SOD3 Expression in Tumor Stroma Provides the Tumor Vessel Maturity in Oral Squamous Cell Carcinoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice and Cells
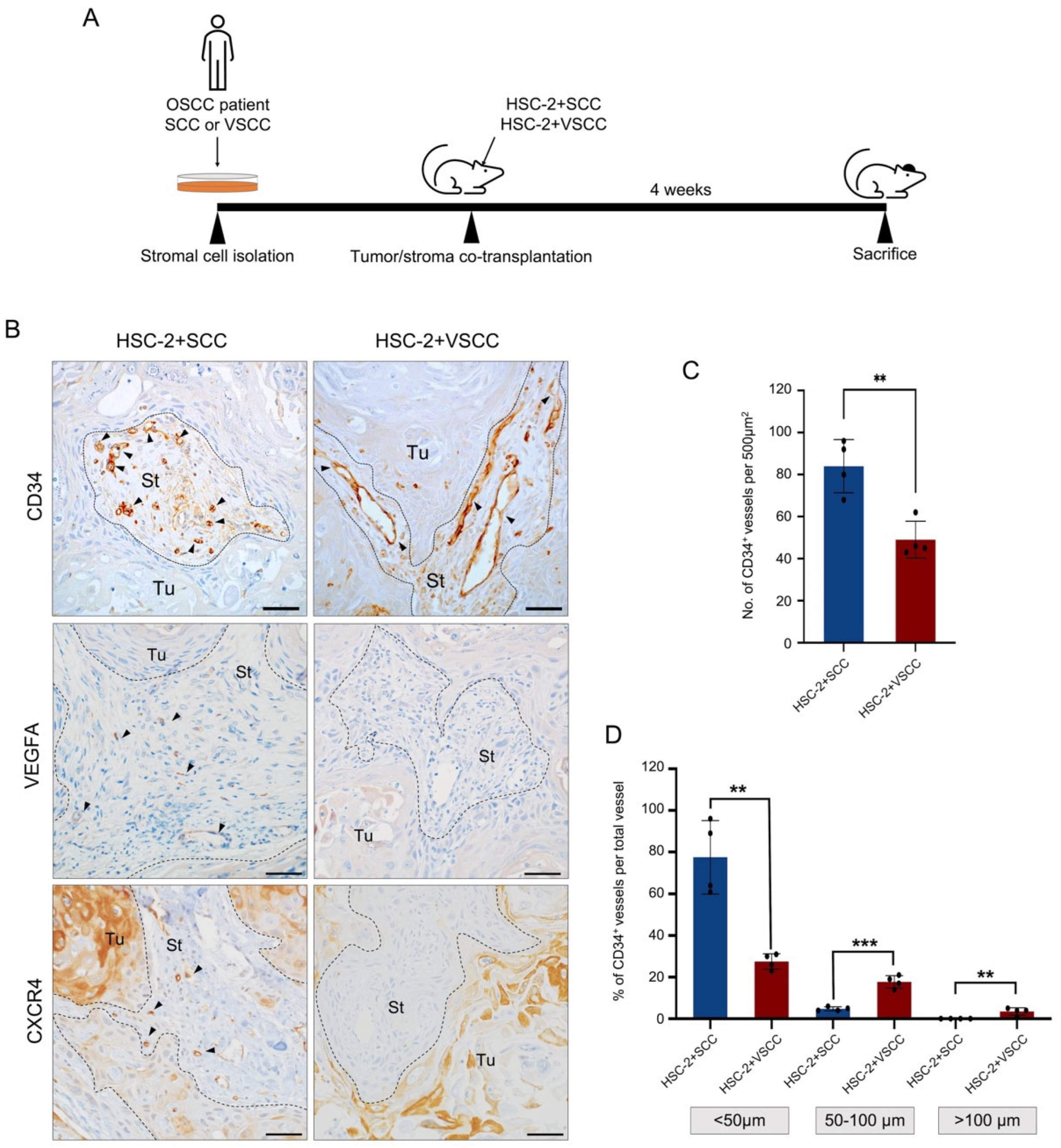
2.2. Tumor/Stroma Co-Xenograft
2.3. Tissue Processing for Histological Analysis
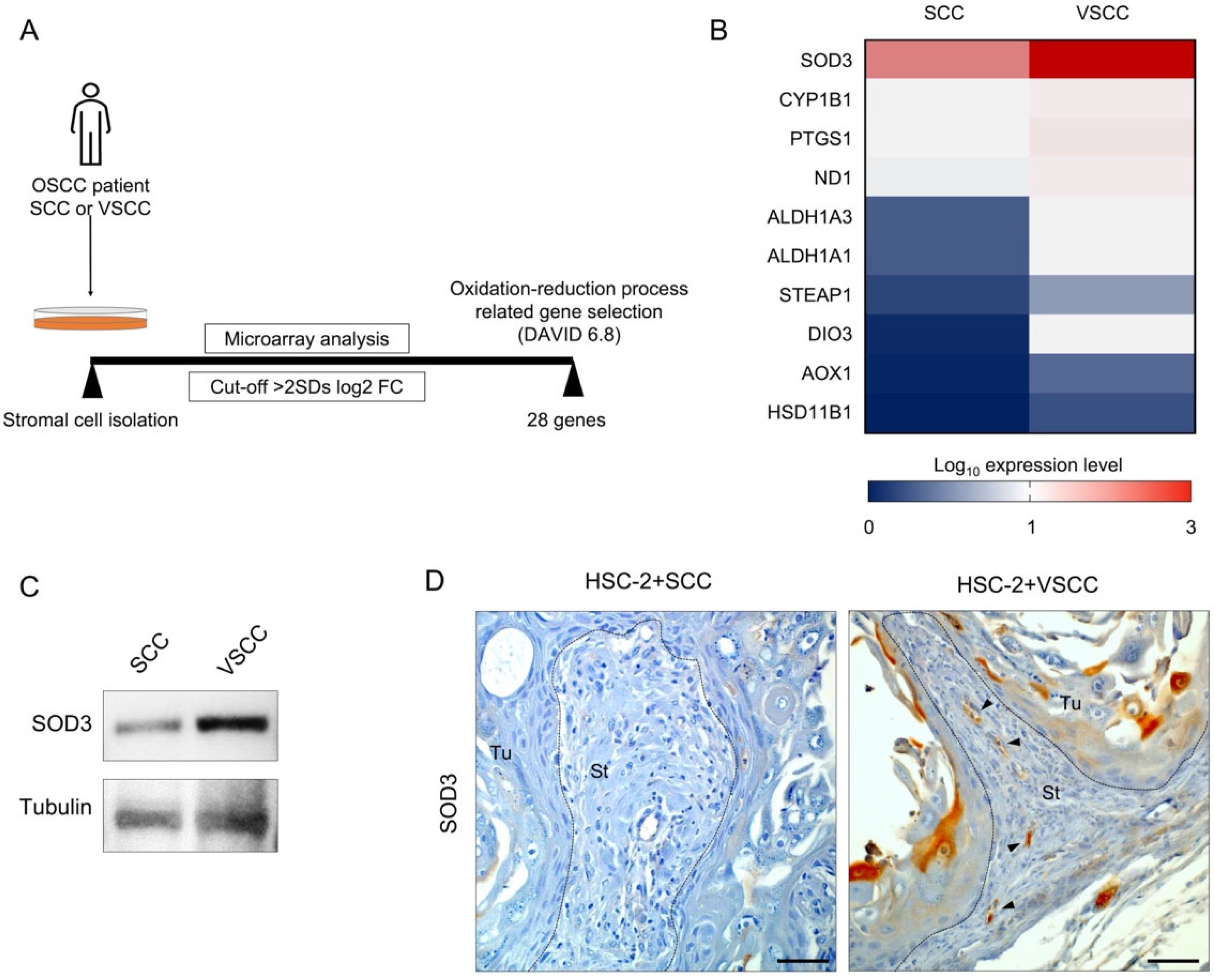
2.4. Immunohistochemistry
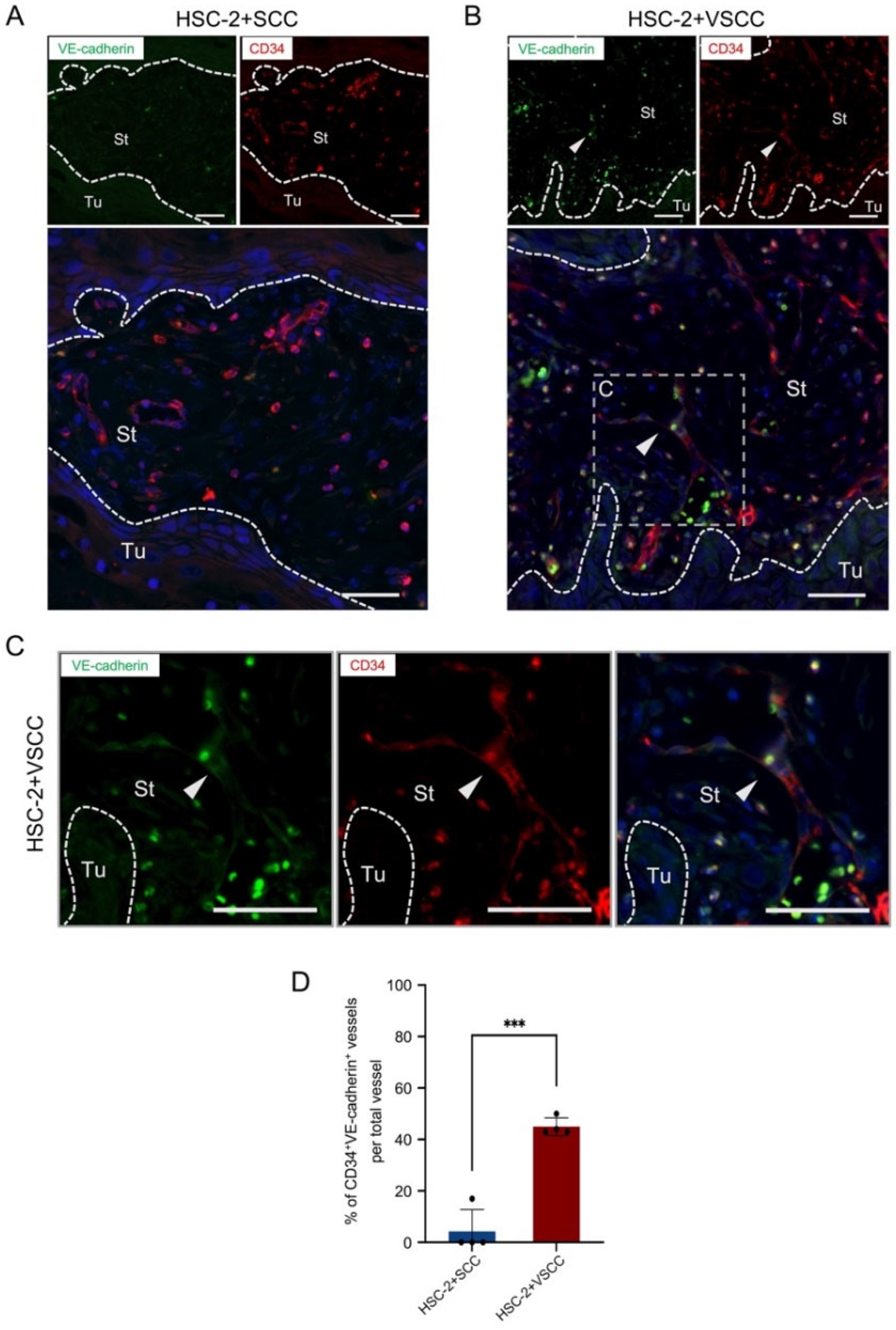
2.5. Double-Fluorescent IHC
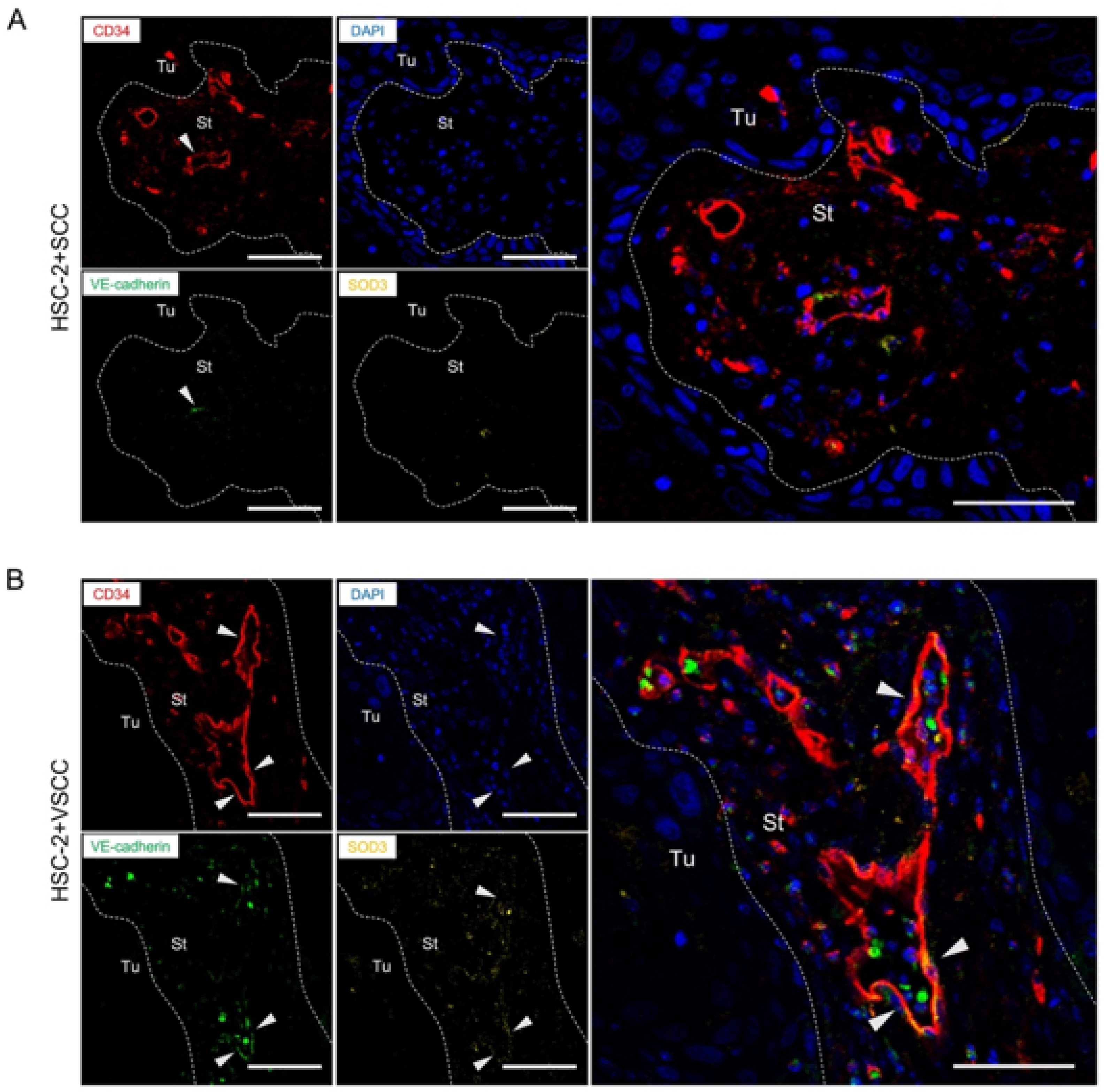
2.6. Multicolor Fluorescent IHC
2.7. Western Blotting
2.8. Microarray Data Analysis
2.9. Quantification and Statistical Analysis
3. Results
3.1. Vascular Architecture Is Different between HSC-2+SCC and HSC-2+VSCC Xenografts
3.2. HSC-2+VSCC Xenograft Includes VE-Cadherin Expressed Vessels More Than HSC-2+SCC Xenograft
3.3. SOD3 Expressed Endothelial Cells Composed of the Vessels in HSC-2+VSCC Xenograft
3.4. VE-Cadherin Expressed Vessels Show the Association with SOD3 Expression in Endothelial Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mueller, M.M.; Fusenig, N.E. Friends or foes-Bipolar effects of the tumour stroma in cancer. Nat. Rev. Cancer 2004, 4, 839–849. [Google Scholar] [CrossRef] [PubMed]
- Takabatake, K.; Kawai, H.; Omori, H.; Qiusheng, S.; Oo, M.W.; Sukegawa, S.; Nakano, K.; Tsujigiwa, H.; Nagatsuka, H. Impact of the stroma on the biological characteristics of the parenchyma in oral squamous cell carcinoma. Int. J. Mol. Sci. 2020, 21, 7714. [Google Scholar] [CrossRef] [PubMed]
