PD1+CD8+ Cells Are an Independent Prognostic Marker in Patients with Head and Neck Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection, Processing, and Characterization
2.2. Tissue Sections Preparation and Antibodies Validation
2.3. Multispectral fIHC Panel Design and Optimization
2.4. Image Acquiring and Processing
2.5. Statistical Analysis
3. Results
3.1. Patients’ Characterization
3.2. HPV+ Tumors Are More Infiltrated by T lymphocytes
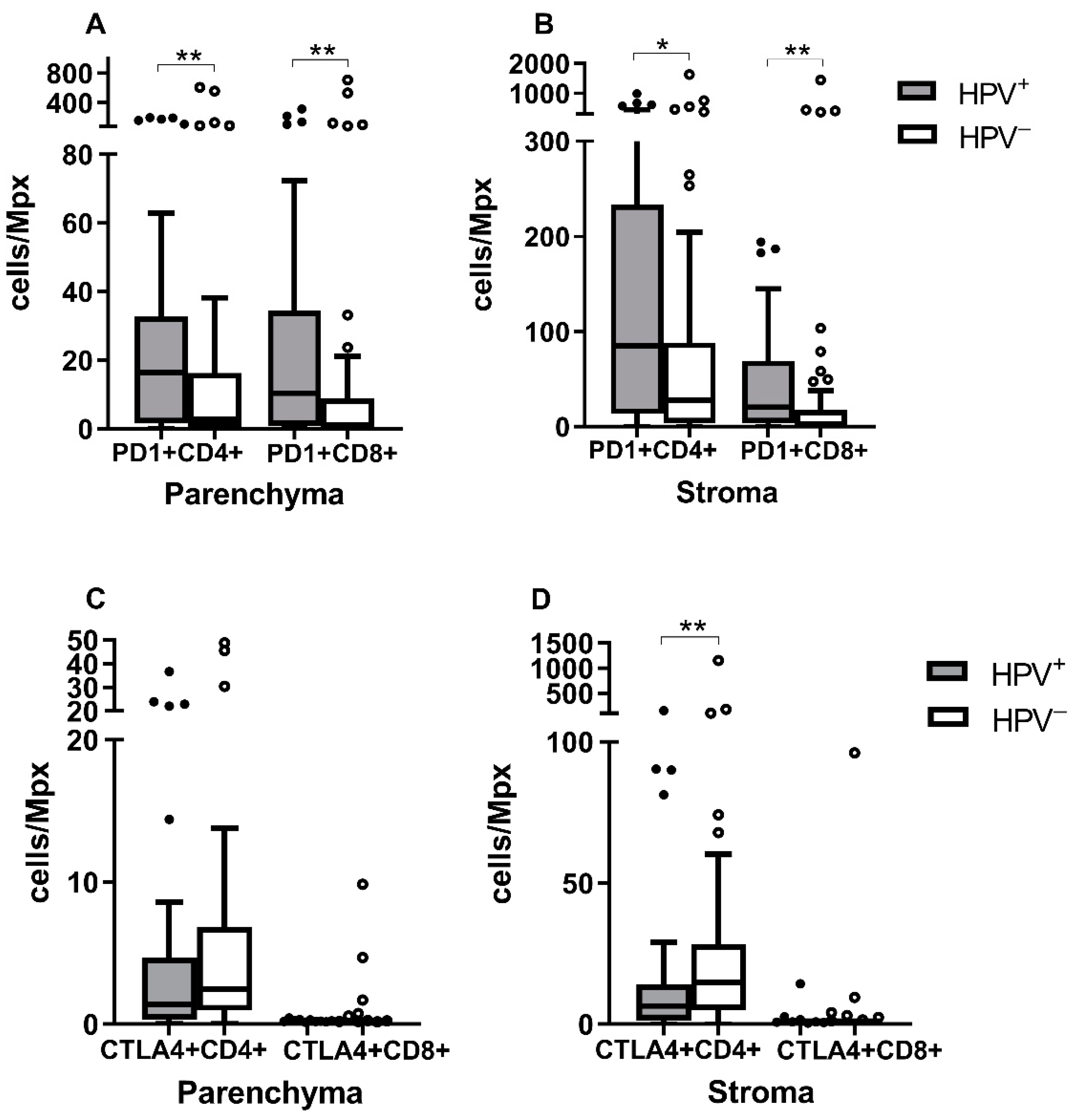
3.3. PD-1+CD4+ and PD-1+CD8+ Levels Are Higher in HPV+ Tumors
3.4. ICOS+ Treg Are More Abundant in HPV− Tumors
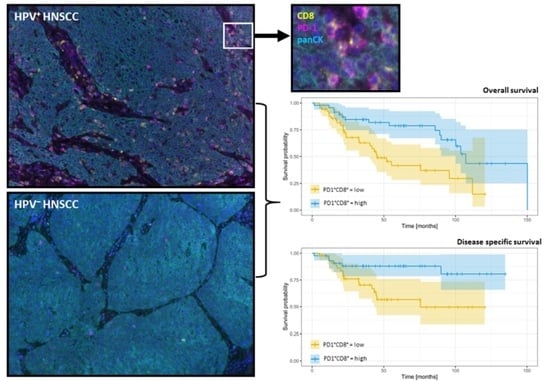
3.5. The Infiltration Level of T Cell Subpopulations Is Prognostic for OS and DSS
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tumban, E. A Current Update on Human Papillomavirus-Associated Head and Neck Cancers. Viruses 2019, 11, 922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gillison, M.L.; Chaturvedi, A.K.; Anderson, W.F.; Fakhry, C. Epidemiology of Human Papillomavirus-Positive Head and Neck Squamous Cell Carcinoma. J. Clin. Oncol. 2015, 33, 3235–3242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koslabova, E.; Hamsikova, E.; Salakova, M.; Klozar, J.; Foltynova, E.; Salkova, E.; Rotnaglova, E.; Ludvikova, V.; Tachezy, R. Markers of HPV infection and survival in patients with head and neck tumors. Int. J. Cancer 2013, 133, 1832–1839. [Google Scholar] [CrossRef]
- Rotnaglova, E.; Tachezy, R.; Salakova, M.; Prochazka, B.; Kosl’abova, E.; Vesela, E.; Ludvikova, V.; Hamsikova, E.; Klozar, J. HPV involvement in tonsillar cancer: Prognostic significance and clinically relevant markers. Int. J. Cancer 2011, 129, 101–110. [Google Scholar] [CrossRef]
- Ward, M.J.; Thirdborough, S.M.; Mellows, T.; Riley, C.; Harris, S.; Suchak, K.; Webb, A.; Hampton, C.; Patel, N.N.; Randall, C.J.; et al. Tumour-infiltrating lymphocytes predict for outcome in HPV-positive oropharyngeal cancer. Br. J. Cancer 2014, 110, 489–500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pagès, F.; Mlecnik, B.; Marliot, F.; Bindea, G.; Ou, F.S.; Bifulco, C.; Lugli, A.; Zlobec, I.; Rau, T.T.; Berger, M.D.; et al. International validation of the consensus Immunoscore for the classification of colon cancer: A prognostic and accuracy study. Lancet 2018, 391, 2128–2139. [Google Scholar] [CrossRef]
