1. Introduction
A healthy dental pulp is crucial in the maintenance of the structural and functional soundness of a tooth. In cases of tooth devitalization due to a deep carious lesion or trauma, newly differentiated odontoblast-like cells migrate toward the site of injury and form reparative dentine to protect pulp tissue from the harmful stimuli. In direct pulp capping, a protective agent is applied to injured pulp tissue to promote pulp healing and preserve the integrity of the tooth. Calcium hydroxide [Ca(OH)
2] and mineral trioxide aggregate (MTA) are the current standard materials used for this treatment [
1,
2]. However, due to their limitations, the clinical performances of these materials are unsatisfactory [
3,
4,
5]. Therefore, researchers continue to develop a novel biocompatible pulp capping material with the goal of regenerating dentin of significantly improved quality.
Dentin phosphophoryn (DPP) is the most abundantly present noncollagenous protein in dentine, and is highly acidic and anionic in nature due to the presence of copious amounts of aspartic acid and phosphoserine in its amino acid structure. Studies have shown that it binds to calcium ions [
6] and mediates reparative dentine formation of improved quality compared to Ca(OH)
2 [
7]. It is a cleaved product of dentin sialophosphoprotein (DSPP), and the absence of DSPP has been associated with dentinogenesis imperfecta type III [
8]. According to a study, dentin sialoprotein (DSP) plays a role in regulating the initiation of dentine mineralization and DPP in the maturation of mineralized teeth [
9]. These findings suggest the importance of DPP in dentinogenesis during developmental stage. Several studies have been conducted to investigate the role of phosphoproteins in the induction of dentine mineralization. It was reported that DPP promotes mineralization of bone and dentine [
6,
10]. DPP induces the formation and growth of hydroxyapatite crystals in dentine when covalently cross-linked to collagen fibrils [
11]. In addition, DPP has acted as a marker for pulp cell differentiation into odontoblasts [
12]. It was found that phosphophoryn regulates gene expression in mesenchymal stem cells via the integrin/mitogen activated protein kinase (MAPK) pathway [
13]. Additionally, DPP elicits signalling via activation of the Smad pathway [
14].
The amino acid chain of human DPP contains a species-conserved arginine-glycine-aspertic acid (RGD) cell attachment domain and a repeated Asp-PSer-PSer sequence. The RGD domain has potential effect in stimulating specific cellular responses, as DPP has been shown to mediate cell adhesion and migration by initiating integrin-mediated signalling on the surface of pulp cells via its RGD motif in vitro [
15]. The osteogenic effects of several RGD peptides from human DPP were investigated. Among the various peptides, RGD-1, RGD-2 and RGD-3 promoted human mesenchymal stem cell differentiation into osteoblasts [
16]. In a later study, human DPP-derived RGD-peptides showed similar effects on the differentiation and mineralization of the rat odontoblast-like cell line MDPC-23. RGD-3 showed remarkable results among the three peptides [
17]. Another study [
18] observed the role of DPP on the differentiation of MDPC-23 cells. From the upregulation of odontogenic gene expressions, it was suggested that DPP might bind integrin receptors on the surface of MDPC-23 cells via its RGD motif.
However, the specific role of the DPP-derived RGD domain has not yet been studied as extensively as the DPP protein. As the background of the present study, a previous study [
16] experimented with various configurations of the amino acid sequence of the RGD domain in human DPP and named them chronologically: RGD-1 (SESDNNSSSRGDASYNSDES), RGD-2 (ANSESDNNSSSRGDA), RGD-3 (SRGDASYNSDESKD), RGD-4 (DNNSSSRGDASYNSD), RGD-5 (SSSRGDASY) and a mutant peptide RGD-6 (SESDNNSSSRADASYNSDES), which was named arginine-alanine-aspertic acid (RAD)-1 in the later study [
17]. Among the peptides that promoted differentiation of mesenchymal stem cells into osteoblasts were RGD-1, RGD-2 and RGD-3. Based on the results of this study, the possible functional significance of these three RGD peptides and their ability to induce proliferation, differentiation and mineralization of odontoblast-like cells were evaluated in one study [
17]. RGD peptides were immobilized onto modified tissue culture polystyrene surfaces, and MDPC-23 cells were cultured onto the surfaces. It was reported that RGD peptides, especially RGD-3-0.5 mg/mL, have significant effect on the differentiation and mineralization of MDPC-23 cells. In the present study, the configurations of RGD-1, RGD-2 and RGD-3 in these previous two studies were used, as well as the peptide named RAD-1 [
17].