- Jakobsson, P.A.; Eneroth, C.M.; Killander, D.; Moberger, G.; Mårtensson, B. Histologic Classification and Grading of Malignancy in Carcinoma of the Larynx. Acta Radiol. Ser. Ther. Phys. Biol. 1973, 12, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Dourado, M.R.; Miwa, K.Y.M.; Hamada, G.B.; Paranaíba, L.M.R.; Sawazaki-Calone, Í.; Domingueti, C.B.; Ervolino de Oliveira, C.; Furlan, E.C.B.; Longo, B.C.; Almangush, A.; et al. Prognostication for oral squamous cell carcinoma patients based on the tumour–stroma ratio and tumour budding. Histopathology 2020, 76, 906–918. [Google Scholar] [CrossRef]
- Almangush, A.; Heikkinen, I.; Bakhti, N.; Mäkinen, L.K.; Kauppila, J.H.; Pukkila, M.; Hagström, J.; Laranne, J.; Soini, Y.; Kowalski, L.P.; et al. Prognostic impact of tumour–stroma ratio in early-stage oral tongue cancers. Histopathology 2018, 72, 1128–1135. [Google Scholar] [CrossRef]
- Oo, M.W.; Kawai, H.; Takabatake, K.; Shan, Q.; Eain, H.S.; Sukegawa, S.; Nakano, K.; Nagatsuka, H. Cancer-associated stromal cells promote the contribution of mmp2-positive bone marrow-derived cells to oral squamous cell carcinoma invasion. Cancers 2022, 14, 137. [Google Scholar] [CrossRef]
- Oo, M.W.; Kawai, H.; Takabatake, K.; Tomida, S.; Eguchi, T.; Ono, K.; Shan, Q.; Ohara, T.; Yoshida, S.; Omori, H.; et al. Resident stroma-secreted chemokine CCL2 governs myeloid-derived suppressor cells in the tumor microenvironment. JCI Insight 2022, 7, e148960. [Google Scholar] [CrossRef]
- Folkman, J. Role of angiogenesis in tumor growth and metastasis. Semin. Oncol. 2002, 29, 15–18. [Google Scholar] [CrossRef]
