Profibrotic Effects of Endothelin-1 on Fibroblasts Are Mediated by Aldosterone In Vitro: Relevance to the Pathogenesis and Therapy of Systemic Sclerosis and Pulmonary Arterial Hypertension
Abstract
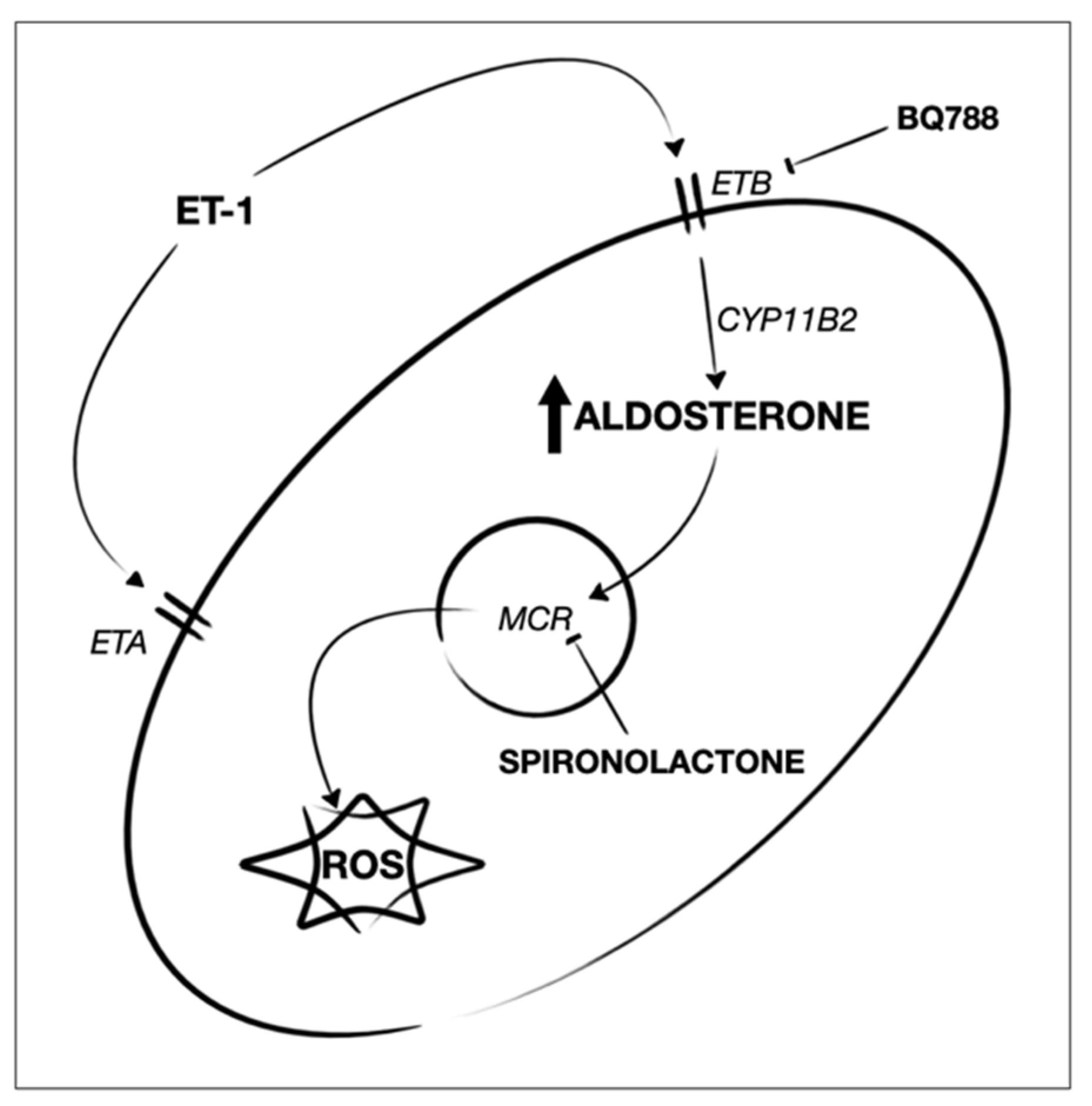
1. Introduction
2. Materials and Methods
2.1. Cell Cultures and Treatments
2.2. Patients and Healthy Donors
2.3. Fibroblasts Isolation
2.4. RNA Isolation and RT-PCR
| ETA: forward 5’-ATGCACAACTATTGCCCACA-3’ reverse 5’-GGACAGGATCCAGATGGAGA-3’ ETB: forward 5’-GCACATCGTCATTGACATCC-3’ reverse 5’-CAGAGGGCAAAGACAAGGAC-3’ MCR: forward 5’-AGGCTACCACAGTCTCCCTG-3’ reverse 5’-GACTGGAGATTTTACACTGC-3’ α-SMA: forward 5’-GGAATCCTGTGAAGCAGCTC-3’ reverse 5’-GAAGGAATAGCCACGCTCAG-3’ |
2.5. Western Blot Analysis
2.6. Flow Cytometry
2.7. Enzyme-Linked Immunosorbent Assay
2.8. Protein Carbonyl Content Assay
2.9. Statistical Analysis
3. Results
3.1. ET-1 Receptors
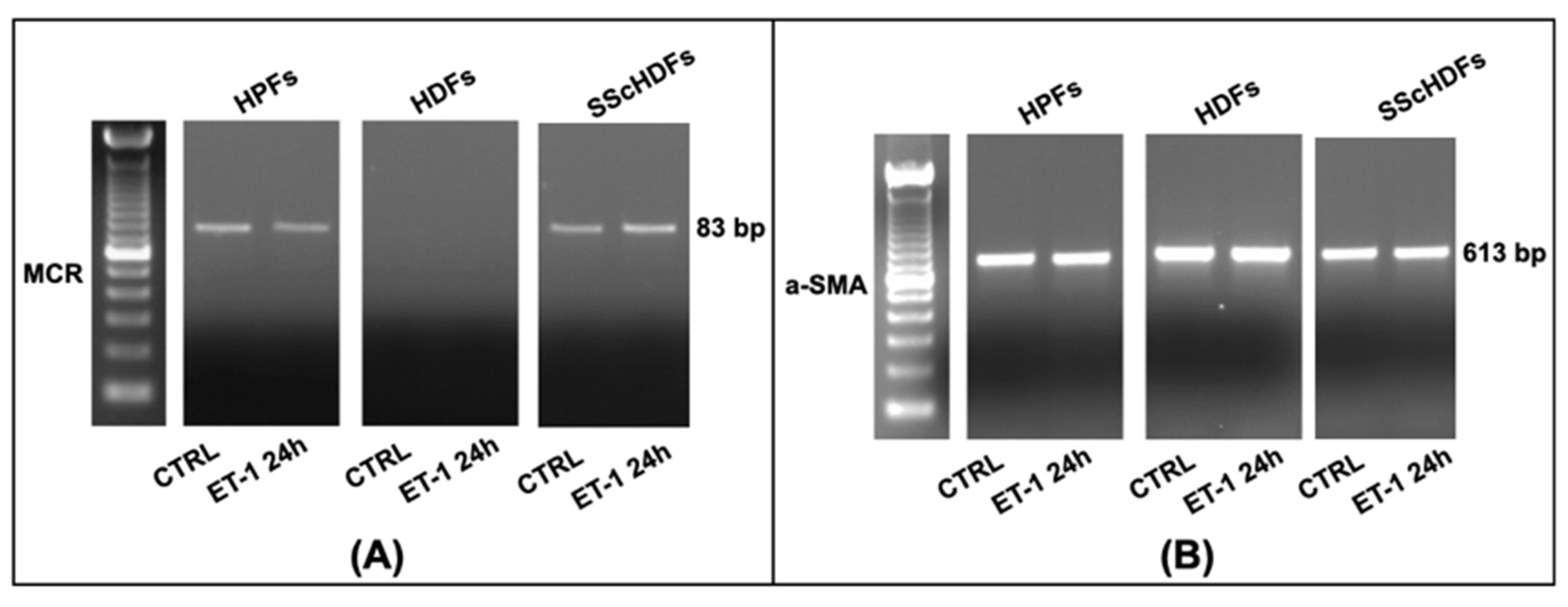
3.2. MCR and CYP11B2
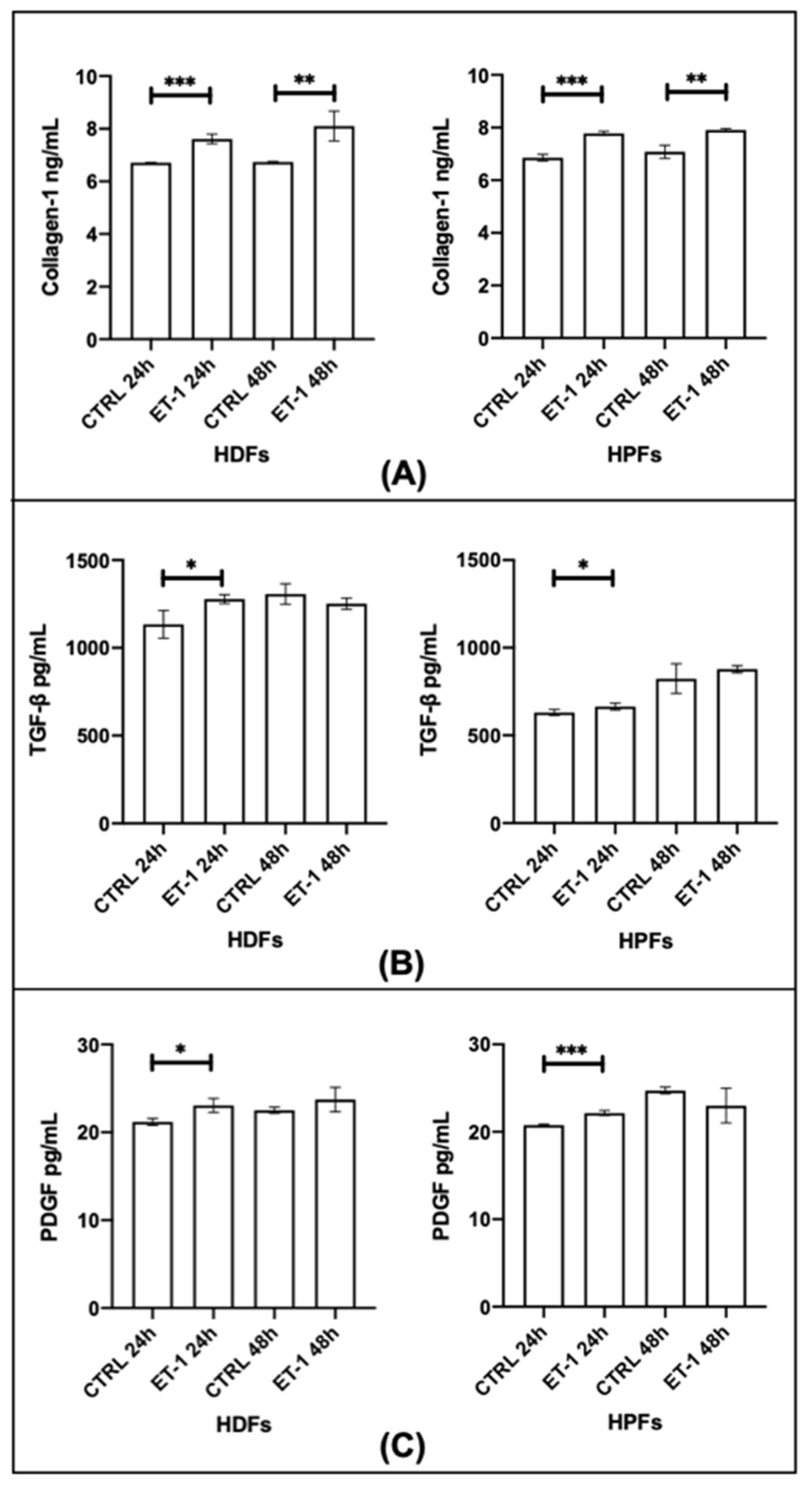
3.3. Trans-Differentiation and Fibroblasts Activation
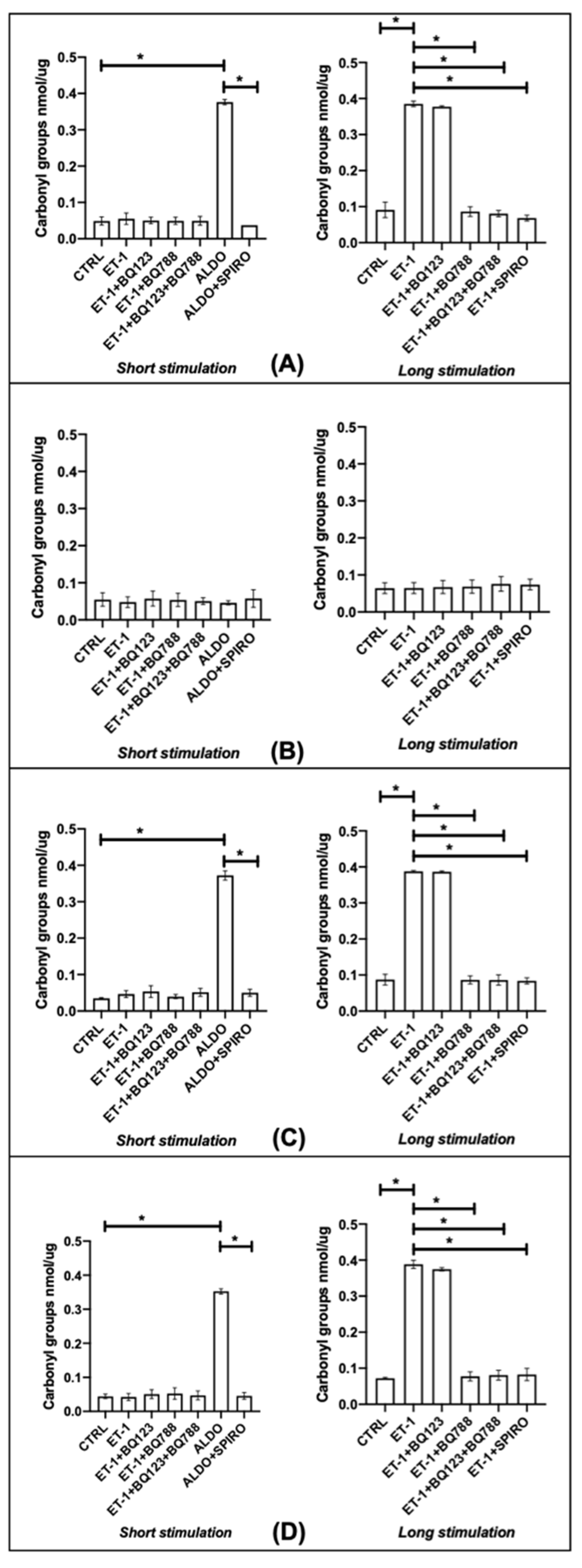
3.4. Oxidative Stress
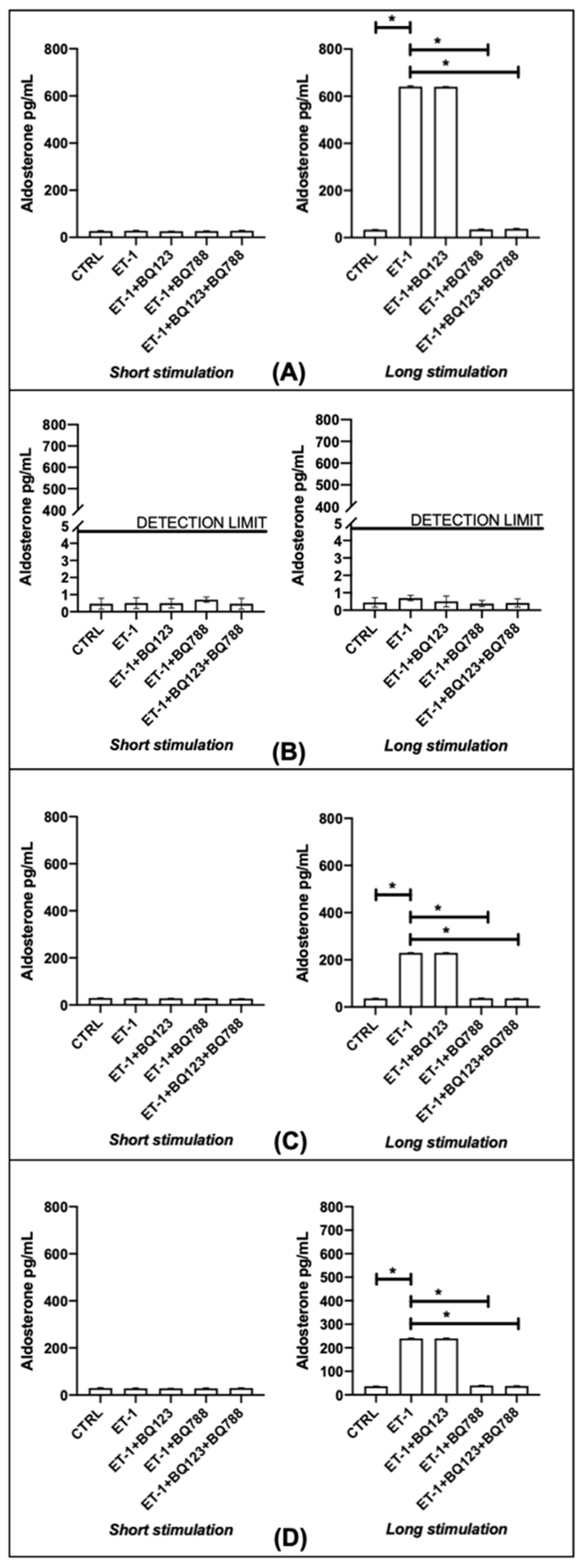
3.5. Aldosterone
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khimji, A.K.; Rockey, D.C. Endothelin-Biology and disease. Cell. Signal. 2010, 22, 1615–1625. [Google Scholar] [CrossRef] [PubMed]
- Maron, B.; Zhang, Y.-Y.; White, K.; Chan, S. Aldosterone inactivates the endothelin-B receptor via a cysteinyl thiol redox switch to decrease pulmonary endothelial nitric oxide levels and modulate pulmonary. Circulation 2012, 126, 963–974. [Google Scholar] [CrossRef] [PubMed]
- Kedzierski, R.M.; Yanagisawa, M. Endothelin System: The Double-Edged Sword in Health and Disease. Ann. Rev. Pharmacol. Toxicol. 2001, 41, 851–876. [Google Scholar] [CrossRef] [PubMed]