Most of the studies conducted regarding DPP have been conducted on animal cells like rat odontoblast-like cells mdpc-23, thus making the results less suitable for human cells. Moreover, the MDPC-23 cells are of terminally differentiated odontoblastic lineage; while the hDPSCs are undifferentiated and can differentiate into some other lineage than odontogenic. Although there have been several studies done with RGD peptides, they were not derived from human DPP. Therefore, the activity of human recombinant DPP, including the specific role of the RGD domain, needed to be established. The aims of this study were to investigate the in vitro effects of human DPP-derived RGD peptides on the proliferation, differentiation and mineralization of hDPSCs, and to explore the mechanism of the peptides’ function in vitro.
2. Materials and Methods
2.1. Coating of RGD Peptides and RAD Peptides
The DPP-derived RGD peptides; RGD-1 (SESDNNSSSRGDASYNSDES), RGD-2 (ANSESDNNSSSRGDA), RGD-3 (SRGDSASYNSDESKD) and RAD peptides; RAD-1 (SESDNNSSSRADASYNSDES), RAD-2 (ANSESDNNSSSRADA) and RAD-3 (SRADSASYNSDESKD) were custom-synthesized by GL Biochem Ltd. (Shanghai, China) and stored at −30 °C until use. The effect of coating of human DPP-derived RGD peptides on non-tissue culture treated polystyrene (non-TCPP, Corning, NY, USA) plates was analysed. The effect of RAD peptides was also investigated, which were synthesized by replacing the RGD domain with the corresponding RAD peptide sequences and coated on non-TCPP plates. Briefly, RGD peptide powder was dissolved in distilled water (dH2O, manufacture) at a 1 M concentration (0.5 mg/mL RGD-1, 0.36 mg/mL RGD-2 and 0.36 mg/mL RGD-3), spread onto non-TCPP plates and then dried at room temperature for two days. RAD-peptides (1 M, 0.52 mg/mL RAD-1, 0.36 mg/mL RAD-2 and 0.37 mg/mL RAD-3) were also coated on plates following a similar technique. dH2O was used as a negative control. In experiment two, RGD-3-coated groups were exposed to certain MAPK inhibitors and their effect was observed. In experiment three, the gene expression levels of integrins in RGD-3-coated group were investigated.
2.2. Cell Culture
Human dental pulp stem cells (hDPSCs) (catalog number: 5025; lot number: 0000361427) were purchased from LONZA (Walkersville, MD, USA). The hDPSCs were cultured in Dulbecco’s modified Eagle’s medium (DMEM, Sigma-Aldrich, St. Louis, MO, USA) supplemented with 10% fetal bovine serum (FBS, Gibco, Grand Island, NY, USA). All media were supplemented with 50 units/mL penicillin and 50 µg/mL streptomycin (Gibco). Culture media was changed every four days. Cell passages from 3 to 5 were used in this study. Cells were inoculated at a density of 4 × 103 cells/well in 96-well plates, 2 × 104 cells/well in 12-well plates, and 3 × 104 cells/well in 6-well plates. Cells were cultivated at 37 °C under humidified 5% CO2 and 95% air atmospheric conditions.
2.3. Cell Proliferation Assay
Cell Counting Kit-8 (CCK-8) was purchased from Dojindo (Kumamoto, Japan). For the cell viability assay, RGD peptides (1 M and their different diluted concentrations) were coated on 96-well microplates and dried for 2 days at room temperature. hDPSCs were cultured in DMEM supplemented with 10% FBS, 50 units/mL penicillin, and 50 μg/mL streptomycin for 4 days. Concentrations were as follows: 1, 0.5, 0.05 and 0.005 mg/mL for RGD-1, 0.72, 0.36, 0.036 and 0.004 mg/mL for RGD-2, and 0.72, 0.36, 0.036 and 0.004 mg/mL for RGD-3. dH2O was used as a control. On day 5, CCK-8 reagent was added to each well (10 µL/well) and incubated with the cells for 80 min in the incubator. The absorbance of lysates was measured using a microplate reader at 450 nm.
2.4. Cell Morphology Observation
hDPSCs were seeded at 3 × 104 cells/well in 6-well microplates. RGD-coated groups (1 M of RGD-1, RGD-2 and RGD-3) were compared with cells in the control group. Cells were visualized at specific time intervals under phase contrast microscopy (Olympus, Tokyo, Japan) on days 1, 3 and 5.
