Exploring the Association between Gut and Urine Microbiota and Prostatic Disease including Benign Prostatic Hyperplasia and Prostate Cancer Using 16S rRNA Sequencing
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Population and Specimen Collection
2.2. Demographics Recording of Patients
2.3. Specimen Sequencing and Molecular Methods
2.4. Statistical Analysis
3. Results
3.1. Demographic and Clinical Characteristics of Participants
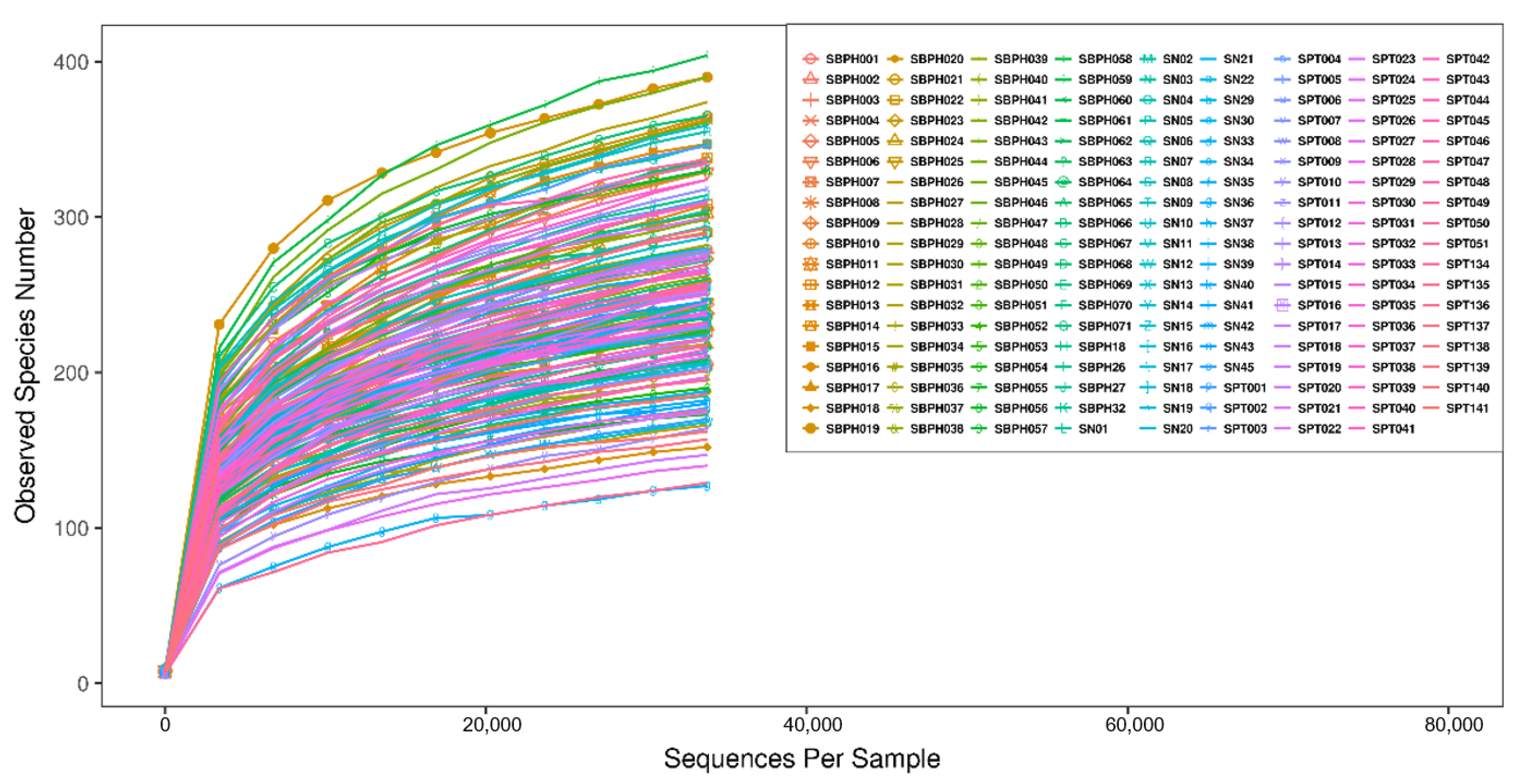
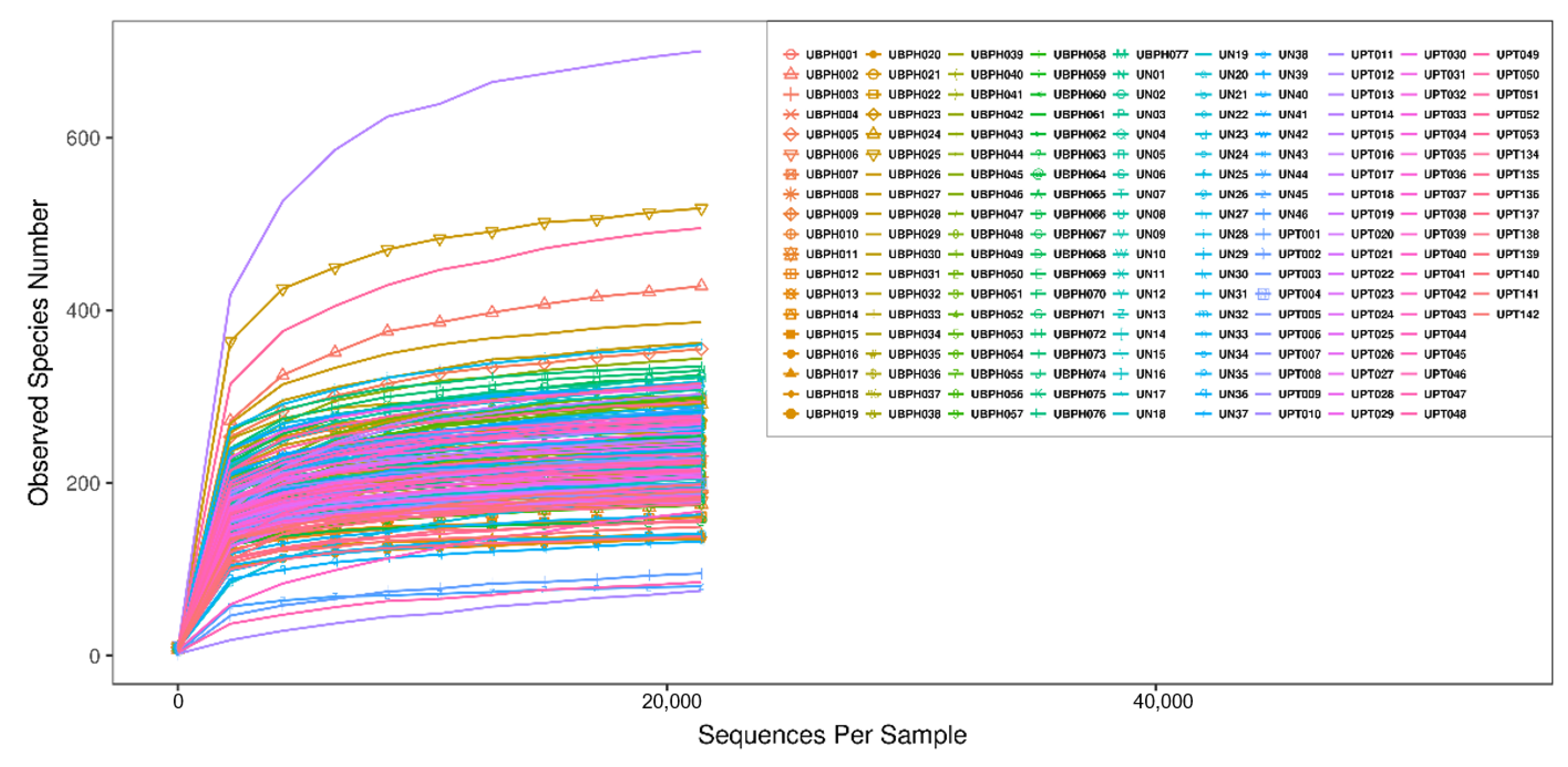
