A Metabolic Signature to Monitor Endothelial Cell Differentiation, Activation, and Vascular Organization
Abstract
1. Introduction
2. Material and Methods
2.1. Monocyte Isolation and Culture
2.2. Endothelial Cell Culture
2.3. Tube Forming Assay Cell
2.4. Rat Aortic Ring Sprouting Assay
2.5. Nuclear Magnetic Resonance (NMR)
2.6. Statistical Analysis
3. Results
3.1. A Metabolic Signature Follows the Monocyte-to-Endothelial-Cell Differentiation
3.2. Upon Hypoxia, Endothelial Cells Present Metabolic Alteration Characteristic of Activation
3.3. Cysteine Does Not Affect the Sprouting of Aortic Rings and Tends to Shift the Metabolic Profile
3.4. CoCl2-Mimicked Hypoxia Inhibits the Sprouting of Aortic Rings
3.5. Different Metabolic Profiles Correspond to the Activation of Endothelial Cell, the Sprouting of Aortic Ring Induced by ROS, and the Inhibition by Propranolol
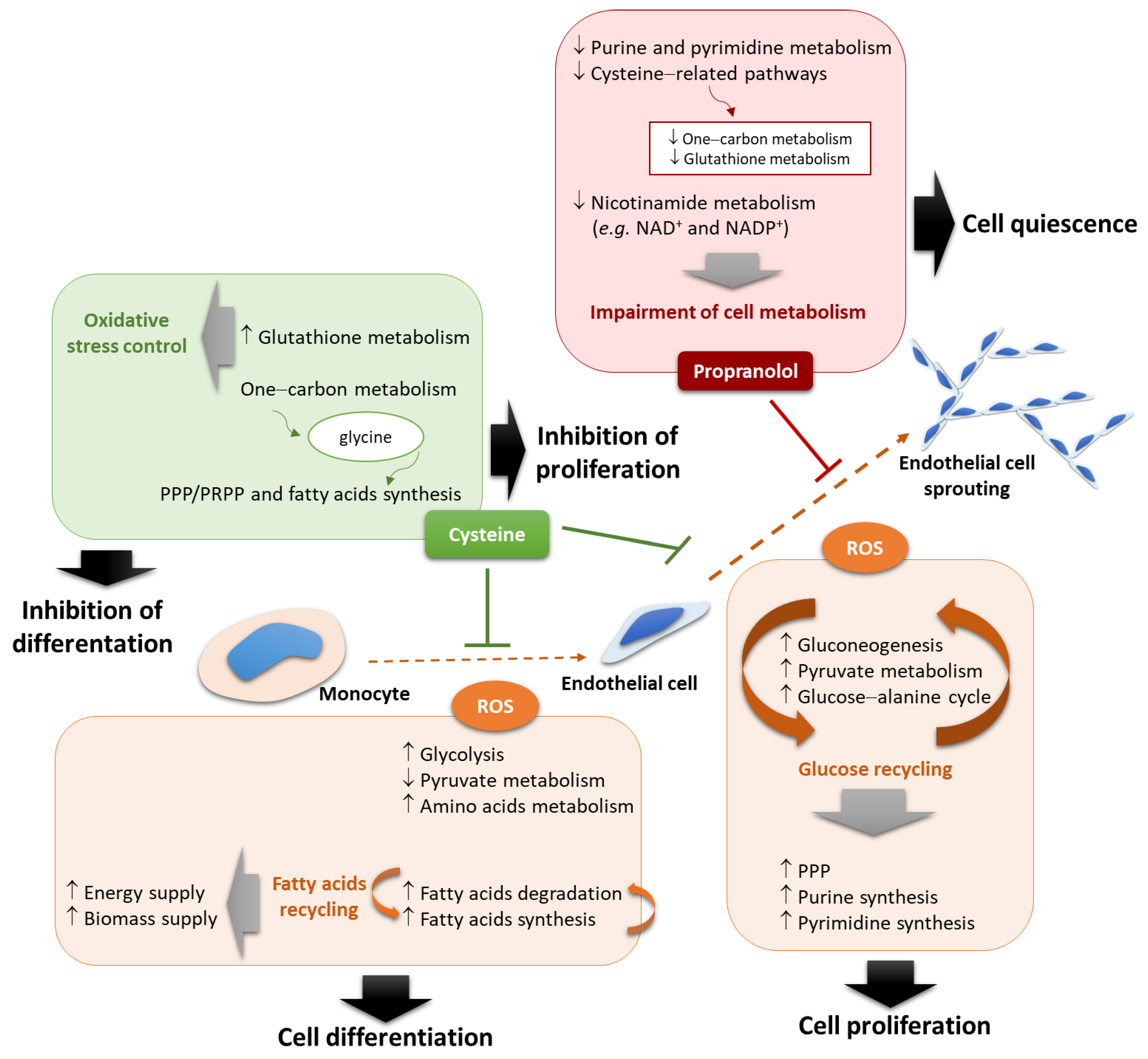
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Caporarello, N.; D’Angeli, F.; Cambria, M.T.; Candido, S.; Giallongo, C.; Salmeri, M.; Lombardo, C.; Longo, A.; Giurdanella, G.; Anfuso, C.D.; et al. Pericytes in Microvessels: From “Mural” Function to Brain and Retina Regeneration. Int. J. Mol. Sci. 2019, 20, 6351. [Google Scholar] [CrossRef] [PubMed]
- Reddy, L.V.K.; Murugan, D.; Mullick, M.; Begum Moghal, E.T.; Sen, D. Recent Approaches for Angiogenesis in Search of Successful Tissue Engineering and Regeneration. Curr. Stem Cell Res. Ther. 2020, 15, 111–134. [Google Scholar] [CrossRef] [PubMed]
- Reinke, J.M.; Sorg, H. Wound Repair and Regeneration. Eur. Surg. Res. 2012, 49, 35–43. [Google Scholar] [CrossRef]
- Kurz, H. Physiology of Angiogenesis. J. Neurooncol. 2000, 50, 17–35. [Google Scholar] [CrossRef]
- Elorza Ridaura, I.; Sorrentino, S.; Moroni, L. Parallels between the Developing Vascular and Neural Systems: Signaling Pathways and Future Perspectives for Regenerative Medicine. Adv. Sci. 2021, 8, 2101837. [Google Scholar] [CrossRef]