2.5. Alkaline Phosphatase (ALP) Activity Assay
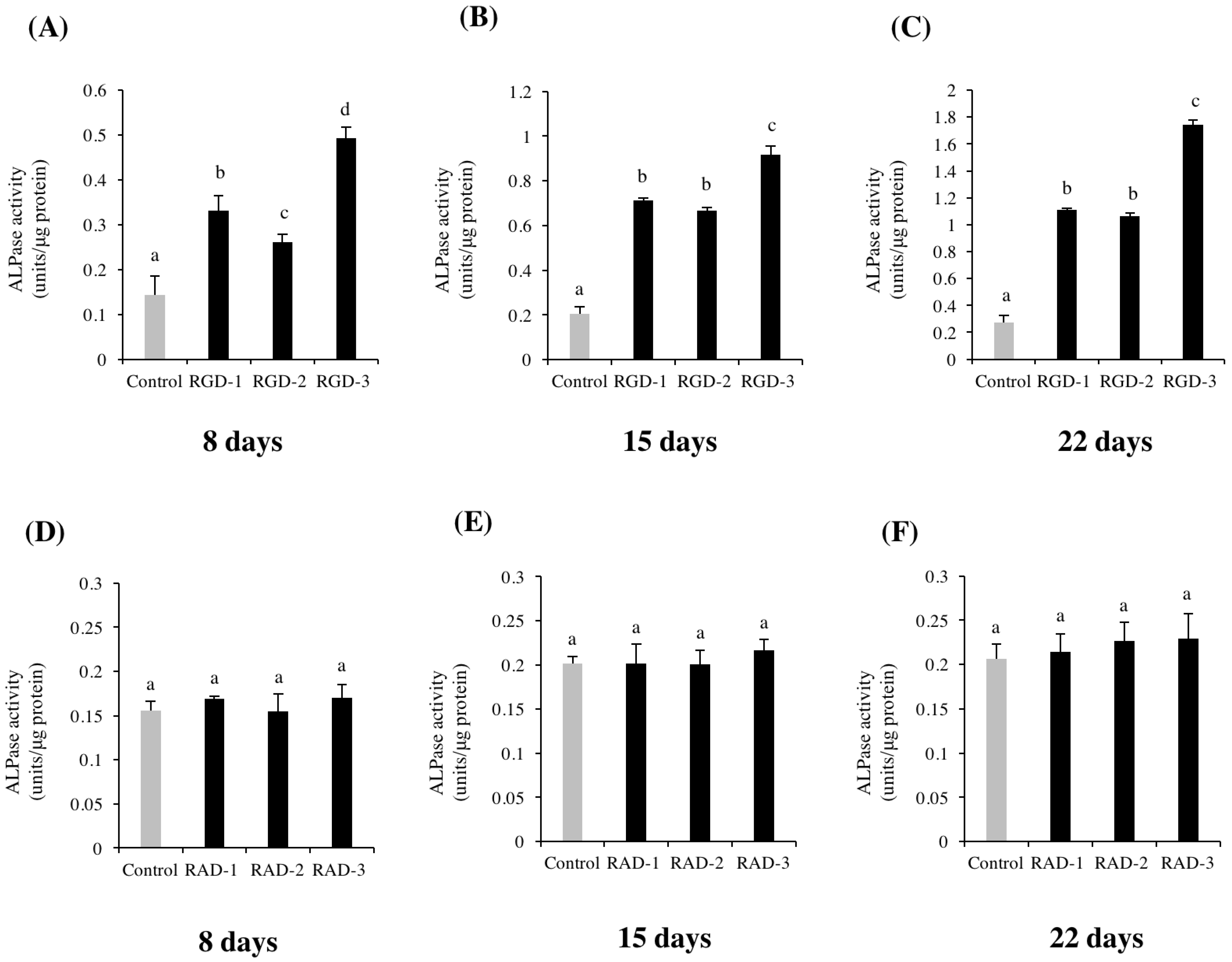
Cells were seeded at 3 × 104 cells/well in 6-well microplates and cultured in DMEM supplemented with 10% FBS, 50 units/mL penicillin and 50 μg/mL streptomycin for six days. Then, 10 mM glycerol-2-phosphate disodium salt n-hydrate (β-GP, Wako, Osaka, Japan) and 50 μg/mL L-ascorbic acid phosphate magnesium salt n-hydrate (AA, Wako) were added on day 7 when the cells reached confluence. For experiment one, non-TCPP plates were coated with 1 M RGD-1, RGD-2 and RGD-3 and 1 M RAD-1, RAD-2 and RAD-3. After incubation for the prescribed number of days, an ALP activity assay was performed on days 8, 15 and 22. Alkaline phosphatase (ALP) assay kit was purchased from Wako (Osaka, Japan). The Pierce BCA protein assay kit was purchased from Thermo Fisher Scientific (Rockford, IL, USA).