- Epstein, F.H.; Levin, E.R. Endothelins. N. Engl. J. Med. 1995, 333, 356–363. [Google Scholar]
- Shi-Wen, X.; Rodriguez-Pascual, F.; Lamas, S.; Holmes, A.; Howat, S.; Pearson, J.D.; Dashwood, M.R.; du Bois, R.M.; Denton, C.P.; Black, C.M.; et al. Constitutive ALK5-independent c-Jun N-terminal kinase activation contributes to endothelin-1 overexpression in pulmonary fibrosis: Evidence of an autocrine endothelin loop operating through the endothelin A and B receptors. Mol. Cell. Biol. 2006, 26, 5518–5527. [Google Scholar] [CrossRef]
- Tinazzi, E.; Puccetti, A.; Patuzzo, G.; Barbieri, A.; Argentino, G.; Confente, F.; Dolcino, M.; Beri, R.; Marchi, G.; Ottia, A.; et al. Endothelin Receptors Expressed by Immune Cells Are Involved in Modulation of Inflammation and in Fibrosis: Relevance to the Pathogenesis of Systemic Sclerosis. J. Immunol. Res. 2015, 2015, 147616. [Google Scholar]
- Rubanyi, G.M.; Polokoff, M. A Endothelins: Molecular biology, biochemistry, pharmacology, physiology, and pathophysiology. Pharmacol. Rev. 1994, 46, 325–415. [Google Scholar]
- Xu, S.W.; Howat, S.L.; Renzoni, E.A.; Holmes, A.; Pearson, J.D.; Dashwood, M.R.; Bou-Gharios, G.; Denton, C.P.; du Bois, R.M.; Black, C.M.; et al. Endothelin-1 induces expression of matrix-associated genes in lung fibroblasts through MEK/ERK. J. Biol. Chem. 2004, 279, 23098–23103. [Google Scholar]
- Shi-Wen, X.; Denton, C.P.; Dashwood, M.R.; Holmes, A.M.; Bou-Gharios, G.; Pearson, J.D.; Black, C.M.; Abraham, D.J. Fibroblast matrix gene expression and connective tissue remodeling: Role of endothelin-1. J. Investig. Dermatol. 2001, 116, 417–425. [Google Scholar] [CrossRef]
- Shi-Wen, X.; Renzoni, E.A.; Kennedy, L.; Howat, S.; Chen, Y.; Pearson, J.D.; Bou-Gharios, G.; Dashwood, M.R.; du Bois, R.M.; Black, C.M.; et al. Endogenous endothelin-1 signaling contributes to type I collagen and CCN2 overexpression in fibrotic fibroblasts. Matrix Biol. 2007, 26, 625–632. [Google Scholar] [CrossRef]