- Lopes-Coelho, F.; Martins, F.; Serpa, J. Endothelial Cells (ECs) Metabolism: A Valuable Piece to Disentangle Cancer Biology. In Tumor Microenvironment; Springer: Berlin/Heidelberg, Germany, 2020; pp. 143–159. [Google Scholar]
- Lopes-Coelho, F.; Martins, F.; Pereira, S.A.; Serpa, J. Anti-Angiogenic Therapy: Current Challenges and Future Perspectives. Int. J. Mol. Sci. 2021, 22, 3765. [Google Scholar] [CrossRef]
- Lopes-Coelho, F.; Silva, F.; Gouveia-Fernandes, S.; Martins, C.; Lopes, N.; Domingues, G.; Brito, C.; Almeida, A.M.; Pereira, S.A.; Serpa, J. Monocytes as Endothelial Progenitor Cells (EPCs), Another Brick in the Wall to Disentangle Tumor Angiogenesis. Cells 2020, 9, 107. [Google Scholar] [CrossRef]
- Gianni-Barrera, R.; Butschkau, A.; Uccelli, A.; Certelli, A.; Valente, P.; Bartolomeo, M.; Groppa, E.; Burger, M.G.; Hlushchuk, R.; Heberer, M.; et al. PDGF-BB Regulates Splitting Angiogenesis in Skeletal Muscle by Limiting VEGF-Induced Endothelial Proliferation. Angiogenesis 2018, 21, 883–900. [Google Scholar] [CrossRef]
- Naito, H.; Iba, T.; Takakura, N. Mechanisms of New Blood-Vessel Formation and Proliferative Heterogeneity of Endothelial Cells. Int. Immunol. 2020, 32, 295–305. [Google Scholar] [CrossRef]
- Potyondy, T.; Uquillas, J.A.; Tebon, P.J.; Byambaa, B.; Hasan, A.; Tavafoghi, M.; Mary, H.; Aninwene, G.E.; Pountos, I.; Khademhosseini, A.; et al. Recent Advances in 3D Bioprinting of Musculoskeletal Tissues. Biofabrication 2021, 13, 022001. [Google Scholar] [CrossRef]
- Laumonier, T.; Menetrey, J. Muscle Injuries and Strategies for Improving Their Repair. J. Exp. Orthop. 2016, 3, 15. [Google Scholar] [CrossRef] [PubMed]
- Subbiah, R.; Ruehle, M.A.; Klosterhoff, B.S.; Lin, A.S.P.; Hettiaratchi, M.H.; Willett, N.J.; Bertassoni, L.E.; García, A.J.; Guldberg, R.E. Triple Growth Factor Delivery Promotes Functional Bone Regeneration Following Composite Musculoskeletal Trauma. Acta Biomater. 2021, 127, 180–192. [Google Scholar] [CrossRef] [PubMed]