Cells were collected and lysed with 0.1% Triton-X-100 (Sigma-Aldrich) in dH2O. Then lysates were sonicated in a sonicator (Bioruptor®, Diagenode, Seraing, Belgium) on ice for 10 min and centrifuged at 12,000 rpm at 4 °C for 15 min, and then the supernatants were collected. ALP activity and protein quantification were determined according to the manufacturers’ instructions. One unit of ALP activity was defined as the release of 1 nmol p-nitrophenol per minute at pH 9.8 and 37 °C. The relative activity was determined as follows: units/μg protein = activity (units/μL)/protein concentration (μg/μL). Absorbance was measured at 405 nm for ALP activity and 570 nm for protein quantification using a microplate reader. Values were averaged from triplicate samples.
2.6. Real-Time Quantitative RT-PCR
Cells were seeded at 3 × 104 cells/well in 6-well microplates and cultured in DMEM containing 10% FBS, 50 units/mL penicillin and 50 μg/mL streptomycin for six days. Then, 10 mM β-GP and 50 μg/mL AA were added on day 7 when the cells reached confluence. For experiment one, non-TCPP plates were coated with 1 M RGD peptides and 1 M RAD peptides.
On day 21, total RNA was isolated using Trizol® (Invitrogen, Carlsbad, CA, USA). The isolated RNA was pelleted, washed in 75% ethanol, and re-suspended in nuclease-free water. A NanoDrop ND-1000 spectrophotometer (Thermo Fisher Scientific) was used to spectroscopically measure the RNA concentration of each sample. Afterward, one microgram of RNA concentration was reverse-transcribed into complimentary DNA (cDNA) using the M-MLV (Invitrogen) reverse transcriptase in a 20 μL reaction system (which included 5× reaction buffer, 500 μg/mL oligo dt, 2.5 mmol/L dNTP mixture, 40 unit/μL RNase inhibitor, 0.1 mol/L DTT, and 1 μL M-MLV) according to the manufacturers’ instructions. The resultant cDNA was used for real-time RT-PCR.