- Wong, P.F.; Wei, W.; Smithy, J.W.; Acs, B.; Toki, M.I.; Blenman, K.R.M.; Zelterman, D.; Kluger, H.M.; Rimm, D.L. Multiplex Quantitative Analysis of Tumor-Infiltrating Lymphocytes and Immunotherapy Outcome in Metastatic Melanoma. Clin. Cancer Res. 2019, 25, 2442–2449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donnem, T.; Hald, S.M.; Paulsen, E.E.; Richardsen, E.; Al-Saad, S.; Kilvaer, T.K.; Brustugun, O.T.; Helland, A.; Lund-Iversen, M.; Poehl, M.; et al. Stromal CD8+ T-cell Density—A Promising Supplement to TNM Staging in Non-Small Cell Lung Cancer. Clin. Cancer Res. 2015, 21, 2635–2643. [Google Scholar] [CrossRef] [Green Version]
- Borsetto, D.; Tomasoni, M.; Payne, K.; Polesel, J.; Deganello, A.; Bossi, P.; Tysome, J.R.; Masterson, L.; Tirelli, G.; Tofanelli, M.; et al. Prognostic Significance of CD4+ and CD8+ Tumor-Infiltrating Lymphocytes in Head and Neck Squamous Cell Carcinoma: A Meta-Analysis. Cancers 2021, 13, 781. [Google Scholar] [CrossRef]
- Simonson, W.T.N.; Allison, K.H. Tumour-infiltrating lymphocytes in cancer: Implications for the diagnostic pathologist. Diagn. Histopathol. 2011, 17, 80–90. [Google Scholar] [CrossRef]
- Nakano, O.; Sato, M.; Naito, Y.; Suzuki, K.; Orikasa, S.; Aizawa, M.; Suzuki, Y.; Shintaku, I.; Nagura, H.; Ohtani, H. Proliferative activity of intratumoral CD8(+) T-lymphocytes as a prognostic factor in human renal cell carcinoma: Clinicopathologic demonstration of antitumor immunity. Cancer Res. 2001, 61, 5132–5136. [Google Scholar]
- Oudejans, J.J.; Jiwa, N.M.; Kummer, J.A.; Ossenkoppele, G.J.; van Heerde, P.; Baars, J.W.; Kluin, P.M.; Kluin-Nelemans, J.C.; van Diest, P.J.; Middeldorp, J.M.; et al. Activated cytotoxic T cells as prognostic marker in Hodgkin’s disease. Blood 1997, 89, 1376–1382. [Google Scholar] [CrossRef] [Green Version]
- de Ruiter, E.J.; Ooft, M.L.; Devriese, L.A.; Willems, S.M. The prognostic role of tumor infiltrating T-lymphocytes in squamous cell carcinoma of the head and neck: A systematic review and meta-analysis. Oncoimmunology 2017, 6, e1356148. [Google Scholar] [CrossRef] [Green Version]
- Webb, E.S.; Liu, P.; Baleeiro, R.; Lemoine, N.R.; Yuan, M.; Wang, Y.H. Immune checkpoint inhibitors in cancer therapy. J. Biomed. Res. 2018, 32, 317–326. [Google Scholar] [CrossRef]
- Amatore, F.; Gorvel, L.; Olive, D. Inducible Co-Stimulator (ICOS) as a potential therapeutic target for anti-cancer therapy. Expert Opin. Ther. Targets 2018, 22, 343–351. [Google Scholar] [CrossRef]
- Vojtechova, Z.; Sabol, I.; Salakova, M.; Turek, L.; Grega, M.; Smahelova, J.; Vencalek, O.; Lukesova, E.; Klozar, J.; Tachezy, R. Analysis of the integration of human papillomaviruses in head and neck tumours in relation to patients’ prognosis. Int. J. Cancer 2016, 138, 386–395. [Google Scholar] [CrossRef] [Green Version]