- Gross-Amat, O.; Guillen, M.; Salmon, D.; Nataf, S.; Auxenfans, C. Characterization of a Topically Testable Model of Burn Injury on Human Skin Explants. Int. J. Mol. Sci. 2020, 21, 6956. [Google Scholar] [CrossRef]
- Hofmann, E.; Fink, J.; Eberl, A.; Prugger, E.-M.; Kolb, D.; Luze, H.; Schwingenschuh, S.; Birngruber, T.; Magnes, C.; Mautner, S.I.; et al. A Novel Human Ex Vivo Skin Model to Study Early Local Responses to Burn Injuries. Sci. Rep. 2021, 11, 364. [Google Scholar] [CrossRef]
- Han, Y.-F.; Han, Y.-Q.; Pan, Y.-G.; Chen, Y.-L.; Chai, J.-K. Transplantation of Microencapsulated Cells Expressing VEGF Improves Angiogenesis in Implanted Xenogeneic Acellular Dermis on Wound. Transplant. Proc. 2010, 42, 1935–1943. [Google Scholar] [CrossRef]
- Edri, R.; Gal, I.; Noor, N.; Harel, T.; Fleischer, S.; Adadi, N.; Green, O.; Shabat, D.; Heller, L.; Shapira, A.; et al. Personalized Hydrogels for Engineering Diverse Fully Autologous Tissue Implants. Adv. Mater. 2019, 31, 1803895. [Google Scholar] [CrossRef]
- Lopes-Coelho, F.; Martins, F.; Hipólito, A.; Mendes, C.; Sequeira, C.O.; Pires, R.F.; Almeida, A.M.; Bonifácio, V.D.B.; Pereira, S.A.; Serpa, J. The Activation of Endothelial Cells Relies on a Ferroptosis-Like Mechanism: Novel Perspectives in Management of Angiogenesis and Cancer Therapy. Front. Oncol. 2021, 11, 1666. [Google Scholar] [CrossRef]
- Graça, G.; Lau, C.-H.E.; Gonçalves, L.G. Exploring Cancer Metabolism: Applications of Metabolomics and Metabolic Phenotyping in Cancer Research and Diagnostics. In Tumor Microenvironment; Springer: Berlin/Heidelberg, Germany, 2020; pp. 367–385. [Google Scholar]
- Murphy, M.P.; Bayir, H.; Belousov, V.; Chang, C.J.; Davies, K.J.A.; Davies, M.J.; Dick, T.P.; Finkel, T.; Forman, H.J.; Janssen-Heininger, Y.; et al. Guidelines for Measuring Reactive Oxygen Species and Oxidative Damage in Cells and in Vivo. Nat. Metab. 2022, 4, 651–662. [Google Scholar] [CrossRef]