To quantify odontogenic gene expressionand gene expression of integrin subunits in hDPSCs, real-time RT-PCR was performed on a LightCycler™ Nano (Roche, Basel, Switzerland). Specific primers for
dentine matrix protein-1 (
DMP-1),
dentine sialophosphoprotein (
DSPP),
ALP,
runt-related transcription factor-2 (
Runx-2),
bone sialoprotein (
BSP),
osteopontin (
OPN),
ITGA1-8,
ITGB1 and
ITGB3 were used. Relative gene expression was calculated using the comparative 2
−∆∆Ct method.
hGAPDH was used as an internal standard to normalize mRNA expression levels. The sequences of primers used for are presented in
Table 1 and
Table 2.
2.7. Arizarin Red S (ARS) Staining
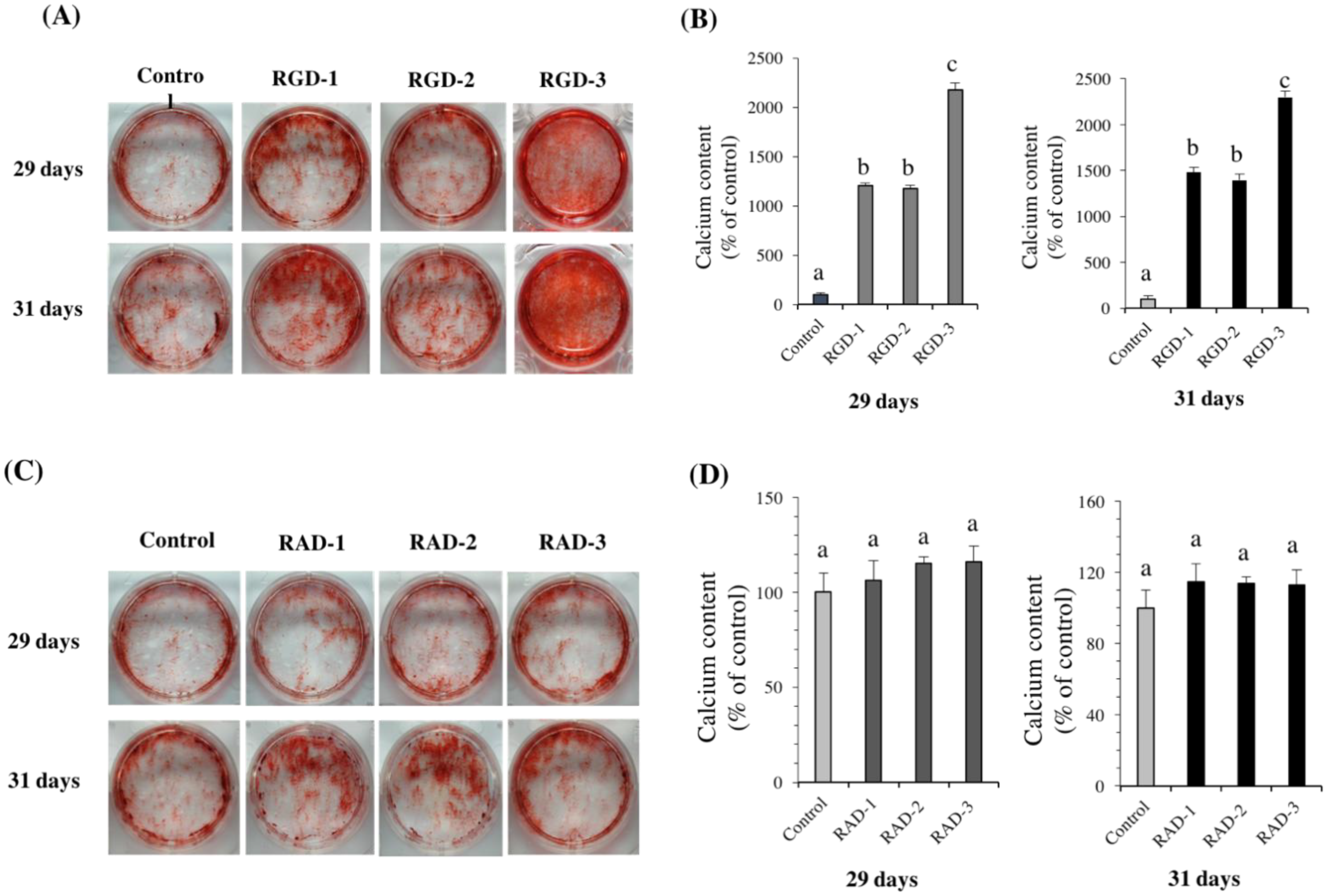
To observe and quantify calcific deposition of hDPSCs, alizarin red S (ARS) staining and the cetylpyridinium chloride (CPC) extraction method were used. Cells were spread at 3 × 104 cells/well in a 6-well microplate and cultured in DMEM supplemented with 10% FBS, 50 units/mL penicillin and 50 μg/mL streptomycin. Then, 10 mM β-GP, 50 μg/mL AA, and 100 nmol/L dexamethasone (Dex, Sigma-Aldrich) were added on day 7 when the cells reached confluence. ARS staining was performed on days 29 and 31.
Cells were fixed with 10% formalin neutral buffer solution (Wako) for 20 min, and washed again with phosphate buffered saline (PBS, pH 7.4, Gibco). ARS solution (1%, pH 4.1, Wako) was added to the cell monolayer, and after 10 min, the staining solution was removed. Then, the cells were washed three times with distilled water and washed thoroughly with PBS to remove the nonspecific background stain. Photographs were taken by a digital imaging system (Funakoshi, Tokyo, Japan). After staining with ARS, CPC (10%, w/v, in distilled water, Sigma-Aldrich) was added to each dish (1 mL per dish) and incubated with the cells for 1 h at 37 °C. After incubation, the transparent CPC solution turned into purple, was diluted by four times in additional CPC solution (10%) and transferred to a 96-well plate (80 μL per well). Absorbance was read at 570 nm.