- Corallo, C.; Franci, B.; Lucani, B.; Montella, A.; Chirico, C.; Gonnelli, S.; Nuti, R.; Giordano, N. From microvasculature to fibroblasts: Contribution of anti-endothelial cell antibodies in systemic sclerosis. Int. J. Immunopathol. Pharmacol. 2015, 28, 93–103. [Google Scholar] [CrossRef]
- Raja, S.G. Endothelin receptor antagonists for pulmonary arterial hypertension: An overview. Cardiovasc. Ther. 2010, 28, e65–e71. [Google Scholar] [CrossRef]
- Razzaq, Z.; Hussain, M.; Naqvi, S.; Aslam, M. Correlation of plasma endothelin-1 levels with pulmonary hypertension after inhaled nitric oxide therapy. J. Ayub Med. Coll. Abbottabad 2009, 21, 106–110. [Google Scholar]
- Akamata, K.; Asano, Y.; Aozasa, N.; Noda, S.; Taniguchi, T.; Takahashi, T.; Ichimura, Y.; Toyama, T.; Sato, S. Bosentan reverses the pro-fibrotic phenotype of systemic sclerosis dermal fibroblasts via increasing DNA binding ability of transcription factor Fli1. Arthritis Res. Ther. 2014, 16, R86. [Google Scholar] [CrossRef]
- Enevoldsen, F.C.; Sahana, J.; Wehland, M.; Grimm, D.; Infanger, M.; Kruger, M. Endothelin Receptor Antagonists: Status Quo and Future Perspectives for Targeted Therapy. J. Clin. Med. 2020, 9, 824. [Google Scholar] [CrossRef]
- Shinde, A.V.; Humeres, C.; Frangogiannis, N.G. The role of α-smooth muscle actin in fibroblast-mediated matrix contraction and remodeling. Biochim. Biophys. Acta. Mol. Basis Dis. 2017, 1863, 298–309. [Google Scholar] [CrossRef]
- Manetti, M.; Romano, E.; Rosa, I.; Guiducci, S.; Bellando-Randone, S.; De Paulis, A.; Ibba-Manneschi, L.; Matucci-Cerinic, M. Endothelial-to-mesenchymal transition contributes to endothelial dysfunction and dermal fibrosis in systemic sclerosis. Ann. Rheum. Dis. 2017, 76, 924–934. [Google Scholar] [CrossRef]