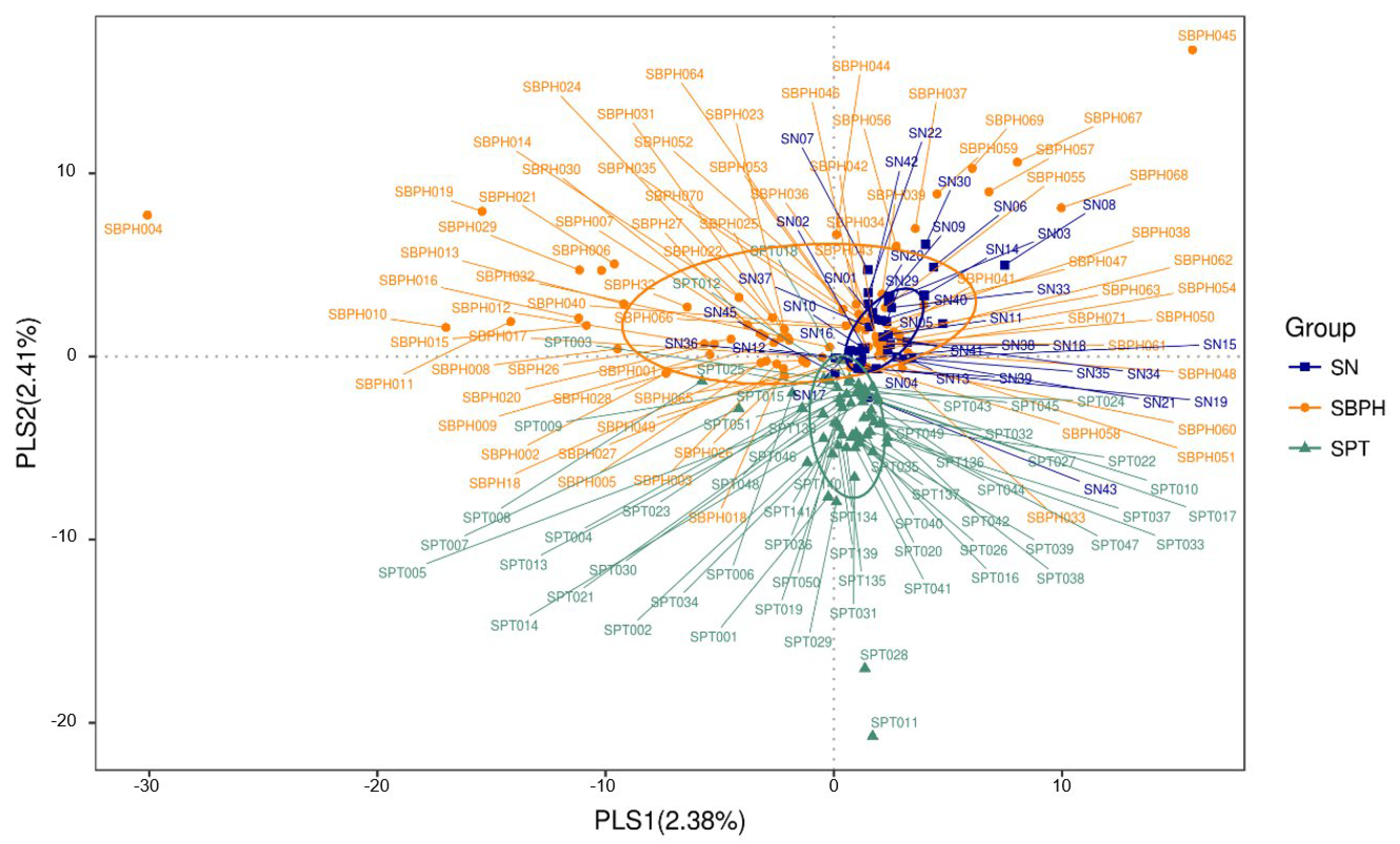
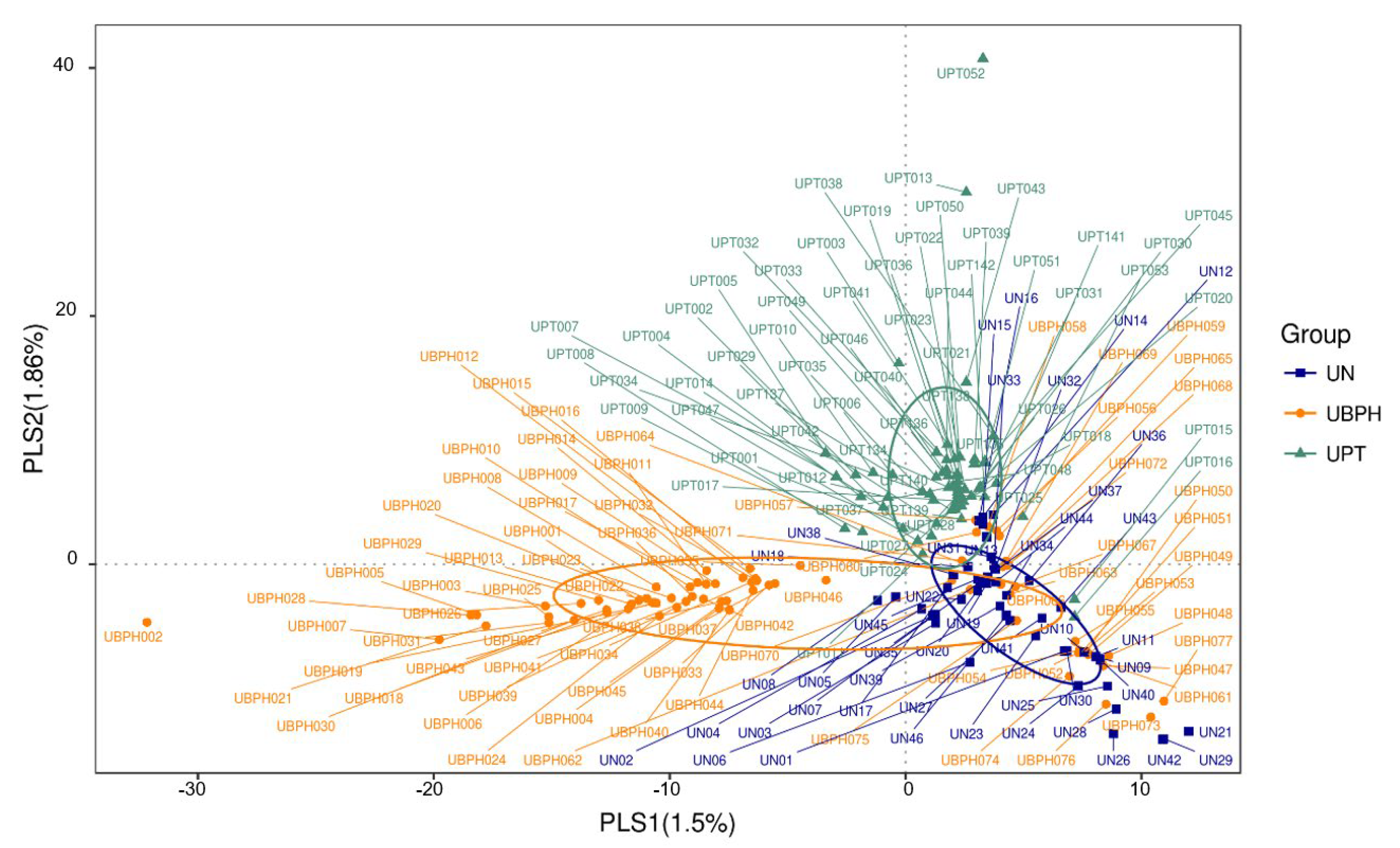