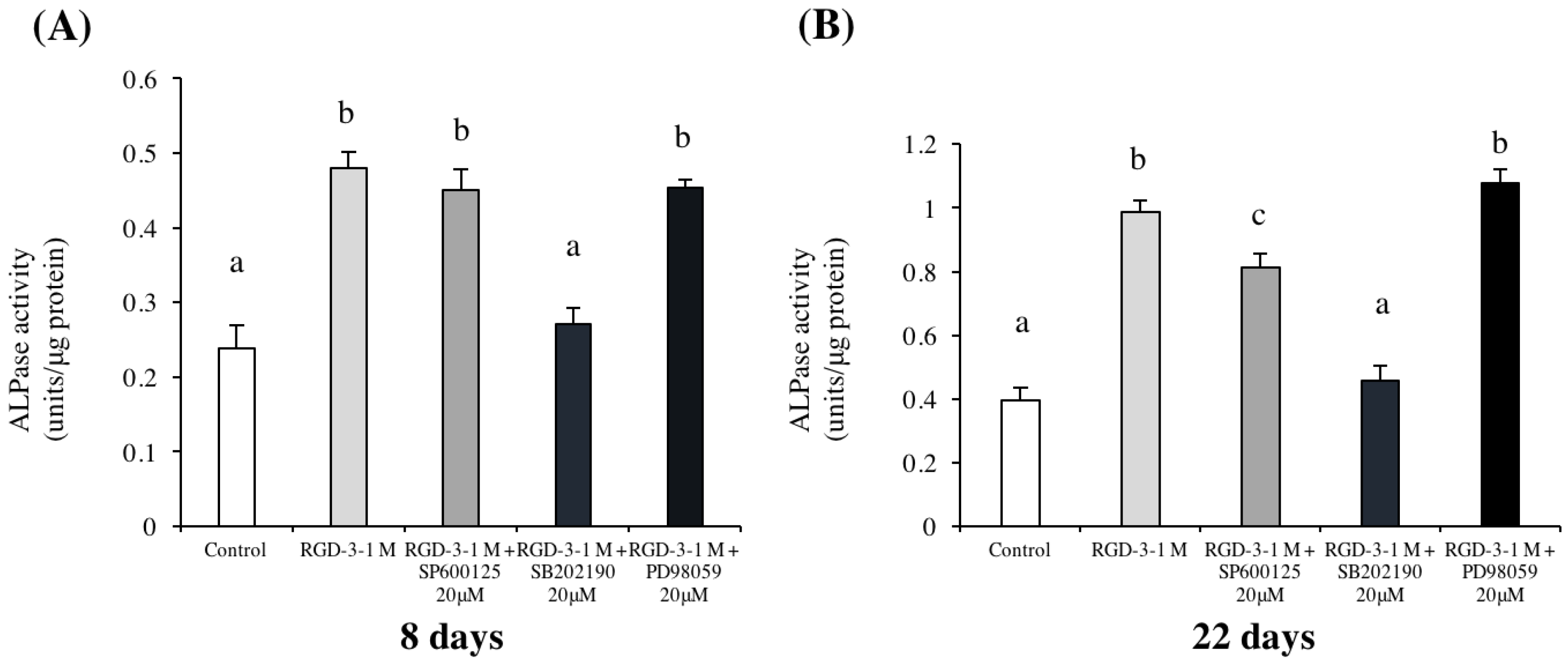
2.8. Effects of MAPK Inhibitors
To investigate the effects of the inhibitors on cell differentiation, cells were seeded at 2 × 104 cells/well in a 1 M RGD-3-coated 12-well microplate. To check the effects of the inhibitor on cell mineralization, cells were seeded at 2 × 104 cells/well in a 1 M RGD-3-coated 6-well microplate. Cells were challenged with 20 μM of one of three MAPK pathway inhibitors on day 6 in serum-free DMEM for 2 h, SP600125 (Cell Signaling Technology, Danvers, MA, USA) for c-Jun N-terminal kinase (JNK), SB202190 (Cell Signaling Technology) for p38 and PD98059 (Cell Signaling Technology) for extracellular signal-regulated kinase (ERK), respectively. Then, the medium was changed to DMEM supplemented with 10% FBS, 50 units/mL penicillin and 50 μg/mL streptomycin, and 10 mM β-GP and 50 μg/mL AA were added on day 7 when the cells reached confluence. ALP activity assays were performed on days 8 and 22. ARS staining was performed on day 31.
2.9. Statistical Analysis
All experiments were carried out in triplicate, and the results are expressed as the mean ± standard deviation. Data were subjected to one-way ANOVA (analysis of variance) and post-hoc Tukey HSD (honestly significant difference) for statistical analysis with significance level at p < 0.05.