- Ranchoux, B.; Antigny, F.; Rucker-Martin, C.; Hautefort, A.; Péchoux, C.; Bogaard, H.J.; Dorfmüller, P.; Remy, S.; Lecerf, F.; Planté, S.; et al. Endothelial-to-mesenchymal transition in pulmonary hypertension. Circulation 2015, 131, 1006–1018. [Google Scholar] [CrossRef]
- Good, R.B.; Gilbane, A.J.; Trinder, S.L.; Denton, C.P.; Coghlan, G.; Abraham, D.J.; Holmes, A.M. Endothelial to Mesenchymal Transition Contributes to Endothelial Dysfunction in Pulmonary Arterial Hypertension. Am. J. Pathol. 2015, 185, 1850–1858. [Google Scholar] [CrossRef]
- Hundemer, G.L.; Curhan, G.C.; Yozamp, N.; Wang, M.; Vaidya, A. Incidence of atrial fibrillation and mineralocorticoid receptor activity in patients with medically and surgically treated primary aldosteronism. JAMA Cardiol. 2018, 3, 768–774. [Google Scholar] [CrossRef]
- Milliez, P.; Girerd, X.; Plouin, P.F.; Blacher, J.; Safar, M.E.; Mourad, J.J. Evidence for an increased rate of cardiovascular events in patients with primary aldosteronism. J. Am. Coll. Cardiol. 2005, 45, 1243–1248. [Google Scholar] [CrossRef] [PubMed]
- Seccia, T.M.; Letizia, C.; Muiesan, M.L.; Lerco, S.; Cesari, M.; Bisogni, V.; Petramala, L.; Maiolino, G.; Volpin, R.; Rossi, G.P. Atrial fibrillation as presenting sign of primary aldosteronism: Results of the Prospective Appraisal on the Prevalence of Primary Aldosteronism in Hypertensive (PAPPHY) Study. J. Hypertens. 2020, 38, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Rossi, G.P.; Seccia, T.M.; Gallina, V.; Muiesan, M.L.; Leoni, L.; Pengo, M.; Ragazzo, F.; Caielli, P.; Belfiore, A.; Bernini, G.; et al. Prospective appraisal of the prevalence of primary aldosteronism in hypertensive patients presenting with atrial flutter or fibrillation (PAPPHY Study): Rationale and study design. J. Hum. Hypertens. 