Hypoxia Induces DPSC Differentiation versus a Neurogenic Phenotype by the Paracrine Mechanism
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.1.1. DPSCs
2.1.2. SH-SY5Y
2.2. DPSC Proliferation Assay
2.3. Phalloidin Staining of the DPSCs
2.4. DPSC Hypoxic Culture Regime
2.5. In Vitro DPSC Neuronal Differentiation
2.6. Preparation of the Conditioned Media from Hypoxic Culture
2.7. Flow Cytometry Analysis
2.8. Reverse Transcription-Quantitative PCR (RT-qPCR) Analysis
2.9. ELISA for EGF and bFGF
2.10. Immunofluorescence Analysis
2.11. Statistical Analysis
3. Results
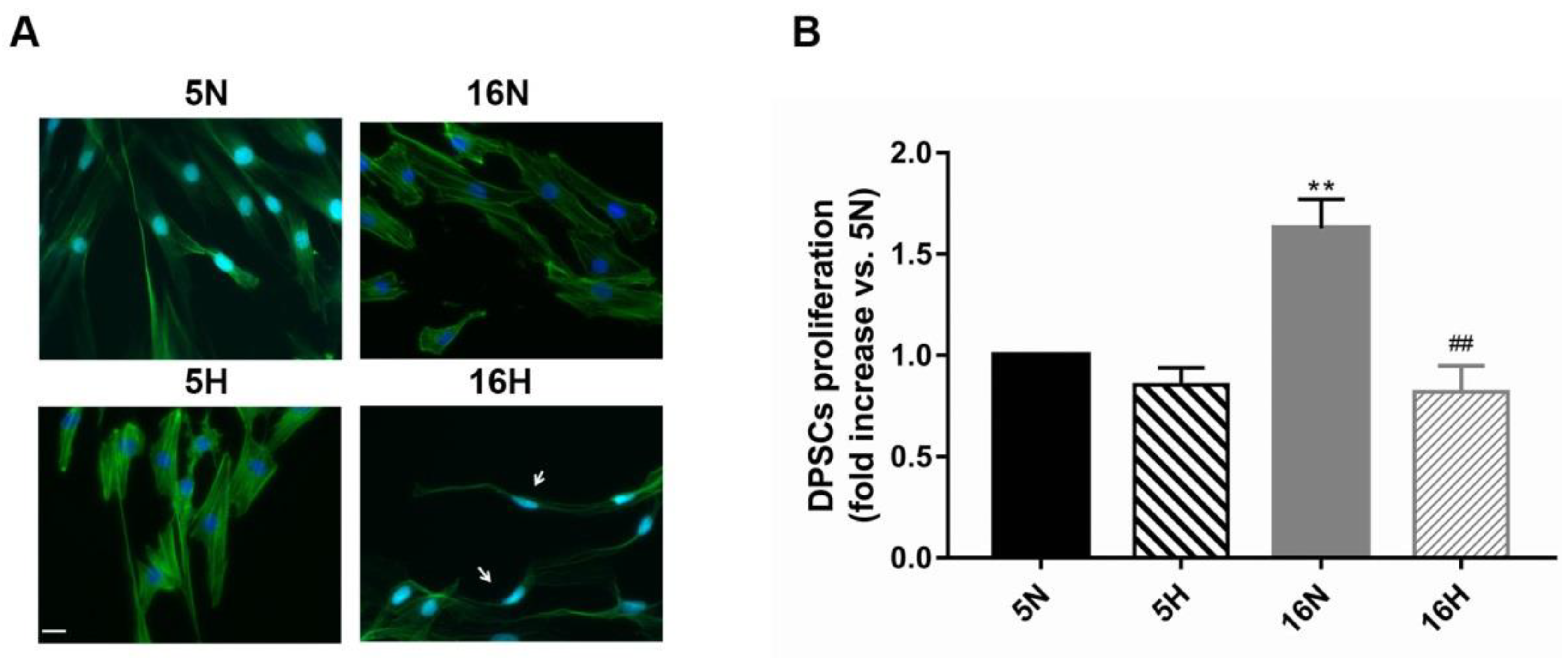
3.1. Determination of Hypoxia Effects on the Proliferation and Morphological Features of DPSCs
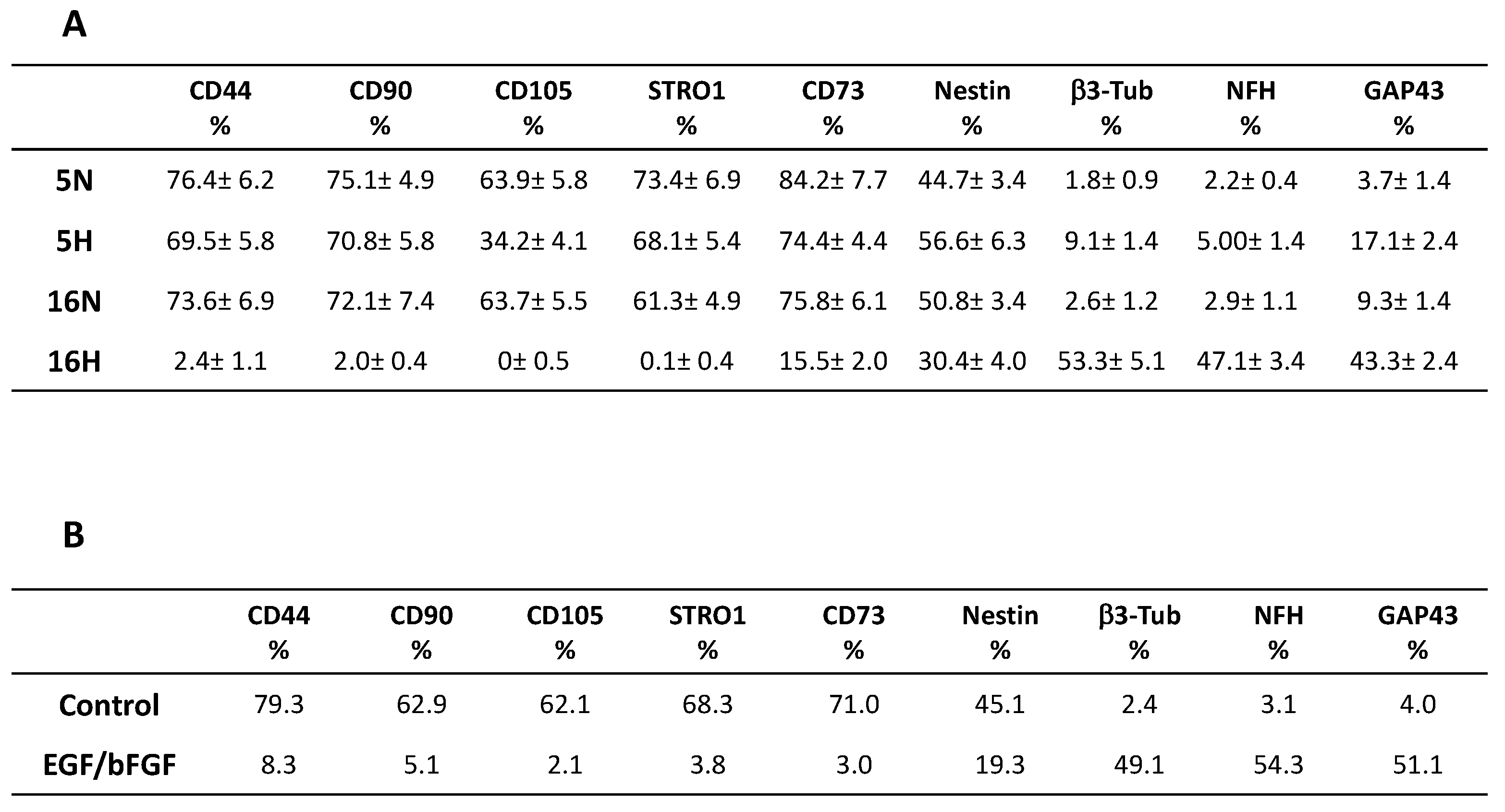
3.2. Comparative Characterization of DPSCs’ Stem and Neuronal Markers by Flow Cytometry
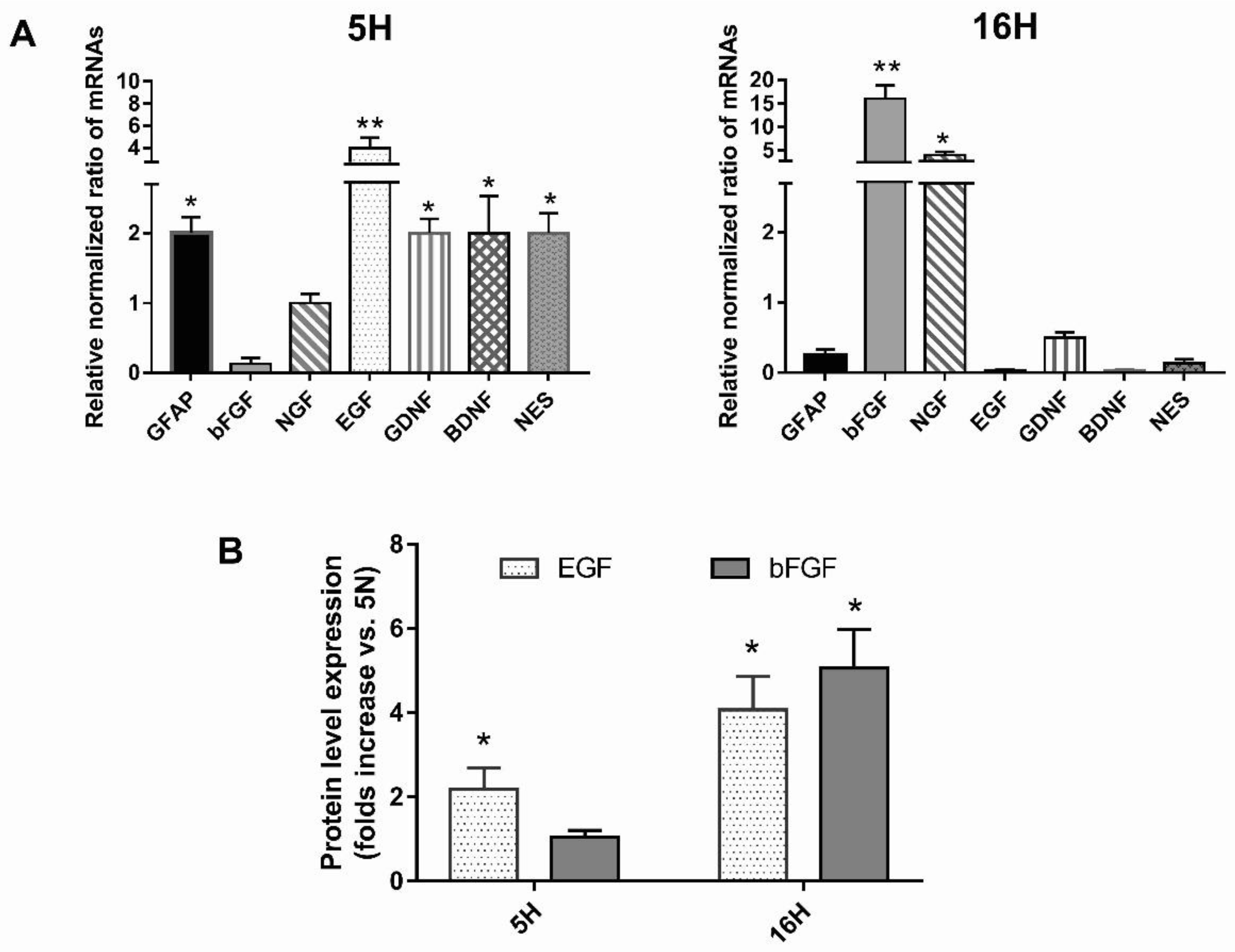
3.3. The Profiling of Differential mRNA Expressed in DPSCs under Hypoxic or Normoxic Conditions
3.4. Growth Factor Expression in Hypoxic or Normoxic DPSCs’ Conditioned Media (CM)
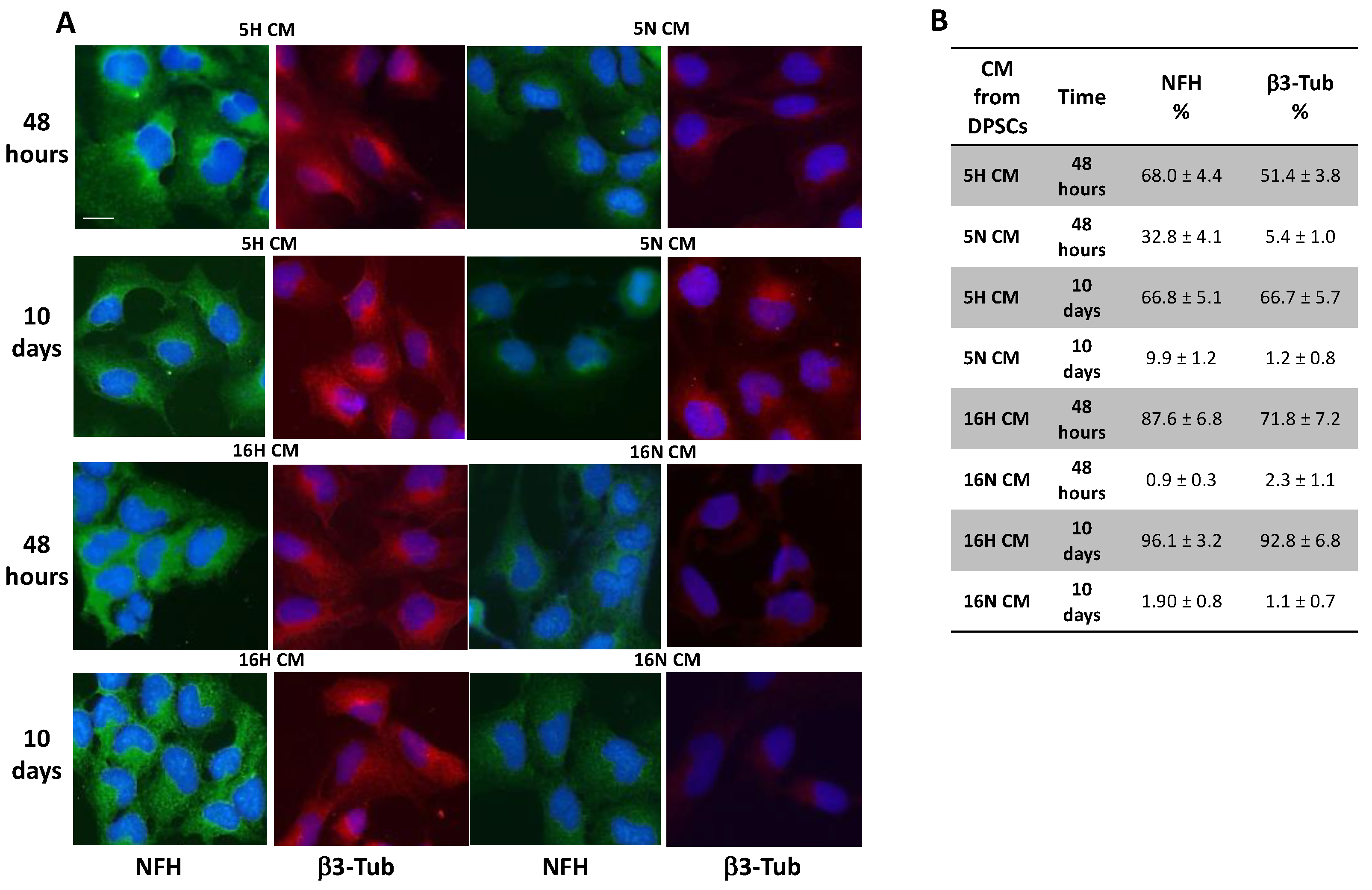
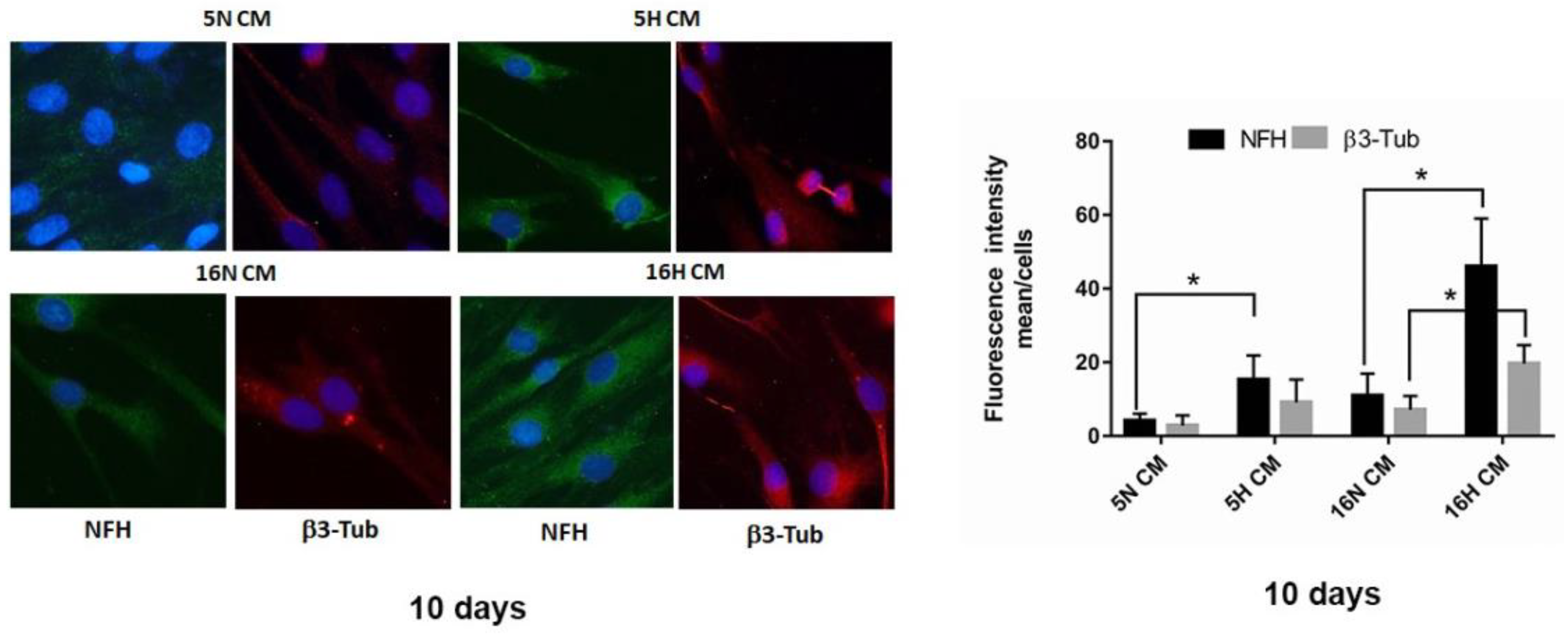
3.5. Analysis of the Paracrine Induction of the Neuronal Phenotype by Immunocytochemistry and Flow Cytometry
3.6. Phenotype Characterization of DPSCs by Flow Cytometry
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Al Madhoun, A.; Sindhu, S.; Haddad, D.; Atari, M.; Ahmad, R.; Al-Mulla, F. Dental Pulp Stem Cells Derived From Adult Human Third Molar Tooth: A Brief Review. Front. Cell Dev. Biol. 2021, 9, 717624. [Google Scholar] [CrossRef] [PubMed]
- Diomede, F.; Fonticoli, L.; Marconi, G.D.; Della Rocca, Y.; Rajan, T.S.; Trubiani, O.; Murmura, G.; Pizzicannella, J. Decellularized Dental Pulp, Extracellular Vesicles, and 5-Azacytidine: A New Tool for Endodontic Regeneration. Biomedicines 2022, 10, 403. [Google Scholar] [CrossRef] [PubMed]
- Tatullo, M.; Marrelli, M.; Shakesheff, K.M.; White, L.J. Dental pulp stem cells: Function, isolation and applications in regenerative medicine. J. Tissue Eng. Regen. Med. 2015, 9, 1205–1216. [Google Scholar] [CrossRef]
- Anitua, E.; Troya, M.; Zalduendo, M. Progress in the use of dental pulp stem cells in regenerative medicine. Cytotherapy 2018, 20, 479–498. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.; McKee, C.; Bakshi, S.; Walker, K.; Hakman, E.; Halassy, S.; Svinarich, D.; Dodds, R.; Govind, C.K.; Chaudhry, G.R. Mesenchymal stem cells: Cell therapy and regeneration potential. J. Tissue Eng. Regen. Med. 2019, 13, 1738–1755. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Lei, T.; Liu, Y.; Yang, Y.; Bi, W.; Du, H. The potential therapy with dental tissue-derived mesenchymal stem cells in Parkinson’s disease. Stem Cell Res. Ther. 2021, 12, 5. [Google Scholar] [CrossRef] [PubMed]