- Weidner, N.; Semple, J.P.; Welch, W.R.; Folkman, J. Tumor Angiogenesis and Metastasis—Correlation in Invasive Breast Carcinoma. N. Engl. J. Med. 1991, 324, 1–8. [Google Scholar] [CrossRef]
- Batlle, R.; Andrés, E.; Gonzalez, L.; Llonch, E.; Igea, A.; Gutierrez-Prat, N.; Berenguer-Llergo, A.; Nebreda, A.R. Regulation of tumor angiogenesis and mesenchymal–endothelial transition by p38α through TGF-β and JNK signaling. Nat. Commun. 2019, 10, 1–18. [Google Scholar] [CrossRef]
- Kim, Y.W.; Byzova, T.V. Oxidative Stress in Angiogenesis and Vascular Disease. Blood 2014, 123, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Giannotta, M.; Trani, M.; Dejana, E. VEC and endothelial adherens junctions: Active guardians of vascular integrity. Dev. Cell 2013, 26, 441–454. [Google Scholar] [CrossRef] [PubMed]
- Gavard, J. Endothelial permeability and VE-cadherin A wacky comradeship. Cell Adhes. Migr. 2014, 8, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Shan, Q.; Takabatake, K.; Omori, H.; Kawai, H.; Oo, M.W.; Nakano, K.; Ibaragi, S.; Sasaki, A.; Nagatsuka, H. Stromal cells in the tumor microenvironment promote the progression of oral squamous cell carcinoma. Int. J. Oncol. 2021, 59, 1–17. [Google Scholar] [CrossRef]
- Xu, J.; Liang, J.; Meng, Y.M.; Yan, J.; Yu, X.J.; Liu, C.Q.; Xu, L.; Zhuang, S.M.; Zheng, L. Vascular CXCR4 expression promotes vessel sprouting and sensitivity to sorafenib treatment in hepatocellular carcinoma. Clin. Cancer Res. 2017, 23, 4482–4492. [Google Scholar] [CrossRef]
- Chauhan, V.P.; Stylianopoulos, T.; Boucher, Y.; Jain, R.K. Delivery of molecular and nanoscale medicine to tumors: Transport barriers and strategies. Annu. Rev. Chem. Biomol. Eng. 2011, 2, 281–298. [Google Scholar] [CrossRef]