2013, 27, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Maron, B.A.; Waxman, A.B.; Opotowsky, A.R.; Gillies, H.; Blair, C.; Aghamohammadzadeh, R.; Loscalzo, J.; Leopold, J.A. Effectiveness of spironolactone plus ambrisentan for treatment of pulmonary arterial hypertension (from the [ARIES] study 1 and 2 trials). Am. J. Cardiol. 2013, 112, 720–725. [Google Scholar] [CrossRef] [PubMed]
- Maron, B.A.; Opotowsky, A.R.; Landzberg, M.J.; Loscalzo, J.; Waxman, A.B.; Leopold, J.A. Plasma aldosterone levels are elevated in patients with pulmonary arterial hypertension in the absence of left ventricular heart failure: A pilot study. Eur. J. Heart Fail. 2013, 15, 277–283. [Google Scholar] [CrossRef]
- Belloni, A.S.; Rossi, G.P.; Andreis, P.G.; Neri, G.; Albertin, G.; Pessina, A.C.; Nussdorfer, G.G. Endothelin adrenocortical secretagogue effect is mediated by the B receptor in rats. Hypertension 1996, 27, 1153–1159. [Google Scholar] [CrossRef]
- Archer, S.L.; Weir, E.K.; Wilkins, M.R. Basic science of pulmonary arterial hypertension for clinicians: New concepts and experimental therapies. Circulation 2010, 121, 2045–2066. [Google Scholar] [CrossRef]
- Farber, H.W.; Loscalzo, J. Pulmonary Arterial Hypertension. N. Engl. J. Med. 2004, 351, 1655–1665. [Google Scholar] [CrossRef]
- Hirata, Y.; Emori, T.; Eguchi, S.; Kanno, K.; Imai, T.; Ohta, K.; Marumo, F. Endothelin receptor subtype B mediates synthesis of nitric oxide by cultured bovine endothelial cells. J. Clin. Investig. 1993, 91, 1367–1373. [Google Scholar] [CrossRef]
- Mazzuca, M.Q.; Khalil, R.A. Vascular endothelin receptor type B: Structure, function and dysregulation in vascular disease. Biochem. Pharmacol. 2012, 84, 147–162. [Google Scholar] [CrossRef]