- Mattei, V.; Santacroce, C.; Tasciotti, V.; Martellucci, S.; Santilli, F.; Manganelli, V.; Piccoli, L.; Misasi, R.; Sorice, M.; Garofalo, T. Role of lipid rafts in neuronal differentiation of dental pulp-derived stem cells. Exp. Cell Res. 2015, 339, 231–240. [Google Scholar] [CrossRef]
- Aydin, S.; Sahin, F. Stem Cells Derived from Dental Tissues. Adv. Exp. Med. Biol. 2019, 1144, 123–132. [Google Scholar] [CrossRef]
- Martellucci, S.; Santacroce, C.; Manganelli, V.; Santilli, F.; Piccoli, L.; Cassetta, M.; Misasi, R.; Sorice, M.; Mattei, V. Isolation, Propagation, and Prion Protein Expression During Neuronal Differentiation of Human Dental Pulp Stem Cells. J. Vis. Exp. JoVE 2019, 18, e59282. [Google Scholar] [CrossRef] [Green Version]
- Delle Monache, S.; Pulcini, F.; Frosini, R.; Mattei, V.; Talesa, V.N.; Antognelli, C. Methylglyoxal-Dependent Glycative Stress Is Prevented by the Natural Antioxidant Oleuropein in Human Dental Pulp Stem Cells through Nrf2/Glo1 Pathway. Antioxidants 2021, 10, 716. [Google Scholar] [CrossRef]
- Liu, J.; Yu, F.; Sun, Y.; Jiang, B.; Zhang, W.; Yang, J.; Xu, G.T.; Liang, A.; Liu, S. Concise reviews: Characteristics and potential applications of human dental tissue-derived mesenchymal stem cells. Stem Cells 2015, 33, 627–638. [Google Scholar] [CrossRef] [PubMed]
- Nuti, N.; Corallo, C.; Chan, B.M.; Ferrari, M.; Gerami-Naini, B. Multipotent Differentiation of Human Dental Pulp Stem Cells: A Literature Review. Stem Cell Rev. Rep. 2016, 12, 511–523. [Google Scholar] [CrossRef] [PubMed]
- Martellucci, S.; Santacroce, C.; Santilli, F.; Piccoli, L.; Delle Monache, S.; Angelucci, A.; Misasi, R.; Sorice, M.; Mattei, V. Cellular and Molecular Mechanisms Mediated by recPrP(C) Involved in the Neuronal Differentiation Process of Mesenchymal Stem Cells. Int. J. Mol. Sci. 2019, 20, 345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martellucci, S.; Manganelli, V.; Santacroce, C.; Santilli, F.; Piccoli, L.; Sorice, M.; Mattei, V. Role of Prion protein-EGFR multimolecular complex during neuronal differentiation of human dental pulp-derived stem cells. Prion 2018, 12, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Delle Monache, S.; Martellucci, S.; Clementi, L.; Pulcini, F.; Santilli, F.; Mei, C.; Piccoli, L.; Angelucci, A.; Mattei, V. In Vitro Conditioning Determines the Capacity of Dental Pulp Stem Cells to Function as Pericyte-Like Cells. Stem Cells Dev. 2019, 28, 695–706. [Google Scholar] [CrossRef]
- Mattei, V.; Martellucci, S.; Pulcini, F.; Santilli, F.; Sorice, M.; Delle Monache, S. Regenerative Potential of DPSCs and Revascularization: Direct, Paracrine or Autocrine Effect? Stem Cell Rev. Rep. 2021, 17, 1635–1646. [Google Scholar] [CrossRef]
- Yoshida, S.; Tomokiyo, A.; Hasegawa, D.; Hamano, S.; Sugii, H.; Maeda, H. Insight into the Role of Dental Pulp Stem Cells in Regenerative Therapy. Biology 2020, 9, 160. [Google Scholar] [CrossRef]