- Lermant, A.; Murdoch, C.E. Cysteine Glutathionylation Acts as a Redox Switch in Endothelial Cells. Antioxidants 2019, 8, 315. [Google Scholar] [CrossRef]
- Shefa, U.; Kim, M.-S.; Jeong, N.Y.; Jung, J. Antioxidant and Cell-Signaling Functions of Hydrogen Sulfide in the Central Nervous System. Oxid. Med. Cell. Longev. 2018, 2018, 1–17. [Google Scholar] [CrossRef]
- Olas, B. Hydrogen Sulfide as a “Double-Faced” Compound. In Advances in Clinical Chemistry; Academic Press: Cambridge, MA, USA, 2017; pp. 187–196. [Google Scholar]
- Povsic, T.J.; Zavodni, K.L.; Kelly, F.L.; Zhu, S.; Goldschmidt-Clermont, P.J.; Dong, C.; Peterson, E.D. Circulating Progenitor Cells Can Be Reliably Identified on the Basis of Aldehyde Dehydrogenase Activity. J. Am. Coll. Cardiol. 2007, 50, 2243–2248. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lamy, S.; Lachambre, M.-P.; Lord-Dufour, S.; Béliveau, R. Propranolol Suppresses Angiogenesis in Vitro: Inhibition of Proliferation, Migration, and Differentiation of Endothelial Cells. Vascul. Pharmacol. 2010, 53, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.; Chong, J.; Zhou, G.; de Lima Morais, D.A.; Chang, L.; Barrette, M.; Gauthier, C.; Jacques, P.-É.; Li, S.; Xia, J. MetaboAnalyst 5.0: Narrowing the Gap between Raw Spectra and Functional Insights. Nucleic Acids Res. 2021, 49, W388–W396. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-J.; Nan, G.-X. Oxidative Stress-Induced Angiogenesis. J. Clin. Neurosci. 2019, 63, 13–16. [Google Scholar] [CrossRef]
- Aoki, M.; Nata, T.; Morishita, R.; Matsushita, H.; Nakagami, H.; Yamamoto, K.; Yamazaki, K.; Nakabayashi, M.; Ogihara, T.; Kaneda, Y. Endothelial Apoptosis Induced by Oxidative Stress Through Activation of NF-ΚB. Hypertension 2001, 38, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Shaito, A.; Aramouni, K.; Assaf, R.; Parenti, A.; Orekhov, A.; El Yazbi, A.; Pintus, G.; Eid, A.H. Oxidative Stress-Induced Endothelial Dysfunction in Cardiovascular Diseases. Front. Biosci. 2022, 27, 0105. [Google Scholar] [CrossRef]