- Tachezy, R.; Klozar, J.; Rubenstein, L.; Smith, E.; Saláková, M.; Smahelová, J.; Ludvíková, V.; Rotnáglová, E.; Kodet, R.; Hamsíková, E. Demographic and risk factors in patients with head and neck tumors. J. Med. Virol. 2009, 81, 878–887. [Google Scholar] [CrossRef]
- Edge, S.B. AJCC Cancer Staging Manual; Springer: Berlin/Heidelberg, Germany, 2010; Volume 7, pp. 97–100. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Khoury, T.; Nagrale, V.; Opyrchal, M.; Peng, X.; Wang, D.; Yao, S. Prognostic Significance of Stromal Versus Intratumoral Infiltrating Lymphocytes in Different Subtypes of Breast Cancer Treated With Cytotoxic Neoadjuvant Chemotherapy. Appl. Immunohistochem. Mol. Morphol. 2018, 26, 523–532. [Google Scholar] [CrossRef]
- Badoual, C.; Hans, S.; Rodriguez, J.; Peyrard, S.; Klein, C.; Agueznay Nel, H.; Mosseri, V.; Laccourreye, O.; Bruneval, P.; Fridman, W.H.; et al. Prognostic value of tumor-infiltrating CD4+ T-cell subpopulations in head and neck cancers. Clin. Cancer Res. 2006, 12, 465–472. [Google Scholar] [CrossRef] [Green Version]
- Oguejiofor, K.; Galletta-Williams, H.; Dovedi, S.J.; Roberts, D.L.; Stern, P.L.; West, C.M. Distinct patterns of infiltrating CD8+ T cells in HPV+ and CD68 macrophages in HPV- oropharyngeal squamous cell carcinomas are associated with better clinical outcome but PD-L1 expression is not prognostic. Oncotarget 2017, 8, 14416–14427. [Google Scholar] [CrossRef] [Green Version]
- Nasman, A.; Romanitan, M.; Nordfors, C.; Grun, N.; Johansson, H.; Hammarstedt, L.; Marklund, L.; Munck-Wikland, E.; Dalianis, T.; Ramqvist, T. Tumor infiltrating CD8+ and Foxp3+ lymphocytes correlate to clinical outcome and human papillomavirus (HPV) status in tonsillar cancer. PLoS ONE 2012, 7, e38711. [Google Scholar] [CrossRef]
- Nordfors, C.; Grun, N.; Tertipis, N.; Ahrlund-Richter, A.; Haeggblom, L.; Sivars, L.; Du, J.; Nyberg, T.; Marklund, L.; Munck-Wikland, E.; et al. CD8+ and CD4+ tumour infiltrating lymphocytes in relation to human papillomavirus status and clinical outcome in tonsillar and base of tongue squamous cell carcinoma. Eur. J. Cancer 2013, 49, 2522–2530. [Google Scholar] [CrossRef] [PubMed]
- Oguejiofor, K.; Hall, J.; Slater, C.; Betts, G.; Hall, G.; Slevin, N.; Dovedi, S.; Stern, P.L.; West, C.M. Stromal infiltration of CD8 T cells is associated with improved clinical outcome in HPV-positive oropharyngeal squamous carcinoma. Br. J. Cancer 2015, 113, 886–893. [Google Scholar] [CrossRef] [PubMed]