- Yoshida, S.; Kawai, H.; Eguchi, T.; Sukegawa, S.; Oo, M.W.; Anqi, C.; Takabatake, K.; Nakano, K.; Okamoto, K.; Nagatsuka, H. Tumor Angiogenic Inhibition Triggered Necrosis (TAITN) in Oral Cancer. Cells 2019, 8, 761. [Google Scholar] [CrossRef]
- Vestweber, D. VE-cadherin: The major endothelial adhesion molecule controlling cellular junctions and blood vessel formation. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 223–232. [Google Scholar] [CrossRef]
- Harris, N.R.; Nielsen, N.R.; Pawlak, J.B.; Aghajanian, A.; Rangarajan, K.; Serafin, D.S.; Farber, G.; Dy, D.M.; Nelson-Maney, N.P.; Xu, W.; et al. VE-Cadherin Is Required for Cardiac Lymphatic Maintenance and Signaling. Circ. Res. 2022, 130, 5–23. [Google Scholar] [CrossRef]
- Vittet, D.; Buchou, T.; Schweitzer, A.; Dejana, E.; Huber, P. Targeted null-mutation in the vascular endothelial-cadherin gene impairs the organization of vascular-like structures in embryoid bodies. Proc. Natl. Acad. Sci. USA 1997, 94, 6273–6278. [Google Scholar] [CrossRef]
- Sherwood, L.M.; Parris, E.E.; Folkman, J. Tumor Angiogenesis: Therapeutic Implications. N. Engl. J. Med. 1971, 285, 1182–1186. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.K.; Duda, D.G.; Clark, J.W.; Loeffler, J.S. Lessons from phase III clinical trials on anti-VEGF therapy for cancer. Nat. Clin. Pract. Oncol. 2006, 63, 24–40. [Google Scholar] [CrossRef] [PubMed]
- Nagy, J.A.; Chang, S.H.; Dvorak, A.M.; Dvorak, H.F. Why are tumour blood vessels abnormal and why is it important to know? Br. J. Cancer 2009, 100, 865–869. [Google Scholar] [CrossRef]