- Yoshimoto, M.; Waki, A.; Yonekura, Y.; Sadato, N.; Murata, T.; Omata, N.; Takahashi, N.; Welch, M.J.; Fujibayashi, Y. Characterization of Acetate Metabolism in Tumor Cells in Relation to Cell Proliferation: Acetate Metabolism in Tumor Cells. Nucl. Med. Biol. 2001, 28, 117–122. [Google Scholar] [CrossRef]
- Meiser, J.; Tumanov, S.; Maddocks, O.; Labuschagne, C.F.; Athineos, D.; Van Den Broek, N.; Mackay, G.M.; Gottlieb, E.; Blyth, K.; Vousden, K.; et al. Serine One-Carbon Catabolism with Formate Overflow. Sci. Adv. 2016, 2, e1601273. [Google Scholar] [CrossRef]
- Yang, M.; Vousden, K.H. Serine and One-Carbon Metabolism in Cancer. Nat. Rev. Cancer 2016, 16, 650–662. [Google Scholar] [CrossRef]
- Hsu, F.-Y.; Liou, J.-Y.; Tang, F.-Y.; Sou, N.-L.; Peng, J.-H.; Chiang, E.-P.I. Ketogenic Diet Consumption Inhibited Mitochondrial One-Carbon Metabolism. Int. J. Mol. Sci. 2022, 23, 3650. [Google Scholar] [CrossRef]
- Vassalli, G. Aldehyde Dehydrogenases: Not Just Markers, but Functional Regulators of Stem Cells. Stem Cells Int. 2019, 2019, 1–15. [Google Scholar] [CrossRef]
- Blix, E.S.; Kildal, A.B.; Bertelsen, E.; Waage, A.; Myklebust, J.H.; Kolstad, A.; Husebekk, A. Content of Endothelial Progenitor Cells in Autologous Stem Cell Grafts Predict Survival after Transplantation for Multiple Myeloma. Biol. Blood Marrow Transplant. 2015, 21, 840–847. [Google Scholar] [CrossRef][Green Version]
- De Bock, K.; Georgiadou, M.; Schoors, S.; Kuchnio, A.; Wong, B.W.; Cantelmo, A.R.; Quaegebeur, A.; Ghesquière, B.; Cauwenberghs, S.; Eelen, G.; et al. Role of PFKFB3-Driven Glycolysis in Vessel Sprouting. Cell 2013, 154, 651–663. [Google Scholar] [CrossRef]
- Hunt, T.K.; Aslam, R.S.; Beckert, S.; Wagner, S.; Ghani, Q.P.; Hussain, M.Z.; Roy, S.; Sen, C.K. Aerobically Derived Lactate Stimulates Revascularization and Tissue Repair via Redox Mechanisms. Antioxid. Redox Signal. 2007, 9, 1115–1124. [Google Scholar] [CrossRef]