- Okamoto, Y.; Ninomiya, H.; Tanioka, M.; Sakamoto, A.; Miwa, S.; Masaki, T. Palmitoylation of human endothelinB. Its critical role in G protein coupling and a differential requirement for the cytoplasmic tail by G protein subtypes. J. Biol. Chem. 1997, 272, 21589–21596. [Google Scholar] [CrossRef][Green Version]
- Aleksiejczuk, M.; Gromotowicz-Poplawska, A.; Marcinczyk, N.; Przylipiak, A.; Chabielska, E. The expression of the renin-angiotensin-aldosterone system in the skin and its effects on skin physiology and pathophysiology. J. Physiol. Pharmacol. 2019, 70, 325–336. [Google Scholar]
- Morrell, N.W.; Atochina, E.N.; Morris, K.G.; Danilov, S.M.; Stenmark, K.R. Angiotensin converting enzyme expression is increased in small pulmonary arteries of rats with hypoxia-induced pulmonary hypertension. J. Clin. Investig. 1995, 96, 1823–1833. [Google Scholar] [CrossRef]
- Van den Hoogen, F.; Baron, M.; Matucci-cerinic, M.; van den Hoogen, F.; Khanna, D.; Fransen, J.; Johnson, S.R.; Baron, M.; Tyndall, A.; Matucci-cerinic, M.; et al. 2013 classification criteria for systemic sclerosis: An American College of Rheumatology/European League against Rheumatism collaborative initiative. Arthritis Rheumatol. 2014, 65, 2737–2747. [Google Scholar] [CrossRef]
- LeRoy, E.C.; Black, C.; Fleischmajer, R.; Jablonska, S.; Krieg, T.; Medsger, T.A.J.; Rowell, N.; Wollheim, F. Scleroderma (systemic sclerosis): Classification, subsets and pathogenesis. J. Rheumatol. 1988, 15, 202–205. [Google Scholar]
- LeRoy, E.C.; Medsger, T.A.J. Criteria for the classification of early systemic sclerosis. J. Rheumatol. 2001, 28, 1573–1576. [Google Scholar]
- Chaisson, N.F.; Hassoun, P.M. Systemic sclerosis-associated pulmonary arterial hypertension. Chest 2013, 144, 1346–1356. [Google Scholar] [CrossRef]