- Succaria, F.; Kvistborg, P.; Stein, J.E.; Engle, E.L.; McMiller, T.L.; Rooper, L.M.; Thompson, E.; Berger, A.E.; van den Brekel, M.; Zuur, C.L.; et al. Characterization of the tumor immune microenvironment in human papillomavirus-positive and -negative head and neck squamous cell carcinomas. Cancer Immunol. Immunother. 2021, 70, 1227–1237. [Google Scholar] [CrossRef] [PubMed]
- Partlova, S.; Boucek, J.; Kloudova, K.; Lukesova, E.; Zabrodsky, M.; Grega, M.; Fucikova, J.; Truxova, I.; Tachezy, R.; Spisek, R.; et al. Distinct patterns of intratumoral immune cell infiltrates in patients with HPV-associated compared to non-virally induced head and neck squamous cell carcinoma. Oncoimmunology 2015, 4, e965570. [Google Scholar] [CrossRef]
- Mito, I.; Takahashi, H.; Kawabata-Iwakawa, R.; Ida, S.; Tada, H.; Chikamatsu, K. Comprehensive analysis of immune cell enrichment in the tumor microenvironment of head and neck squamous cell carcinoma. Sci. Rep. 2021, 11, 16134. [Google Scholar] [CrossRef]
- Poropatich, K.; Fontanarosa, J.; Swaminathan, S.; Dittmann, D.; Chen, S.; Samant, S.; Zhang, B. Comprehensive T-cell immunophenotyping and next-generation sequencing of human papillomavirus (HPV)-positive and HPV-negative head and neck squamous cell carcinomas. J. Pathol. 2017, 243, 354–365. [Google Scholar] [CrossRef]
- Badoual, C.; Hans, S.; Merillon, N.; Van Ryswick, C.; Ravel, P.; Benhamouda, N.; Levionnois, E.; Nizard, M.; Si-Mohamed, A.; Besnier, N.; et al. PD-1-expressing tumor-infiltrating T cells are a favorable prognostic biomarker in HPV-associated head and neck cancer. Cancer Res. 2013, 73, 128–138. [Google Scholar] [CrossRef] [Green Version]
- Kansy, B.A.; Concha-Benavente, F.; Srivastava, R.M.; Jie, H.B.; Shayan, G.; Lei, Y.; Moskovitz, J.; Moy, J.; Li, J.; Brandau, S.; et al. PD-1 Status in CD8(+) T Cells Associates with Survival and Anti-PD-1 Therapeutic Outcomes in Head and Neck Cancer. Cancer Res. 2017, 77, 6353–6364. [Google Scholar] [CrossRef] [Green Version]
- Lechner, A.; Schlößer, H.; Rothschild, S.I.; Thelen, M.; Reuter, S.; Zentis, P.; Shimabukuro-Vornhagen, A.; Theurich, S.; Wennhold, K.; Garcia-Marquez, M.; et al. Characterization of tumor-associated T-lymphocyte subsets and immune checkpoint molecules in head and neck squamous cell carcinoma. Oncotarget 2017, 8, 44418–44433. [Google Scholar] [CrossRef] [Green Version]