- Bakopoulou, A.; About, I. Stem Cells of Dental Origin: Current Research Trends and Key Milestones towards Clinical Application. Stem Cells Int. 2016, 2016, 4209891. [Google Scholar] [CrossRef] [Green Version]
- Liu, P.; Zhang, Y.; Ma, Y.; Tan, S.; Ren, B.; Liu, S.; Dai, H.; Xu, Z. Application of dental pulp stem cells in oral maxillofacial tissue engineering. Int. J. Med. Sci. 2022, 19, 310–320. [Google Scholar] [CrossRef]
- Zhai, Q.; Dong, Z.; Wang, W.; Li, B.; Jin, Y. Dental stem cell and dental tissue regeneration. Front. Med. 2019, 13, 152–159. [Google Scholar] [CrossRef]
- Kawashima, N.; Okiji, T. Odontoblasts: Specialized hard-tissue-forming cells in the dentin-pulp complex. Congenit. Anom. 2016, 56, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Saito, M.T.; Silverio, K.G.; Casati, M.Z.; Sallum, E.A.; Nociti, F.H., Jr. Tooth-derived stem cells: Update and perspectives. World J. Stem Cells 2015, 7, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Botelho, J.; Cavacas, M.A.; Machado, V.; Mendes, J.J. Dental stem cells: Recent progresses in tissue engineering and regenerative medicine. Ann. Med. 2017, 49, 644–651. [Google Scholar] [CrossRef] [PubMed]
- Navabazam, A.R.; Sadeghian Nodoshan, F.; Sheikhha, M.H.; Miresmaeili, S.M.; Soleimani, M.; Fesahat, F. Characterization of mesenchymal stem cells from human dental pulp, preapical follicle and periodontal ligament. Iran. J. Reprod. Med. 2013, 11, 235–242. [Google Scholar]
- Luo, J.; Dou, L.; Yang, Z.; Zhou, Z.; Huang, H. CBFA2T2 promotes adipogenic differentiation of mesenchymal stem cells by regulating CEBPA. Biochem. Biophys. Res. Commun. 2020, 529, 133–139. [Google Scholar] [CrossRef]
- Honda, R. Role of the Disulfide Bond in Prion Protein Amyloid Formation: A Thermodynamic and Kinetic Analysis. Biophys. J. 2018, 114, 885–892. [Google Scholar] [CrossRef] [Green Version]
- Akpinar, G.; Kasap, M.; Aksoy, A.; Duruksu, G.; Gacar, G.; Karaoz, E. Phenotypic and proteomic characteristics of human dental pulp derived mesenchymal stem cells from a natal, an exfoliated deciduous, and an impacted third molar tooth. Stem Cells Int. 2014, 2014, 457059. [Google Scholar] [CrossRef]
- Delle Monache, S.; Calgani, A.; Sanita, P.; Zazzeroni, F.; Gentile Warschauer, E.; Giuliani, A.; Amicucci, G.; Angelucci, A. Adipose-derived stem cells sustain prolonged angiogenesis through leptin secretion. Growth Factors 2016, 34, 87–96. [Google Scholar] [CrossRef]
- Xing, J.; Lian, M.; Shen, Q.; Feng, G.; Huang, D.; Lu, X.; Gu, Z.; Li, L.; Zhang, J.; Huang, S.; et al. AGS3 is involved in TNF-alpha medicated osteogenic differentiation of human dental pulp stem cells. Differ. Res. Biol. Divers. 2015, 89, 128–136. [Google Scholar] [CrossRef]
- Mortada, I.; Mortada, R. Dental pulp stem cells and osteogenesis: An update. Cytotechnology 2018, 70, 1479–1486. [Google Scholar] [CrossRef]