- Morelli, S.; Ferri, C.; Polettini, E.; Bellini, C.; Gualdi, G.F.; Pittoni, V.; Valesini, G.; Santucci, A. Plasma endothelin-1 levels, pulmonary hypertension, and lung fibrosis in patients with systemic sclerosis. Am. J. Med. 1995, 99, 255–260. [Google Scholar] [CrossRef]
- Kuryliszyn-Moskal, A.; Klimiuk, P.A.; Sierakowski, S. Soluble adhesion molecules (sVCAM-1, sE-selectin), vascular endothelial growth factor (VEGF) and endothelin-1 in patients with systemic sclerosis: Relationship to organ systemic involvement. Clin. Rheumatol. 2005, 24, 111–116. [Google Scholar] [CrossRef]
- Rubens, C.; Ewert, R.; Halank, M.; Wensel, R.; Orzechowski, H.-D.; Schultheiss, H.-P.; Hoeffken, G. Big Endothelin-1 and Endothelin-1 Plasma Levels Are Correlated With the Severity of Primary Pulmonary Hypertension. Chest 2001, 120, 1562–1569. [Google Scholar] [CrossRef]
- Cipriani, P.; Di Benedetto, P.; Ruscitti, P.; Campese, A.F.; Liakouli, V.; Carubbi, F.; Pantano, I.; Berardicurti, O.; Screpanti, I.; Giacomelli, R.; et al. Impaired Endothelium-Mesenchymal Stem Cells Cross-talk in Systemic Sclerosis: A Link Between Vascular and Fibrotic Features. Arthritis Res. Ther. 2014, 16, 442. [Google Scholar] [CrossRef] [PubMed]
- Safdar, Z.; Frost, A.; Basant, A.; Deswal, A.; O’Brian Smith, E.; Entman, M. Spironolactone in pulmonary arterial hypertension: Results of a cross-over study. Pulm. Circ. 2020, 10, 2045894019898030. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, J.; Deng, L.; Suennen, C.; Koca, D.; Meral, D.; Bode, C.; Hein, L.; Lother, A. Eplerenone Improves Pulmonary Vascular Remodeling and Hypertension by Inhibition of the Mineralocorticoid Receptor in Endothelial Cells. Hypertension 2021, 78, 456–465. [Google Scholar] [CrossRef] [PubMed]



| Sex | Age | Disease Form | Disease Duration | |
|---|---|---|---|---|
| Patients | 1 Male 3 Female | 34–51 | 2 dSSc 2 lSSc | 3 < 5 years 1 > 5 years |