- Spector, M.E.; Bellile, E.; Amlani, L.; Zarins, K.; Smith, J.; Brenner, J.C.; Rozek, L.; Nguyen, A.; Thomas, D.; McHugh, J.B.; et al. Prognostic Value of Tumor-Infiltrating Lymphocytes in Head and Neck Squamous Cell Carcinoma. JAMA Otolaryngol. Head Neck Surg. 2019, 145, 1012–1019. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, G.; Bag, S.; Chakraborty, P.; Dey, D.; Roy, S.; Jain, P.; Roy, P.; Soong, R.; Majumder, P.P.; Dutt, S. Density of CD3+ and CD8+ cells in gingivo-buccal oral squamous cell carcinoma is associated with lymph node metastases and survival. PLoS ONE 2020, 15, e0242058. [Google Scholar] [CrossRef] [PubMed]
- Solomon, B.; Young, R.J.; Bressel, M.; Urban, D.; Hendry, S.; Thai, A.; Angel, C.; Haddad, A.; Kowanetz, M.; Fua, T.; et al. Prognostic Significance of PD-L1(+) and CD8(+) Immune Cells in HPV(+) Oropharyngeal Squamous Cell Carcinoma. Cancer Immunol. Res. 2018, 6, 295–304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quan, H.; Shan, Z.; Liu, Z.; Liu, S.; Yang, L.; Fang, X.; Li, K.; Wang, B.; Deng, Z.; Hu, Y.; et al. The repertoire of tumor-infiltrating lymphocytes within the microenvironment of oral squamous cell carcinoma reveals immune dysfunction. Cancer Immunol. Immunother. 2020, 69, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Poropatich, K.; Hernandez, D.; Fontanarosa, J.; Brown, K.; Woloschak, G.; Paintal, A.; Raparia, K.; Samant, S. Peritumoral cuffing by T-cell tumor-infiltrating lymphocytes distinguishes HPV-related oropharyngeal squamous cell carcinoma from oral cavity squamous cell carcinoma. J. Oral. Pathol. Med. 2017, 46, 972–978. [Google Scholar] [CrossRef]
- Wolf, G.T.; Chepeha, D.B.; Bellile, E.; Nguyen, A.; Thomas, D.; McHugh, J. Tumor infiltrating lymphocytes (TIL) and prognosis in oral cavity squamous carcinoma: A preliminary study. Oral. Oncol. 2015, 51, 90–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, K.; Fu, Y.; Han, Y.; Xia, R.; Xu, S.; Duan, S.; Zhang, Z.; Li, J. Fewer tumour-specific PD-1(+)CD8(+) TILs in high-risk "Infiltrating" HPV(-) HNSCC. Br. J. Cancer 2020, 123, 932–941. [Google Scholar] [CrossRef]
- Lukesova, E.; Boucek, J.; Rotnaglova, E.; Salakova, M.; Koslabova, E.; Grega, M.; Eckschlager, T.; Rihova, B.; Prochazka, B.; Klozar, J.; et al. High level of Tregs is a positive prognostic marker in patients with HPV-positive oral and oropharyngeal squamous cell carcinomas. Biomed. Res. Int. 2014, 2014, 303929. [Google Scholar] [CrossRef] [Green Version]
- De Ruiter, E.J.; de Roest, R.H.; Brakenhoff, R.H.; Leemans, C.R.; de Bree, R.; Terhaard, C.H.J.; Willems, S.M. Digital pathology-aided assessment of tumor-infiltrating T lymphocytes in advanced stage, HPV-negative head and neck tumors. Cancer Immunol. Immunother. 2020, 69, 581–591. [Google Scholar] [CrossRef]