4. Discussion
In the present study, the significance of RGD peptides in DPP sequence were observed in terms of differentiation and mineralization and the mechanism behind their effects was explored as well. The effects of RGD peptides were investigated by dissolving the RGD peptide powders with dH
2O and physically coating non-TCPS. The surfaces were left unwashed after the coating was dried, leaving all the peptides on the surfaces. Afterwards, hDPSCs were grown on the coated surfaces and their responses were observed. There was a noticeable difference in the number of cells attached on to the surfaces, starting from day one. This supports the previous statements from multiple studies that the RGD contains cell attachment properties, which may have played an active role in attracting more cells to the surface and eventually spreading out the cells faster. According to a study [
19], initial cell attachment rate is an indication of matrix mineralization. Thus, if human DPP-derived RGD peptides were to be included in future pulp capping materials, it could act as an attractor of odontoblasts in the site of exposed pulp and aid in a quicker wound healing.
The RGD peptides promoted the differentiation of hDPSCs, observed by the promotion of ALP activity and enhanced mRNA expressions of odontogenic genes
DMP-1,
DSPP,
ALP and
BSP. Among the three RGD peptides, RGD-3 induced the strongest ALP activity on all days of the experiment, the highest value on day 22 compared to the control. The isolated RNA samples showed significantly upregulated mRNA expressions in all three RGD peptides, specifically RGD-3 compared to the control. Related results were observed in mineralization experiment where the RGD-3 had the most prominent calcific stain on both days 29 and 31. One probable reason for this result could be that RGD-3 contains a lysine residue at the C-terminus (SRGDASYNSDESKD) in its amino acid chain. A study compared different peptides including RGD, and observed their attachment to self-assembled monolayers. Among the different flanking sequences, lysine-containing RGDSPK performed better than the other three peptides [
20]. In a further study, RGDSPK induced the highest level of ALP activity and mineralization of MC3T3-E1 osteoblastic cells [
21]. Based on these studies, it was suggested that the extra amine in the side chain of lysine becomes positively charged and nonspecifically interacts with the negatively charged glycocalyx on the cell surface. Thus, the specific binding of RGD-3 to cell integrins is supplemented by the nonspecific binding of lysine residues to the cell membrane, which explains the importance of the specific conformation of RGD-3. As a result, cell adhesion and subsequent differentiation and mineralization were enhanced in the RGD-3 group.
The RAD groups were not significantly different than the dH2O-coated control in differentiation and mineralization, which suggests the importance of the RGD domain and the specific conformation of RGD-3 in the DPP sequence, which may play a predominant role in extracellular matrix-mediated signalling and the differentiation of dental pulp stem cells into odontoblasts. Moreover, all three RGD-peptides induced enhanced ALP activity and led to strong calcific staining compared to the control, which may be related to their individual conformation in the DPP sequence. To better understand their mechanism and effective clinical application, further study is required.