- Labedz-Maslowska, A.; Bryniarska, N.; Kubiak, A.; Kaczmarzyk, T.; Sekula-Stryjewska, M.; Noga, S.; Boruczkowski, D.; Madeja, Z.; Zuba-Surma, E. Multilineage Differentiation Potential of Human Dental Pulp Stem Cells-Impact of 3D and Hypoxic Environment on Osteogenesis In Vitro. Int. J. Mol. Sci. 2020, 21, 6172. [Google Scholar] [CrossRef] [PubMed]
- Ching, H.S.; Luddin, N.; Rahman, I.A.; Ponnuraj, K.T. Expression of Odontogenic and Osteogenic Markers in DPSCs and SHED: A Review. Curr. Stem Cell Res. Ther. 2017, 12, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Wang, J.; Lu, J.; Zou, D.; Sun, H.; Dong, Y.; Yu, H.; Zhang, L.; Yang, T.; Zhang, X.; et al. The effect of co-culturing costal chondrocytes and dental pulp stem cells combined with exogenous FGF9 protein on chondrogenesis and ossification in engineered cartilage. Biomaterials 2012, 33, 7699–7711. [Google Scholar] [CrossRef]
- Janebodin, K.; Horst, O.V.; Ieronimakis, N.; Balasundaram, G.; Reesukumal, K.; Pratumvinit, B.; Reyes, M. Isolation and characterization of neural crest-derived stem cells from dental pulp of neonatal mice. PLoS ONE 2011, 6, e27526. [Google Scholar] [CrossRef] [PubMed]
- Darvishi, M.; Hamidabadi, H.G.; Bojnordi, M.N.; Saeednia, S.; Zahiri, M.; Niapour, A.; Alizadeh, R. Differentiation of human dental pulp stem cells into functional motor neuron: In vitro and ex vivo study. Tissue Cell 2021, 72, 101542. [Google Scholar] [CrossRef]
- Yang, C.; Li, X.; Sun, L.; Guo, W.; Tian, W. Potential of human dental stem cells in repairing the complete transection of rat spinal cord. J. Neural Eng. 2017, 14, 026005. [Google Scholar] [CrossRef]
- Xiao, L.; Ide, R.; Saiki, C.; Kumazawa, Y.; Okamura, H. Human Dental Pulp Cells Differentiate toward Neuronal Cells and Promote Neuroregeneration in Adult Organotypic Hippocampal Slices In Vitro. Int. J. Mol. Sci. 2017, 18, 1745. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Zou, X.Y.; El-Ayachi, I.; Romero, L.O.; Yu, Z.; Iglesias-Linares, A.; Cordero-Morales, J.F.; Huang, G.T. Human Dental Pulp Stem Cells and Gingival Mesenchymal Stem Cells Display Action Potential Capacity In Vitro after Neuronogenic Differentiation. Stem Cell Rev. Rep. 2019, 15, 67–81. [Google Scholar] [CrossRef]
- Vaseenon, S.; Chattipakorn, N.; Chattipakorn, S.C. The possible role of basic fibroblast growth factor in dental pulp. Arch. Oral Biol. 2020, 109, 104574. [Google Scholar] [CrossRef]
- Zheng, K.; Feng, G.; Zhang, J.; Xing, J.; Huang, D.; Lian, M.; Zhang, W.; Wu, W.; Hu, Y.; Lu, X.; et al. Basic fibroblast growth factor promotes human dental pulp stem cells cultured in 3D porous chitosan scaffolds to neural differentiation. Int. J. Neurosci. 2021, 131, 625–633. [Google Scholar] [CrossRef]