- Ruan, G.-X.; Kazlauskas, A. Lactate Engages Receptor Tyrosine Kinases Axl, Tie2, and Vascular Endothelial Growth Factor Receptor 2 to Activate Phosphoinositide 3-Kinase/Akt and Promote Angiogenesis. J. Biol. Chem. 2013, 288, 21161–21172. [Google Scholar] [CrossRef]
- Végran, F.; Boidot, R.; Michiels, C.; Sonveaux, P.; Feron, O. Lactate Influx through the Endothelial Cell Monocarboxylate Transporter MCT1 Supports an NF-ΚB/IL-8 Pathway That Drives Tumor Angiogenesis. Cancer Res. 2011, 71, 2550–2560. [Google Scholar] [CrossRef]
- Yu, P.; Wilhelm, K.; Dubrac, A.; Tung, J.K.; Alves, T.C.; Fang, J.S.; Xie, Y.; Zhu, J.; Chen, Z.; De Smet, F.; et al. FGF-Dependent Metabolic Control of Vascular Development. Nature 2017, 545, 224–228. [Google Scholar] [CrossRef]
- Parra-Bonilla, G.; Alvarez, D.F.; Al-Mehdi, A.-B.; Alexeyev, M.; Stevens, T. Critical Role for Lactate Dehydrogenase A in Aerobic Glycolysis That Sustains Pulmonary Microvascular Endothelial Cell Proliferation. Am. J. Physiol. Cell. Mol. Physiol. 2010, 299, L513–L522. [Google Scholar] [CrossRef]
- Peters, K.; Kamp, G.; Berz, A.; Unger, R.E.; Barth, S.; Salamon, A.; Rychly, J.; Kirkpatrick, C.J. Changes in Human Endothelial Cell Energy Metabolic Capacities during in Vitro Cultivation. The Role of “Aerobic Glycolysis” and Proliferation. Cell. Physiol. Biochem. 2009, 24, 483–492. [Google Scholar] [CrossRef]
- Schoors, S.; Bruning, U.; Missiaen, R.; Queiroz, K.C.S.; Borgers, G.; Elia, I.; Zecchin, A.; Cantelmo, A.R.; Christen, S.; Goveia, J.; et al. Fatty Acid Carbon Is Essential for DNTP Synthesis in Endothelial Cells. Nature 2015, 520, 192–197. [Google Scholar] [CrossRef]
- Gerbod-Giannone, M.-C.; Dallet, L.; Naudin, G.; Sahin, A.; Decossas, M.; Poussard, S.; Lambert, O. Involvement of Caveolin-1 and CD36 in Native LDL Endocytosis by Endothelial Cells. Biochim. Biophys. Acta Gen. Subj. 2019, 1863, 830–838. [Google Scholar] [CrossRef] [PubMed]