| Name | Clone | Manufacturer |
|---|---|---|
| CD3e | SP7 | ThermoFisher Scientific (Waltham, MA, USA) |
| CD4 | EP204 | Zeta Corporation (Arcadia, CA, USA) |
| CD8-α | C8/144B | Santa Cruz Biotechnology (Dallas, TX, USA) |
| FoxP3 | 206D | BioLegend (San Diego, CA, USA) |
| PD-1 | EPR4877(2) | Abcam (Cambridge, United Kingdom) |
| CTLA4 | F-8 | Santa Cruz Biotechnology (Dallas, TX, USA) |
| ICOS | SP98 | Abcam (Cambridge, United Kingdom) |
| VEGF | EP1176Y | Biocare Medical (Pacheco, CA, USA) |
| Ki67 | sc-23900 | Santa Cruz Biotechnology (Dallas, TX, USA) |
| CCR4 | polyclonal | Novus Biological (Centennial, CO, USA) |
| Cytokeratin Pan Type I/II | AE1/AE3 | ThermoFisher Scientific (Waltham, MA, USA) |
| Markers | Markers | ||
|---|---|---|---|
| Panel 1 | Th: CD3+CD4+ | Panel 3 | CD3+ |
| Tc: CD3+CD8+ | Ki67+ | ||
| Treg: FOXP3+CD3+CD4+ | VEGF+ | ||
| Panel 2 | CD4+ | Panel 4 | CD4+ |
| PD-1+CD4+ | FOXP3+CD4+ | ||
| CTLA4+CD4+ | ICOS+CD4+ | ||
| CD8+ | ICOS+FOXP3+CD4+ | ||
| PD-1+CD8+ | CCR4+ | ||
| CTLA4+CD8+ | CD4+FOXP3+CCR4+ |
| Characteristics | Total | HPV+ | HPV− | |
|---|---|---|---|---|
| No. (%) | No. (%) | No. (%) | ||
| No. of patients | 97 (100%) | 45 (46%) | 52 (54%) | |
| Age | Mean age | 57.23 | 57.98 | 56.58 |
| (years) | Median age | 58 | 59 | 56 |
| Gender | female | 19 (20%) | 10 (22%) | 9 (17%) |
| male | 78 (80%) | 35 (78%) | 43 (83%) | |
| Tumor location | oropharynx | 83 (86%) | 45 (100%) | 38 (73%) |
| oral cavity | 14 (14%) | 0 (0%) | 14 (27%) | |
| Education a | >12 years | 33 (34%) | 18 (41%) | 15 (29%) |
| ≤12 years | 63 (66%) | 26 (59%) | 37 (71%) | |
| Smoking | never | 19 (20%) | 15 (33%) | 4 (8%) |
| past | 32 (33%) | 19 (42%) | 13 (25%) | |
| current | 46 (47%) | 11 (25%) | 35 (67%) | |
| Alcohol | never | 20 (21%) | 12 (27%) | 8 (15%) |
| consumption | past | 13 (13%) | 7 (16%) | 6 (12%) |
| current | 64 (66%) | 26 (57%) | 38 (73%) | |
| No. of sex partners | >7 | 41 (46%) | 15 (35%) | 26 (55%) |
| ≤6 | 49 (54%) | 28 (65%) | 21 (45%) | |
| Tumor size | T1 | 17 (18%) | 7 (16%) | 10 (19%) |
| (pT) | T2 | 61 (63%) | 27 (60%) | 34 (65%) |
| T3 | 13 (13%) | 9 (20%) | 4 (8%) | |
| T4 | 6 (6%) | 2 (4%) | 4 (8%) | |
| Nodal status | N0 | 31 (32%) | 9 (20%) | 22 (42%) |
| (pN) | N1 | 16 (16.5%) | 5 (11%) | 11 (21%) |
| N2 | 47 (48.5%) | 28 (62%) | 19 (37%) | |
| N3 | 3 (3%) | 3 (7%) | 0 (0%) | |
| Metastasis | 0 | 97 (100%) | 45 (100%) | 52 (100%) |
| (M) | 1 | 0 (0%) | 0 (0%) | 0 (0%) |
| Tumor stage | I | 5 (5%) | 1 (2%) | 4 (8%) |
| (S) | II | 24 (25%) | 6 (13%) | 18 (35%) |