- Rafiee, F.; Pourteymourfard-Tabrizi, Z.; Mahmoudian-Sani, M.R.; Mehri-Ghahfarrokhi, A.; Soltani, A.; Hashemzadeh-Chaleshtori, M.; Jami, M.S. Differentiation of dental pulp stem cells into neuron-like cells. Int. J. Neurosci. 2020, 130, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Rocco, M.L.; Soligo, M.; Manni, L.; Aloe, L. Nerve Growth Factor: Early Studies and Recent Clinical Trials. Curr. Neuropharmacol. 2018, 16, 1455–1465. [Google Scholar] [CrossRef] [PubMed]
- Alastra, G.; Aloe, L.; Baldassarro, V.A.; Calza, L.; Cescatti, M.; Duskey, J.T.; Focarete, M.L.; Giacomini, D.; Giardino, L.; Giraldi, V.; et al. Nerve Growth Factor Biodelivery: A Limiting Step in Moving Toward Extensive Clinical Application? Front. Neurosci. 2021, 15, 695592. [Google Scholar] [CrossRef] [PubMed]
- Werle, S.B.; Chagastelles, P.; Pranke, P.; Casagrande, L. The effects of hypoxia on in vitro culture of dental-derived stem cells. Arch. Oral Biol. 2016, 68, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Michiels, C. Physiological and pathological responses to hypoxia. Am. J. Pathol. 2004, 164, 1875–1882. [Google Scholar] [CrossRef] [Green Version]
- Cristofaro, I.; Limongi, C.; Piscopo, P.; Crestini, A.; Guerriero, C.; Fiore, M.; Conti, L.; Confaloni, A.; Tata, A.M. M2 Receptor Activation Counteracts the Glioblastoma Cancer Stem Cell Response to Hypoxia Condition. Int. J. Mol. Sci. 2020, 21, 1700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lendahl, U.; Lee, K.L.; Yang, H.; Poellinger, L. Generating specificity and diversity in the transcriptional response to hypoxia. Nat. Rev. Genet. 2009, 10, 821–832. [Google Scholar] [CrossRef]
- Ahmed, N.E.; Murakami, M.; Kaneko, S.; Nakashima, M. The effects of hypoxia on the stemness properties of human dental pulp stem cells (DPSCs). Sci. Rep. 2016, 6, 35476. [Google Scholar] [CrossRef] [Green Version]
- Kwon, S.Y.; Chun, S.Y.; Ha, Y.S.; Kim, D.H.; Kim, J.; Song, P.H.; Kim, H.T.; Yoo, E.S.; Kim, B.S.; Kwon, T.G. Hypoxia Enhances Cell Properties of Human Mesenchymal Stem Cells. Tissue Eng. Regen. Med. 2017, 14, 595–604. [Google Scholar] [CrossRef]
- Sarra, G.; Machado, M.E.L.; Caballero-Flores, H.V.; Moreira, M.S.; Pedroni, A.C.F.; Marques, M.M. Effect of human dental pulp stem cell conditioned medium in the dentin-pulp complex regeneration: A pilot in vivo study. Tissue Cell 2021, 72, 101536. [Google Scholar] [CrossRef]
- Gunawardena, T.N.A.; Rahman, M.T.; Abdullah, B.J.J.; Abu Kasim, N.H. Conditioned media derived from mesenchymal stem cell cultures: The next generation for regenerative medicine. J. Tissue Eng. Regen. Med. 2019, 13, 569–586. [Google Scholar] [CrossRef] [PubMed]
- Li, X.X.; Yuan, X.J.; Zhai, Y.; Yu, S.; Jia, R.X.; Yang, L.P.; Ma, Z.Z.; Zhao, Y.M.; Wang, Y.X.; Ge, L.H. Treatment with Stem Cells from Human Exfoliated Deciduous Teeth and Their Derived Conditioned Medium Improves Retinal Visual Function and Delays the Degeneration of Photoreceptors. Stem Cells Dev. 2019, 28, 1514–1526. [Google Scholar] [CrossRef] [PubMed]