- Magnussen, A.L.; Mills, I.G. Vascular normalisation as the stepping stone into tumour microenvironment transformation. Br. J. Cancer 2021, 125, 324–336. [Google Scholar] [CrossRef]
- Jones, D.P. Redefining oxidative stress. Antioxid. Redox Signal. 2006, 8, 1865–1879. [Google Scholar] [CrossRef] [PubMed]
- Betteridge, D.J. What is oxidative stress? Metab. Clin. Exp. 2000, 49, 3–8. [Google Scholar] [CrossRef]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef]
- Sena, C.M.; Leandro, A.; Azul, L.; Seiça, R.; Perry, G. Vascular oxidative stress: Impact and therapeutic approaches. Front. Physiol. 2018, 9, 1668. [Google Scholar] [CrossRef]
- Chaiswing, L.; Zhong, W.; Cullen, J.J.; Oberley, L.W.; Oberley, T.D. Extracellular redox state regulates features associated with prostate cancer cell invasion. Cancer Res. 2008, 68, 5820–5826. [Google Scholar] [CrossRef]
- O’Leary, B.R.; Fath, M.A.; Bellizzi, A.M.; Hrabe, J.E.; Button, A.M.; Allen, B.G.; Case, A.J.; Altekruse, S.; Wagner, B.A.; Buettner, G.R.; et al. Loss of SOD3 (EcSOD) expression promotes an aggressive phenotype in human pancreatic ductal adenocarcinoma. Clin. Cancer Res. 2015, 21, 1741–1751. [Google Scholar] [CrossRef]
- Teoh-Fitzgerald, M.L.T.; Fitzgerald, M.P.; Jensen, T.J.; Futscher, B.W.; Domann, F.E. Genetic and epigenetic inactivation of extracellular superoxide dismutase promotes an invasive phenotype in human lung cancer by disrupting ECM homeostasis. Mol. Cancer Res. 2012, 10, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Bhat, H.K. Superoxide dismutase 3 is induced by antioxidants, inhibits oxidative DNA damage and is associated with inhibition of estrogen-induced breast cancer. Carcinogenesis 2012, 33, 2601–2610. [Google Scholar] [CrossRef] [PubMed]