| III | 16 (16%) | 7 (16%) | 9 (17%) | |
| IV | 52 (54%) | 31 (69%) | 21 (40%) | |
| Tumor grade | 1 | 14 (14%) | 3 (7%) | 11 (21%) |
| 2 | 57 (59%) | 25 (55%) | 32 (62%) | |
| 3 | 26 (27%) | 17 (38%) | 9 (17%) | |
| Relapse | no | 82 (85%) | 41 (91%) | 41 (79%) |
| yes | 15 (15%) | 4 (9%) | 11 (21%) |
| Models Including HPV Status, IHC Markers Evaluated with Respect to the Stromal or Parenchymal Location. | |||
|---|---|---|---|
| OS | DSS | ||
| BIC = 308.96 | HR (p value) | BIC = 169.16 | HR (p value) |
| HPV RNA+ | 0.26 (0.0003) | HPV RNA+ | 0.15 (0.0011) |
| Increasing tumor stage | 1.55 (0.0179) | CD3+CD4+/Treg ratio | 3.18 (0.0016) |
| Parenchymal PD1+CD8+ T | 0.53 (0.0003) | Stromal PD1+CD8+ T | 0.44 (0.0007) |
| Models including HPV status, the IHC markers counted regardless of location. | |||
| OS | DSS | ||
| BIC = 307.31 | HR (p value) | BIC = 171.50 | HR (p value) |
| HPV RNA+ | 0.26 (0.0004) | HPV RNA+ | 0.22 (0.0057) |
| Increasing tumor stage | 1.57 (0.0160) | CD3+ T | 2.79 (0.0094) |
| PD1+CD8+ T | 0.52 (0.0001) | PD1+CD8+ T | 0.36 (0.0001) |
| Models without HPV status inclusion, the IHC markers evaluated with respect to the stromal or parenchymal location. | |||
| OS | DSS | ||
| BIC = 313.13 | HR (p value) | BIC = 174.57 | HR (p value) |
| Smoking (No) | 0.37 (0.0055) | Increasing age | 0.93 (0.0032) |
| Increasing tumor size | 1.44 (0.0709) | Stromal CD8+ T | 2.19 (0.0165) |
| Parenchymal PD1+CD8+ T | 0.52 (0.0003) | Stromal PD1+CD8+ T | 0.30 (0.0000) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pokrývková, B.; Grega, M.; Klozar, J.; Vencálek, O.; Nunvář, J.; Tachezy, R. PD1+CD8+ Cells Are an Independent Prognostic Marker in Patients with Head and Neck Cancer. Biomedicines 2022, 10, 2794. https://doi.org/10.3390/biomedicines10112794
Pokrývková B, Grega M, Klozar J, Vencálek O, Nunvář J, Tachezy R. PD1+CD8+ Cells Are an Independent Prognostic Marker in Patients with Head and Neck Cancer. Biomedicines. 2022; 10(11):2794. https://doi.org/10.3390/biomedicines10112794
Chicago/Turabian StylePokrývková, Barbora, Marek Grega, Jan Klozar, Ondřej Vencálek, Jaroslav Nunvář, and Ruth Tachezy. 2022. "PD1+CD8+ Cells Are an Independent Prognostic Marker in Patients with Head and Neck Cancer" Biomedicines 10, no. 11: 2794. https://doi.org/10.3390/biomedicines10112794
APA StylePokrývková, B., Grega, M., Klozar, J., Vencálek, O., Nunvář, J., & Tachezy, R. (2022). PD1+CD8+ Cells Are an Independent Prognostic Marker in Patients with Head and Neck Cancer. Biomedicines, 10(11), 2794. https://doi.org/10.3390/biomedicines10112794