- Gugliandolo, A.; Fonticoli, L.; Trubiani, O.; Rajan, T.S.; Marconi, G.D.; Bramanti, P.; Mazzon, E.; Pizzicannella, J.; Diomede, F. Oral Bone Tissue Regeneration: Mesenchymal Stem Cells, Secretome, and Biomaterials. Int. J. Mol. Sci. 2021, 22, 5236. [Google Scholar] [CrossRef] [PubMed]
- Bar, J.K.; Lis-Nawara, A.; Grelewski, P.G. Dental Pulp Stem Cell-Derived Secretome and Its Regenerative Potential. Int. J. Mol. Sci. 2021, 22, 12018. [Google Scholar] [CrossRef]
- Lin, H.; Chen, H.; Zhao, X.; Chen, Z.; Zhang, P.; Tian, Y.; Wang, Y.; Ding, T.; Wang, L.; Shen, Y. Advances in mesenchymal stem cell conditioned medium-mediated periodontal tissue regeneration. J. Transl. Med. 2021, 19, 456. [Google Scholar] [CrossRef]
- Arthur, A.; Rychkov, G.; Shi, S.; Koblar, S.A.; Gronthos, S. Adult human dental pulp stem cells differentiate toward functionally active neurons under appropriate environmental cues. Stem Cells 2008, 26, 1787–1795. [Google Scholar] [CrossRef] [Green Version]
- Kanao, S.; Ogura, N.; Takahashi, K.; Ito, K.; Suemitsu, M.; Kuyama, K.; Kondoh, T. Capacity of Human Dental Follicle Cells to Differentiate into Neural Cells In Vitro. Stem Cells Int. 2017, 2017, 8371326. [Google Scholar] [CrossRef] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−∆∆C(T) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Couto de Carvalho, L.A.; Tosta Dos Santos, S.L.; Sacramento, L.V.; de Almeida, V.R.J.; de Aquino Xavier, F.C.; Dos Santos, J.N.; Gomes Henriques Leitao, A.C. Mesenchymal stem cell markers in periodontal tissues and periapical lesions. Acta Histochem. 2020, 122, 151636. [Google Scholar] [CrossRef]
- Kalinovskii, A.P.; Osmakov, D.I.; Koshelev, S.G.; Lubova, K.I.; Korolkova, Y.V.; Kozlov, S.A.; Andreev, Y.A. Retinoic Acid-Differentiated Neuroblastoma SH-SY5Y Is an Accessible In Vitro Model to Study Native Human Acid-Sensing Ion Channels 1a (ASIC1a). Biology 2022, 11, 167. [Google Scholar] [CrossRef]
- Lavorato, A.; Raimondo, S.; Boido, M.; Muratori, L.; Durante, G.; Cofano, F.; Vincitorio, F.; Petrone, S.; Titolo, P.; Tartara, F.; et al. Mesenchymal Stem Cell Treatment Perspectives in Peripheral Nerve Regeneration: Systematic Review. Int. J. Mol. Sci. 2021, 22, 572. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, Z.; Jiang, Y.; Niu, Z.; Fu, L.; Luo, Z.; Cooper, P.R.; Smith, A.J.; He, W. Nuclear Factor I-C promotes proliferation and differentiation of apical papilla-derived human stem cells in vitro. Exp. Cell Res. 2015, 332, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Vaidya, M. Hypoxia inhibits mesenchymal stem cell proliferation through HIF1alpha-dependent regulation of P27. Mol. Cell. Biochem. 