- Sah, S.K.; Agrahari, G.; Kim, T.Y. Insights into superoxide dismutase 3 in regulating biological and functional properties of mesenchymal stem cells. Cell Biosci. 2020, 10, 22. [Google Scholar] [CrossRef]
- Yokoe, H.; Nomura, H.; Yamano, Y.; Fushimi, K.; Sakamoto, Y.; Ogawara, K.; Shiiba, M.; Bukawa, H.; Uzawa, K.; Takiguchi, Y.; et al. Alteration of extracellular superoxide dismutase expression is associated with an aggressive phenotype of oral squamous-cell carcinoma. Exp. Ther. Med. 2010, 1, 585–590. [Google Scholar] [CrossRef] [PubMed]
- Emami Nejad, A.; Najafgholian, S.; Rostami, A.; Sistani, A.; Shojaeifar, S.; Esparvarinha, M.; Nedaeinia, R.; Haghjooy Javanmard, S.; Taherian, M.; Ahmadlou, M.; et al. The role of hypoxia in the tumor microenvironment and development of cancer stem cell: A novel approach to developing treatment. Cancer Cell Int. 2021, 21, 62. [Google Scholar] [CrossRef] [PubMed]
- Nagy, J.A.; Morgan, E.S.; Herzberg, K.T.; Manseau, E.J.; Dvorak, A.M.; Dvorak, H.F. Pathogenesis of Ascites Tumor Growth: Angiogenesis, Vascular Remodeling, and Stroma Formation in the Peritoneal Lining. Cancer Res. 1995, 55, 376–385. [Google Scholar]
- Dvorak, H.F. How tumors make bad blood vessels and stroma. Am. J. Pathol. 2003, 162, 1747–1757. [Google Scholar] [CrossRef]