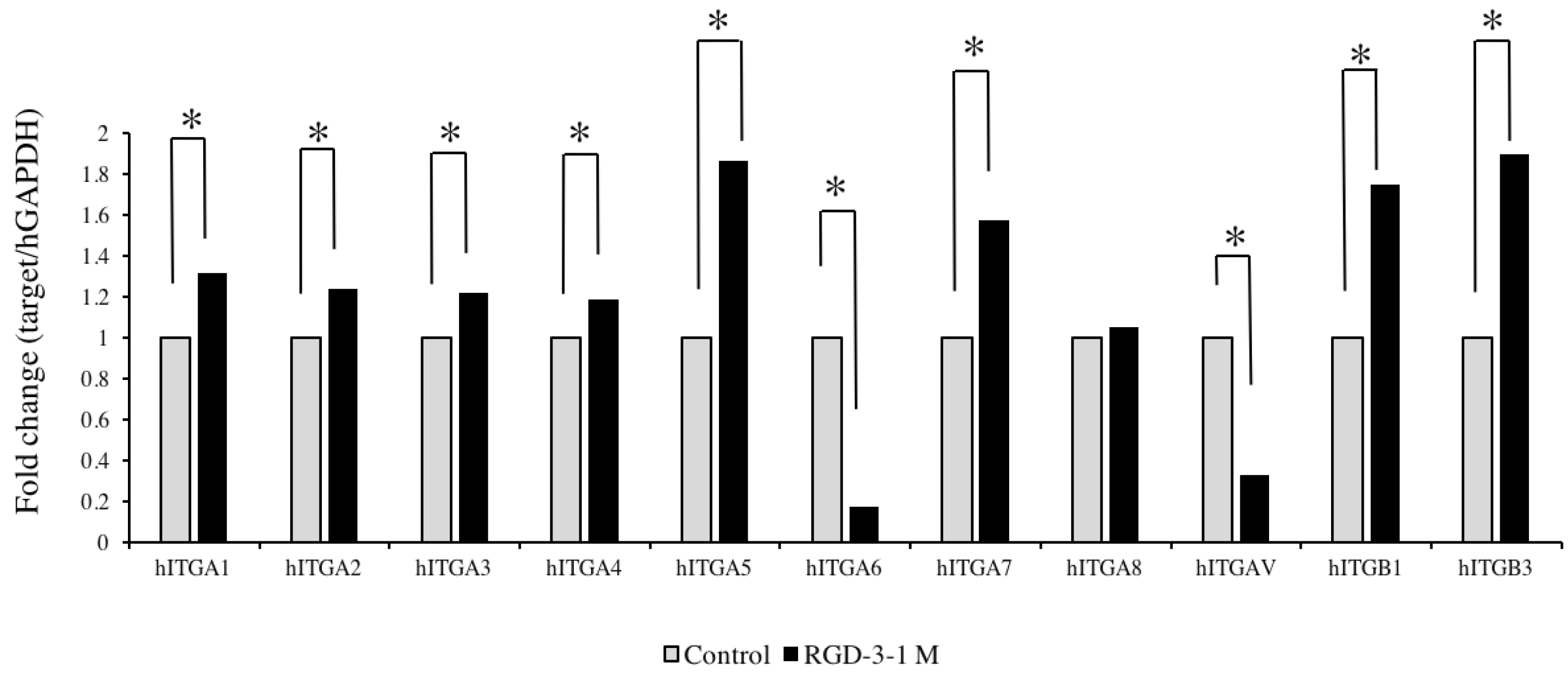
In continuation of the effects of human DPP-derived RGD peptides on hDPSCs, it was necessary to examine exactly how the signals transferred from extracellular space in to the cells, and if the RGD domain was involved in this process. That is where integrins and cell-signalling pathways come into discussion. Integrins, the cell adhesion receptors can transfer signals in and out of cells. Most integrins recognize multiple ECM proteins, and the specific configuration of their α and β structural subunits dictates the binding specificity and signalling properties of an individual integrin [
22]. There are different cell signal transduction pathways for integrins. The MAPK pathways are established signal-transduction pathways [
23,
24]. There have been reports that these pathways become activated during tertiary dentinogenesis stage [
25]. The three primary members of the protein kinase cascades in the MAPK pathways have vastly diverse regulatory domains, which enables them to respond to a wide range of stimulations [
26]. However, the fact that a single specific MAPK protein is activated in response to a specific ECM protein is associated with scaffolding or anchoring proteins. The MAPK pathways have been linked to the SIBLING family of proteins as the pathway for elucidating specific cellular responses. In the present study, three specific inhibitors were chosen for the three main MAPK pathways: the highly selective SP600125 inhibitor of JNK1/2/3 (Jun amino-terminal kinase), the cell permeable SB202190 inhibitor of p38 α and β subunits which lack the ability to impact other protein kinases; and lastly PD98059 that blocks ERK (extracellular signal regulated kinase) activation [
27]. It has been observed that Dex plays a role in promoting the differentiation and mineralization of rat odontoblast-like cells through MAPK pathway [
28], so to observe the unbiased effect, Dex was not present in the mineralization-inducing medium. Based on the results of the present study experiments, RGD-3 was selected for the experiments regarding MAPK inhibitors, and an ALP activity assay and Alizarin red S staining were performed. The inhibition of p38 signalling had an evident effect in suppression of the differentiation and mineralization, which were accelerated multiple folds by RGD-3. The ALP activity was reduced to a level comparable to the control, starting as early as day 8. Interestingly, inhibition of JNK pathway had also reduced ALP activity on day 22 but unlike the complete inhibitory effect of p38 inhibitor, the JNK inhibitor had partial inhibitory effect.
The communication between cell-surface integrins and ECM proteins is crucial to mediating signals inside of the cells. In a study by Jadlowiec et al. [
13], it was reported that phosphophoryn might function in osteoblastic gene expression by binding to certain αβ integrin receptors on the cell surface. Runx-2 mRNA expression was investigated and in response to an integrin-blocking α
vβ
3 antibody, the mRNA expression of Runx-2 decreased by almost 60% in human mesenchymal stem cells. In the present study, RGD-3 triggered the upregulation of
ITGA1-5,
ITGA7,
ITGB1 and
ITGB3 compared to the control. Based on the remarkable upregulation in the present study, there is a possibility that integrins
ITGA5,
ITGA7,
ITGB1 and
ITGB3, either alone or in combination, might be involved in intracellular signalling in hDPSCs.