(11):2796. https://doi.org/10.3390/biomedicines10112796
Chicago/Turabian StyleArooj, Sadia, Saif ur Rehman, Azhar Imran, Abdullah Almuhaimeed, A. Khuzaim Alzahrani, and Abdulkareem Alzahrani. 2022. "A Deep Convolutional Neural Network for the Early Detection of Heart Disease" Biomedicines 10, no. 11: 2796. https://doi.org/10.3390/biomedicines10112796
APA StyleArooj, S., Rehman, S. u., Imran, A., Almuhaimeed, A., Alzahrani, A. K., & Alzahrani, A. (2022). A Deep Convolutional Neural Network for the Early Detection of Heart Disease. Biomedicines, 10(11), 2796. https://doi.org/10.3390/biomedicines10112796