2016, 415, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Zainal Ariffin, S.H.; Kermani, S.; Zainol Abidin, I.Z.; Megat Abdul Wahab, R.; Yamamoto, Z.; Senafi, S.; Zainal Ariffin, Z.; Abdul Razak, M. Differentiation of dental pulp stem cells into neuron-like cells in serum-free medium. Stem Cells Int. 2013, 2013, 250740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Lian, M.; Cao, P.; Bao, G.; Xu, G.; Sun, Y.; Wang, L.; Chen, J.; Wang, Y.; Feng, G.; et al. Effects of Nerve Growth Factor and Basic Fibroblast Growth Factor Promote Human Dental Pulp Stem Cells to Neural Differentiation. Neurochem. Res. 2017, 42, 1015–1025. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Albashari, A.A.; Wang, X.; Jin, L.; Zhang, Y.; Zheng, L.; Xia, J.; Xu, H.; Zhao, Y.; Xiao, J.; et al. Effects of Transplanted Heparin-Poloxamer Hydrogel Combining Dental Pulp Stem Cells and bFGF on Spinal Cord Injury Repair. Stem Cells Int. 2018, 2018, 2398521. [Google Scholar] [CrossRef] [Green Version]
- Kovalevich, J.; Langford, D. Considerations for the use of SH-SY5Y neuroblastoma cells in neurobiology. Methods Mol. Biol. 2013, 1078, 9–21. [Google Scholar] [CrossRef] [Green Version]
- Nosrat, I.V.; Smith, C.A.; Mullally, P.; Olson, L.; Nosrat, C.A. Dental pulp cells provide neurotrophic support for dopaminergic neurons and differentiate into neurons in vitro; implications for tissue engineering and repair in the nervous system. Eur. J. Neurosci. 2004, 19, 2388–2398. [Google Scholar] [CrossRef] [Green Version]
- Ueda, T.; Inden, M.; Ito, T.; Kurita, H.; Hozumi, I. Characteristics and Therapeutic Potential of Dental Pulp Stem Cells on Neurodegenerative Diseases. Front. Neurosci. 2020, 14, 407. [Google Scholar] [CrossRef]
- Ullah, I.; Subbarao, R.B.; Kim, E.J.; Bharti, D.; Jang, S.J.; Park, J.S.; Shivakumar, S.B.; Lee, S.L.; Kang, D.; Byun, J.H.; et al. In vitro comparative analysis of human dental stem cells from a single donor and its neuronal differentiation potential evaluated by electrophysiology. Life Sci. 2016, 154, 39–51. [Google Scholar] [CrossRef] [Green Version]
- Chun, S.Y.; Soker, S.; Jang, Y.J.; Kwon, T.G.; Yoo, E.S. Differentiation of Human Dental Pulp Stem Cells into Dopaminergic Neuron-like Cells in Vitro. J. Korean Med. Sci. 2016, 31, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Gancheva, M.R.; Kremer, K.L.; Gronthos, S.; Koblar, S.A. Using Dental Pulp Stem Cells for Stroke Therapy. Front. Neurol. 2019, 10, 422. [Google Scholar] [CrossRef] [PubMed]
- Mead, B.; Logan, A.; Berry, M.; Leadbeater, W.; Scheven, B.A. Intravitreally transplanted dental pulp stem cells promote neuroprotection and axon regeneration of retinal ganglion cells after optic nerve injury. Investig. Ophthalmol. Vis. Sci. 2013, 54, 7544–7556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Sanchez, D.; Fernandez, D.; Rodriguez-Rey, J.C.; Perez-Campo, F.M. Enhancing survival, engraftment, and osteogenic potential of mesenchymal stem cells. World J. Stem Cells 2019, 11, 748–763. [Google Scholar] [CrossRef] [PubMed]