- Azzi, S.; Hebda, J.K.; Gavard, J. Vascular permeability and drug delivery in cancers. Front. Oncol. 2013, 3, 211. [Google Scholar] [CrossRef]
- Carmeliet, P.; Jain, R.K. Principles and mechanisms of vessel normalization for cancer and other angiogenic diseases. Nat. Rev. Drug Discov. 2011, 10, 417–427. [Google Scholar] [CrossRef]
- Mira, E.; Carmona-Rodríguez, L.; Pérez-Villamil, B.; Casas, J.; Fernández-Aceñero, M.J.; Martínez-Rey, D.; Martín-González, P.; Heras-Murillo, I.; Paz-Cabezas, M.; Tardáguila, M.; et al. SOD3 improves the tumor response to chemotherapy by stabilizing endothelial HIF-2α. Nat. Commun. 2018, 9, 575. [Google Scholar] [CrossRef]
- Demchenko, I.T.; Oury, T.D.; Crapo, J.D.; Piantadosi, C.A. Regulation of the brain’s vascular responses to oxygen. Circ. Res. 2002, 91, 1031–1037. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Rey, D.; Carmona-Rodríguez, L.; Fernández-Aceñero, M.J.; Mira, E.; Mañes, S. Extracellular Superoxide Dismutase, the Endothelial Basement Membrane, and the WNT Pathway: New Players in Vascular Normalization and Tumor Infiltration by T-Cells. Front. Immunol. 2020, 11, 579552. [Google Scholar] [CrossRef] [PubMed]

| Primary Antibody | Immunized Animal | Antigen Retrieval | Dilution | Supplier |
|---|---|---|---|---|
| CD34 | Rabbit | Microwave heating in 0.01 mol/L citrate buffer (pH 6.0) at 100 °C for 1 min | 1:500 | ab81289 1 |
| VEGFA | Rabbit | Microwave heating in 0.01 mol/L citrate buffer (pH 6.0) at 100 °C for 1 min | 1:200 | ab183100 1 |
| CXCR4 | Rabbit | Microwave heating in 0.01 mol/L citrate buffer (pH 6.0) at 100 °C for 1 min | 1:300 | ab124824 1 |