- Cho, S. CD36 as a Therapeutic Target for Endothelial Dysfunction in Stroke. Curr. Pharm. Des. 2012, 18, 3721–3730. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Silverstein, R.L.; Febbraio, M. CD36, a Scavenger Receptor Involved in Immunity, Metabolism, Angiogenesis, and Behavior. Sci. Signal. 2009, 2, re3. [Google Scholar] [CrossRef]
- Elmasri, H.; Karaaslan, C.; Teper, Y.; Ghelfi, E.; Weng, M.; Ince, T.A.; Kozakewich, H.; Bischoff, J.; Cataltepe, S. Fatty Acid Binding Protein 4 Is a Target of VEGF and a Regulator of Cell Proliferation in Endothelial Cells. FASEB J. 2009, 23, 3865–3873. [Google Scholar] [CrossRef]
- Yetkin-Arik, B.; Vogels, I.M.C.; Neyazi, N.; van Duinen, V.; Houtkooper, R.H.; van Noorden, C.J.F.; Klaassen, I.; Schlingemann, R.O. Endothelial Tip Cells in Vitro Are Less Glycolytic and Have a More Flexible Response to Metabolic Stress than Non-Tip Cells. Sci. Rep. 2019, 9, 10414. [Google Scholar] [CrossRef]
- Elmasri, H.; Ghelfi, E.; Yu, C.; Traphagen, S.; Cernadas, M.; Cao, H.; Shi, G.-P.; Plutzky, J.; Sahin, M.; Hotamisligil, G.; et al. Endothelial Cell-Fatty Acid Binding Protein 4 Promotes Angiogenesis: Role of Stem Cell Factor/c-Kit Pathway. Angiogenesis 2012, 15, 457–468. [Google Scholar] [CrossRef]
- Huang, H.; Vandekeere, S.; Kalucka, J.; Bierhansl, L.; Zecchin, A.; Brüning, U.; Visnagri, A.; Yuldasheva, N.; Goveia, J.; Cruys, B.; et al. Role of Glutamine and Interlinked Asparagine Metabolism in Vessel Formation. EMBO J. 2017, 36, 2334–2352. [Google Scholar] [CrossRef]
- Kim, B.; Li, J.; Jang, C.; Arany, Z. Glutamine Fuels Proliferation but Not Migration of Endothelial Cells. EMBO J. 2017, 36, 2321–2333. [Google Scholar] [CrossRef]
- Ludin, A.; Gur-Cohen, S.; Golan, K.; Kaufmann, K.B.; Itkin, T.; Medaglia, C.; Lu, X.-J.; Ledergor, G.; Kollet, O.; Lapidot, T. Reactive Oxygen Species Regulate Hematopoietic Stem Cell Self-Renewal, Migration and Development, As Well As Their Bone Marrow Microenvironment. Antioxid. Redox Signal. 2014, 21, 1605–1619. [Google Scholar] [CrossRef]