- Ide, C.; Nakai, Y.; Nakano, N.; Seo, T.B.; Yamada, Y.; Endo, K.; Noda, T.; Saito, F.; Suzuki, Y.; Fukushima, M.; et al. Bone marrow stromal cell transplantation for treatment of sub-acute spinal cord injury in the rat. Brain Res. 2010, 1332, 32–47. [Google Scholar] [CrossRef]
- Zhang, X.M.; Ouyang, Y.J.; Yu, B.Q.; Li, W.; Yu, M.Y.; Li, J.Y.; Jiao, Z.M.; Yang, D.; Li, N.; Shi, Y.; et al. Therapeutic potential of dental pulp stem cell transplantation in a rat model of Alzheimer’s disease. Neural Regen. Res. 2021, 16, 893–898. [Google Scholar] [CrossRef]

| Abbreviations | Type of Treatment |
|---|---|
| 5N | DPSCs for 5 days in normoxia |
| 5H | DPSCs for 5 days in hypoxia |
| 16N | DPSCs for 16 days in normoxia |
| 16H | DPSCs for 16 days in hypoxia |
| Abbreviations | Type of Conditioned Media |
|---|---|
| 5N CM | Conditioned media derived from normoxic DPSCs for 5 days |
| 5H CM | Conditioned media derived from hypoxic DPSCs for 5 days |
| 16N CM | Conditioned media derived from normoxic DPSCs for 16 days |
| 16H CM | Conditioned media derived from hypoxic DPSCs for 16 days |
| Gene | Forward Primer | Reverse Primer | Tm Value |
|---|---|---|---|
| GAPDH | AGGTGAAGGTCGGAGTCAAC | CCATGTAGTTGAGGTCAATGAAG | 58–65 |
| GFAP | TAGAGGGCGAGGAGAACCG | GTGGCCTTCTGACACAGACTTG | 64 |
| bFGF | CTGTACTGCAAAAACGGG | AAAGTATAGCTTTCTGCC | 63 |
| NGF | CATGCTGGACCCAAGCTCA | GACATTACGCTATGCACCTCAGTG | 60 |
| EGF | GGTCAATGCAACCAACTTCA | GGCATTGAGTAGGTGATTAG | 63 |
| GDNF | TCAAATATGCCAGAGGATTATCCTG | GCCATTTGTTTATCTGGTGACCTT | 64 |
| BDNF | AGCTCCGGGTTGGTATACT | CCTGGTGGAACTTCTTTGCG | 64 |
| NES | TGGCAAAGGAGCCTACTCCAAGAA | ATCGGGATTCAGCTGACTTAGCCT | 65 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delle Monache, S.; Pulcini, F.; Santilli, F.; Martellucci, S.; Santacroce, C.; Fabrizi, J.; Angelucci, A.; Sorice, M.; Mattei, V. Hypoxia Induces DPSC Differentiation versus a Neurogenic Phenotype by the Paracrine Mechanism. Biomedicines 2022, 10, 1056. https://doi.org/10.3390/biomedicines10051056
Delle Monache S, Pulcini F, Santilli F, Martellucci S, Santacroce C, Fabrizi J, Angelucci A, Sorice M, Mattei V. Hypoxia Induces DPSC Differentiation versus a Neurogenic Phenotype by the Paracrine Mechanism. Biomedicines. 2022; 10(5):1056. https://doi.org/10.3390/biomedicines10051056
Chicago/Turabian StyleDelle Monache, Simona, Fanny Pulcini, Francesca Santilli, Stefano Martellucci, Costantino Santacroce, Jessica Fabrizi, Adriano Angelucci, Maurizio Sorice, and Vincenzo Mattei. 2022. "Hypoxia Induces DPSC Differentiation versus a Neurogenic Phenotype by the Paracrine Mechanism" Biomedicines 10, no. 5: 1056. https://doi.org/10.3390/biomedicines10051056
APA StyleDelle Monache, S., Pulcini, F., Santilli, F., Martellucci, S., Santacroce, C., Fabrizi, J., Angelucci, A., Sorice, M., & Mattei, V. (2022). Hypoxia Induces DPSC Differentiation versus a Neurogenic Phenotype by the Paracrine Mechanism. Biomedicines, 10(5), 1056. https://doi.org/10.3390/biomedicines10051056