| VE-cadherin | Rat | Microwave heating in 0.01 mol/L citrate buffer (pH 6.0) at 100 °C for 1 min | 1:50 | ab282277 1 |
| SOD3 | Mouse | Microwave heating in 0.01 mol/L citrate buffer (pH 6.0) at 100 °C for 1 min | 1:50 | Sc271170 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oo, M.W.; Kawai, H.; Eain, H.S.; Soe, Y.; Takabatake, K.; Sanou, S.; Shan, Q.; Inada, Y.; Fujii, M.; Fukuhara, Y.; et al. SOD3 Expression in Tumor Stroma Provides the Tumor Vessel Maturity in Oral Squamous Cell Carcinoma. Biomedicines 2022, 10, 2729. https://doi.org/10.3390/biomedicines10112729
Oo MW, Kawai H, Eain HS, Soe Y, Takabatake K, Sanou S, Shan Q, Inada Y, Fujii M, Fukuhara Y, et al. SOD3 Expression in Tumor Stroma Provides the Tumor Vessel Maturity in Oral Squamous Cell Carcinoma. Biomedicines. 2022; 10(11):2729. https://doi.org/10.3390/biomedicines10112729
Chicago/Turabian StyleOo, May Wathone, Hotaka Kawai, Htoo Shwe Eain, Yamin Soe, Kiyofumi Takabatake, Sho Sanou, Qiusheng Shan, Yasunori Inada, Masae Fujii, Yoko Fukuhara, and et al. 2022. "SOD3 Expression in Tumor Stroma Provides the Tumor Vessel Maturity in Oral Squamous Cell Carcinoma" Biomedicines 10, no. 11: 2729. https://doi.org/10.3390/biomedicines10112729
APA StyleOo, M. W., Kawai, H., Eain, H. S., Soe, Y., Takabatake, K., Sanou, S., Shan, Q., Inada, Y., Fujii, M., Fukuhara, Y., Wang, Z., Sukegawa, S., Ono, M., Nakano, K., & Nagatsuka, H. (2022). SOD3 Expression in Tumor Stroma Provides the Tumor Vessel Maturity in Oral Squamous Cell Carcinoma. Biomedicines, 10(11), 2729. https://doi.org/10.3390/biomedicines10112729