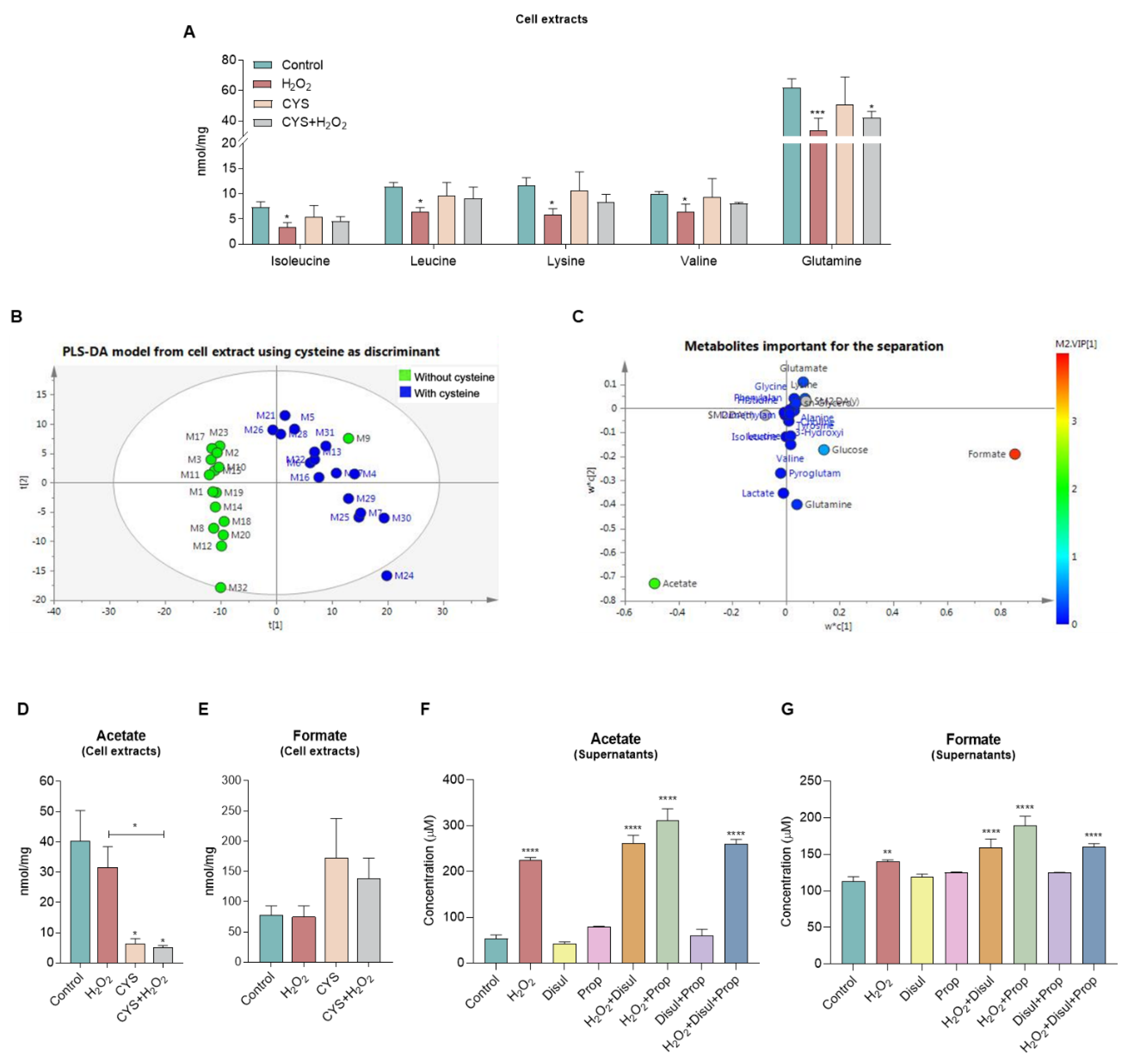
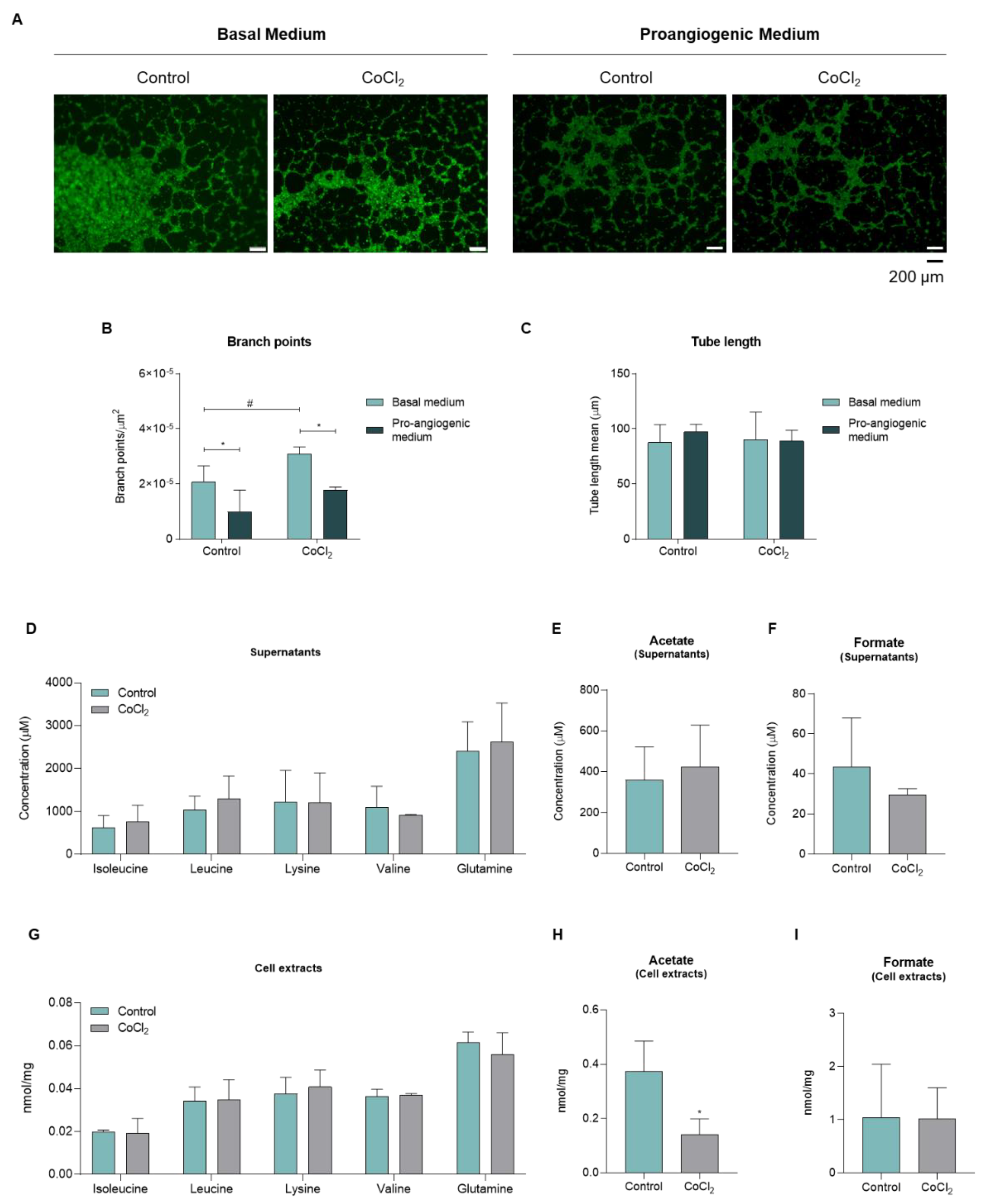
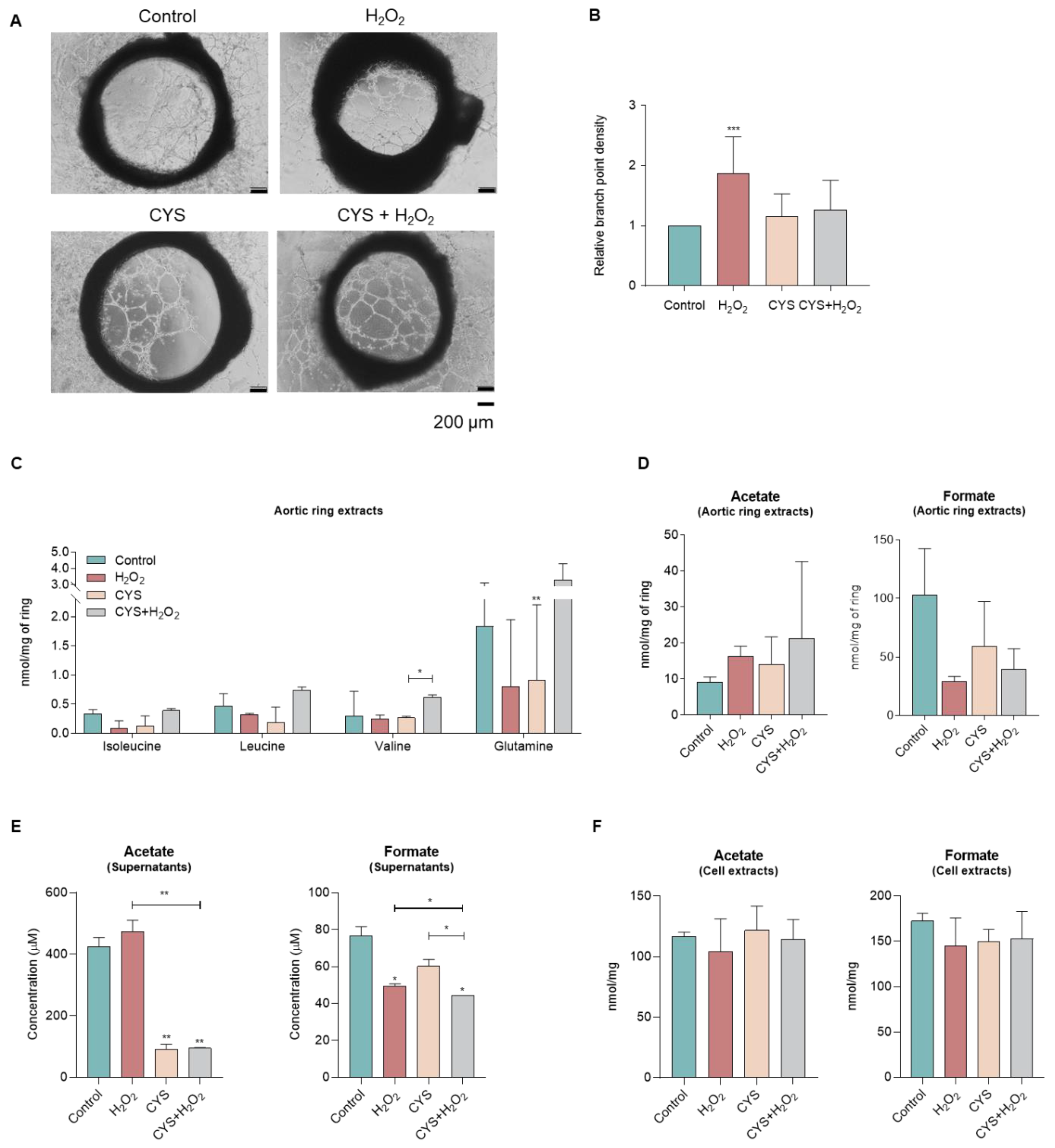

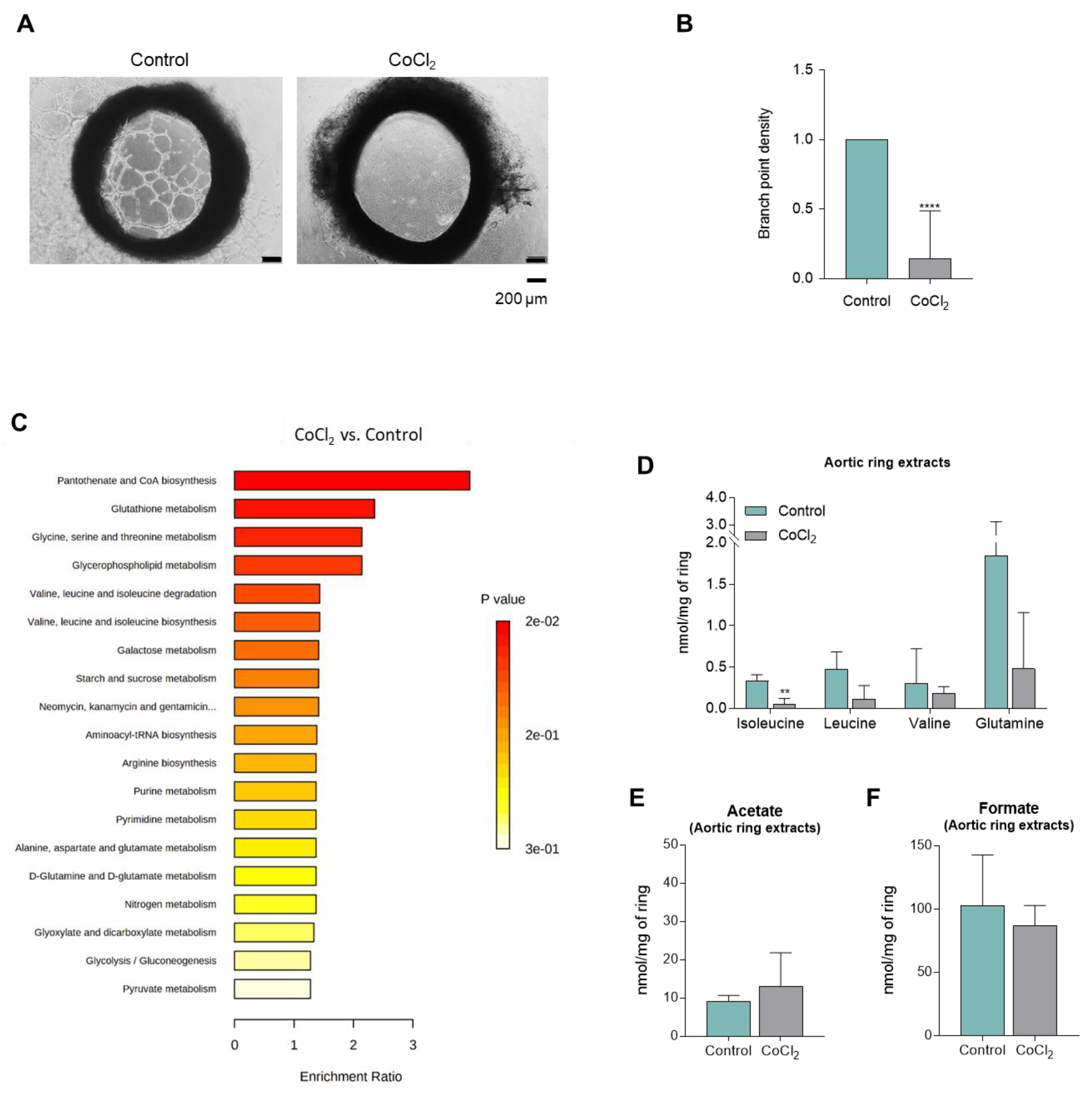

| Supernatants | Cell Extracts | ||||
|---|---|---|---|---|---|
| Culture Condition | Acetate | Formate | BCAA | Putative Biological Process | |
| Monocytes | ROS (H2O2) | ↑ | ↑ | ↓ | ↑ Differentiation |
| Cysteine | ↓ | ↑ | ↑ | ↓ Differentiation ↑ Proliferation | |
| Disulfiram | = | = | NT | ↓ Differentiation | |
| Propranolol | = | = | NT | No effect | |
| HUVECs | CoCl2 | ↑ | ↓ | ↑ | ↑ Proliferation |
| ECAR | ROS (H2O2) | ↑ | ↓ | ↓ | ↑ Proliferation |
| Cysteine | ↓ | ↑ | = | ↓ Proliferation | |
| Propranolol | NT | NT | = | ↓ Proliferation | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopes-Coelho, F.; Martins, F.; Hipólito, A.; Conde, S.V.; Pereira, S.A.; Gonçalves, L.G.; Serpa, J. A Metabolic Signature to Monitor Endothelial Cell Differentiation, Activation, and Vascular Organization. Biomedicines 2022, 10, 2293. https://doi.org/10.3390/biomedicines10092293
Lopes-Coelho F, Martins F, Hipólito A, Conde SV, Pereira SA, Gonçalves LG, Serpa J. A Metabolic Signature to Monitor Endothelial Cell Differentiation, Activation, and Vascular Organization. Biomedicines. 2022; 10(9):2293. https://doi.org/10.3390/biomedicines10092293
Chicago/Turabian StyleLopes-Coelho, Filipa, Filipa Martins, Ana Hipólito, Sílvia V. Conde, Sofia A. Pereira, Luís G. Gonçalves, and Jacinta Serpa. 2022. "A Metabolic Signature to Monitor Endothelial Cell Differentiation, Activation, and Vascular Organization" Biomedicines 10, no. 9: 2293. https://doi.org/10.3390/biomedicines10092293
APA StyleLopes-Coelho, F., Martins, F., Hipólito, A., Conde, S. V., Pereira, S. A., Gonçalves, L. G., & Serpa, J. (2022). A Metabolic Signature to Monitor Endothelial Cell Differentiation, Activation, and Vascular Organization. Biomedicines, 10(9), 2293. https://doi.org/10.3390/biomedicines10092293