| Healthy donors | 1 Male 1 Female | 32–45 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Argentino, G.; Barbieri, A.; Beri, R.; Bason, C.; Ruzzenente, A.; Olivieri, O.; Tinazzi, E.; Puccetti, A.; Vitali, C.; Del Papa, N.; et al. Profibrotic Effects of Endothelin-1 on Fibroblasts Are Mediated by Aldosterone In Vitro: Relevance to the Pathogenesis and Therapy of Systemic Sclerosis and Pulmonary Arterial Hypertension. Biomedicines 2022, 10, 2765. https://doi.org/10.3390/biomedicines10112765
Argentino G, Barbieri A, Beri R, Bason C, Ruzzenente A, Olivieri O, Tinazzi E, Puccetti A, Vitali C, Del Papa N, et al. Profibrotic Effects of Endothelin-1 on Fibroblasts Are Mediated by Aldosterone In Vitro: Relevance to the Pathogenesis and Therapy of Systemic Sclerosis and Pulmonary Arterial Hypertension. Biomedicines. 2022; 10(11):2765. https://doi.org/10.3390/biomedicines10112765
Chicago/Turabian StyleArgentino, Giuseppe, Alessandro Barbieri, Ruggero Beri, Caterina Bason, Andrea Ruzzenente, Oliviero Olivieri, Elisa Tinazzi, Antonio Puccetti, Claudio Vitali, Nicoletta Del Papa, and et al. 2022. "Profibrotic Effects of Endothelin-1 on Fibroblasts Are Mediated by Aldosterone In Vitro: Relevance to the Pathogenesis and Therapy of Systemic Sclerosis and Pulmonary Arterial Hypertension" Biomedicines 10, no. 11: 2765. https://doi.org/10.3390/biomedicines10112765
APA StyleArgentino, G., Barbieri, A., Beri, R., Bason, C., Ruzzenente, A., Olivieri, O., Tinazzi, E., Puccetti, A., Vitali, C., Del Papa, N., Friso, S., & Lunardi, C. (2022). Profibrotic Effects of Endothelin-1 on Fibroblasts Are Mediated by Aldosterone In Vitro: Relevance to the Pathogenesis and Therapy of Systemic Sclerosis and Pulmonary Arterial Hypertension. Biomedicines, 10(11), 2765. https://doi.org/10.3390/biomedicines10112765