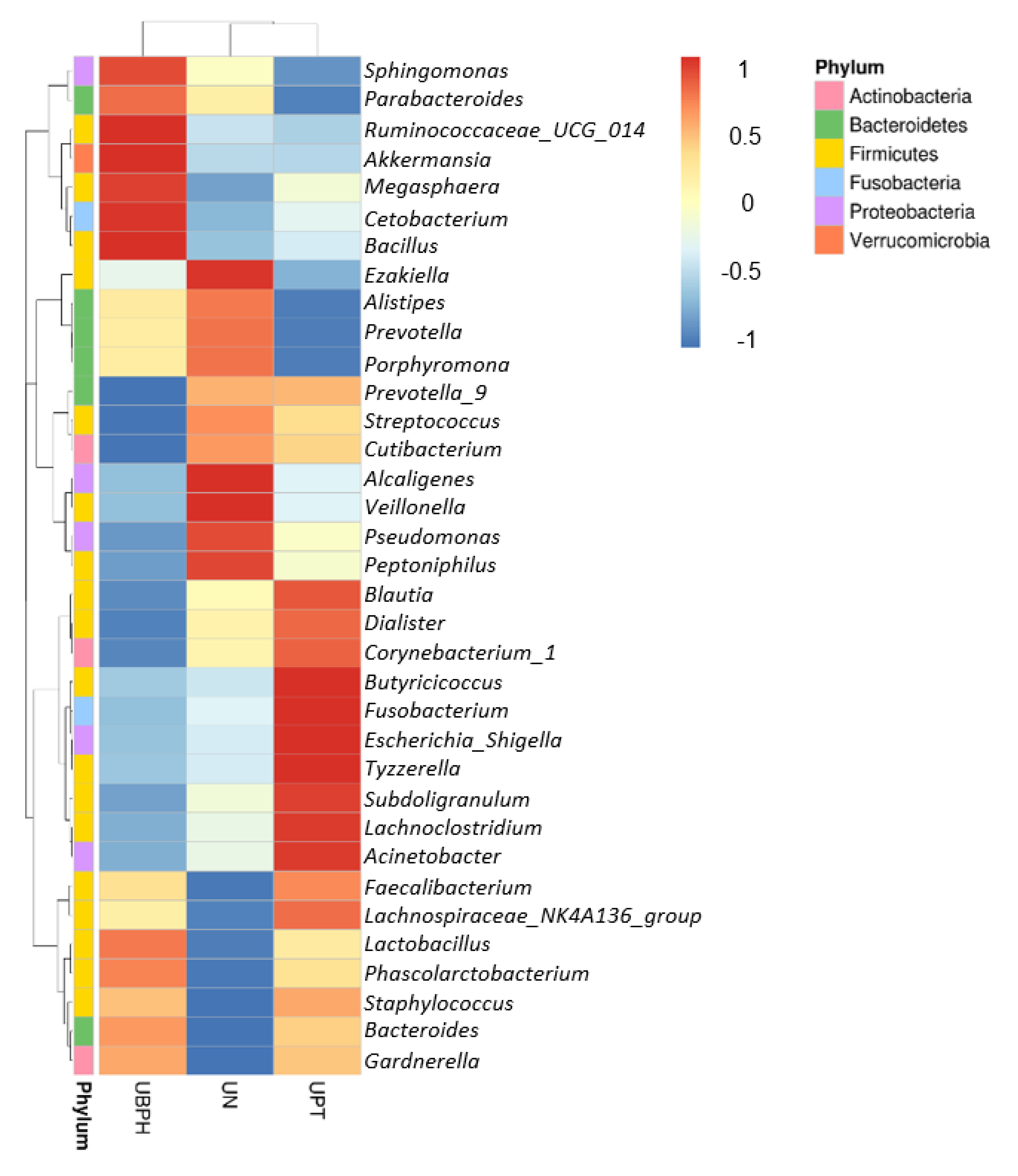
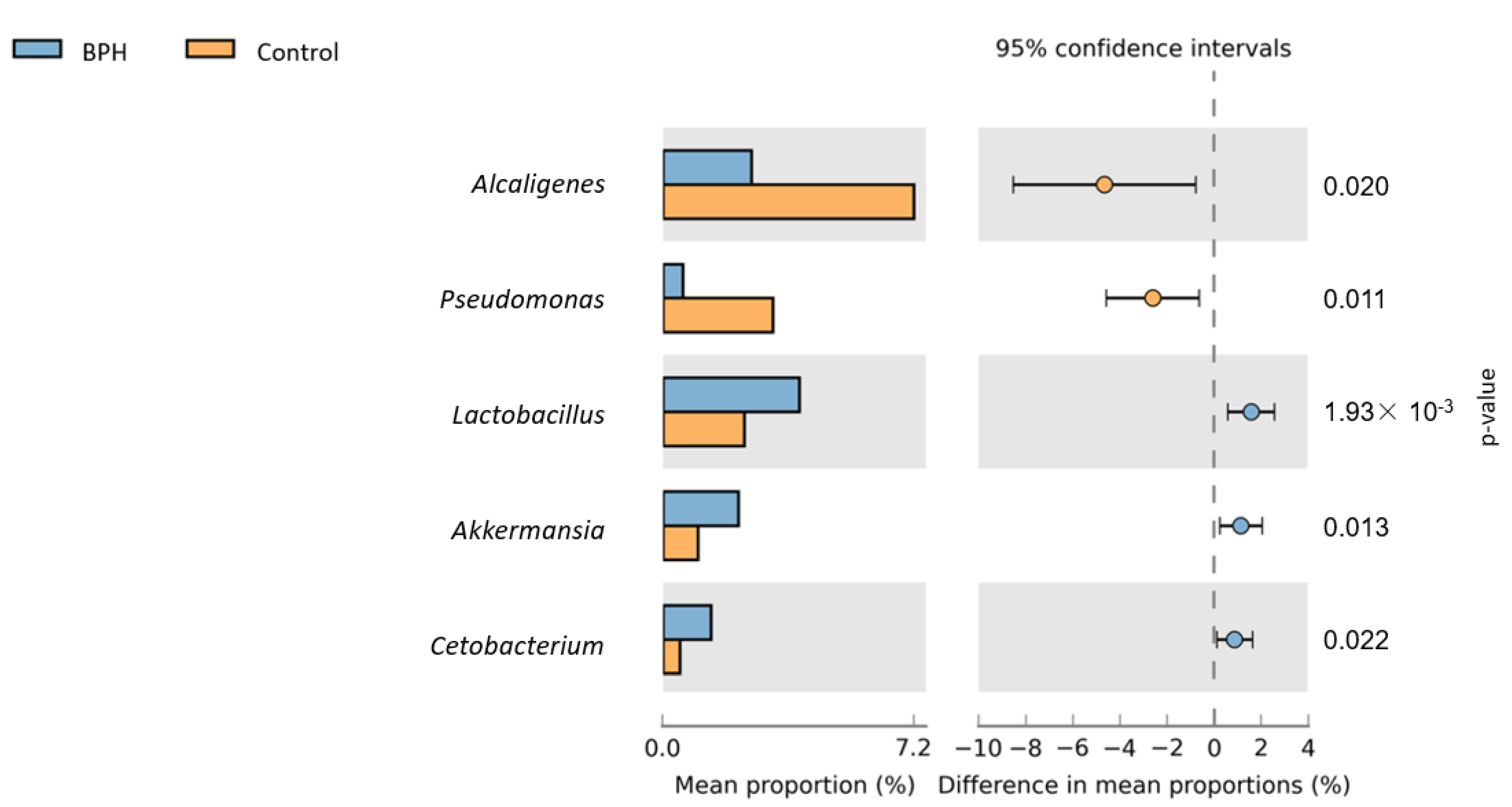
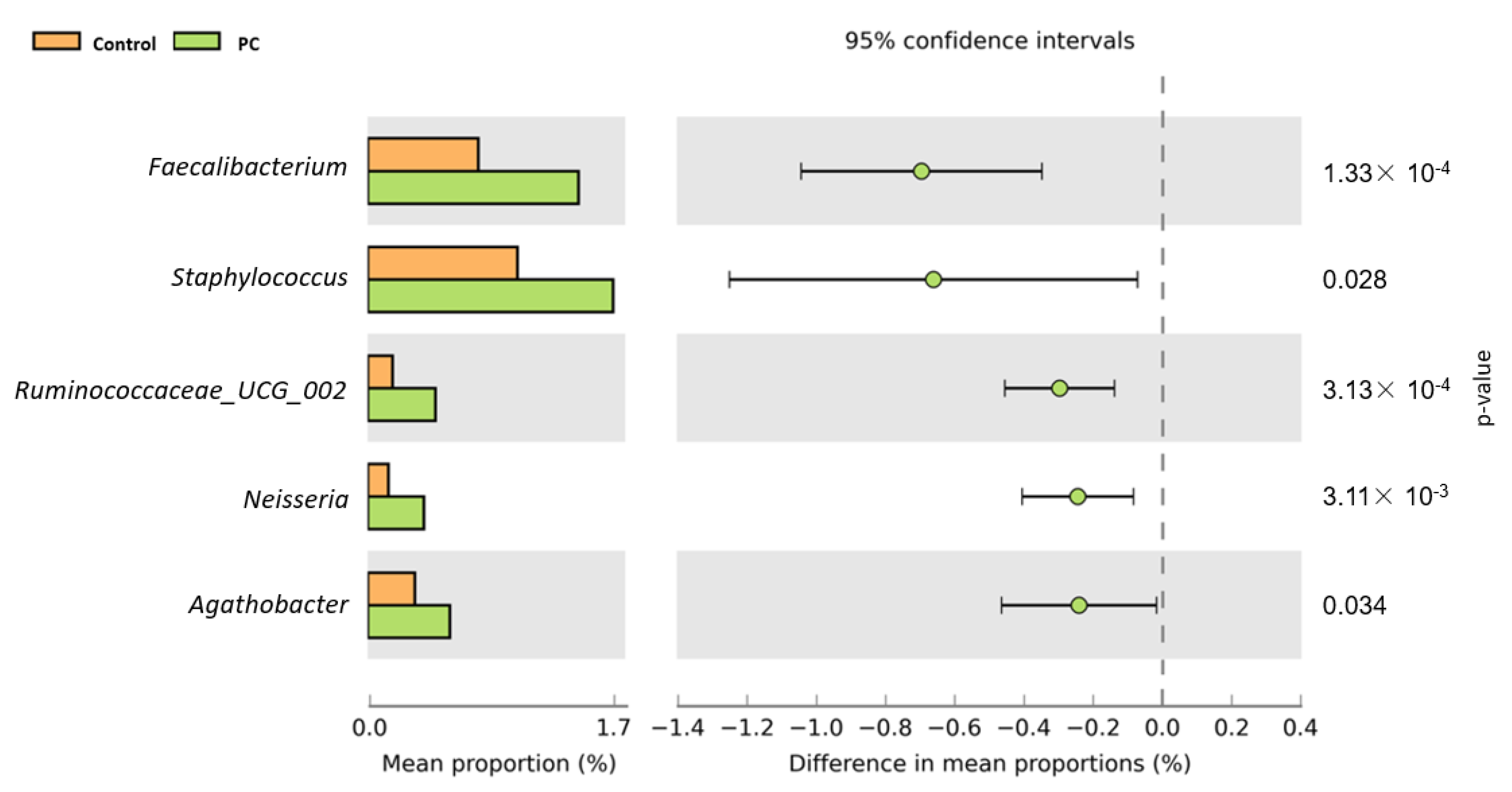
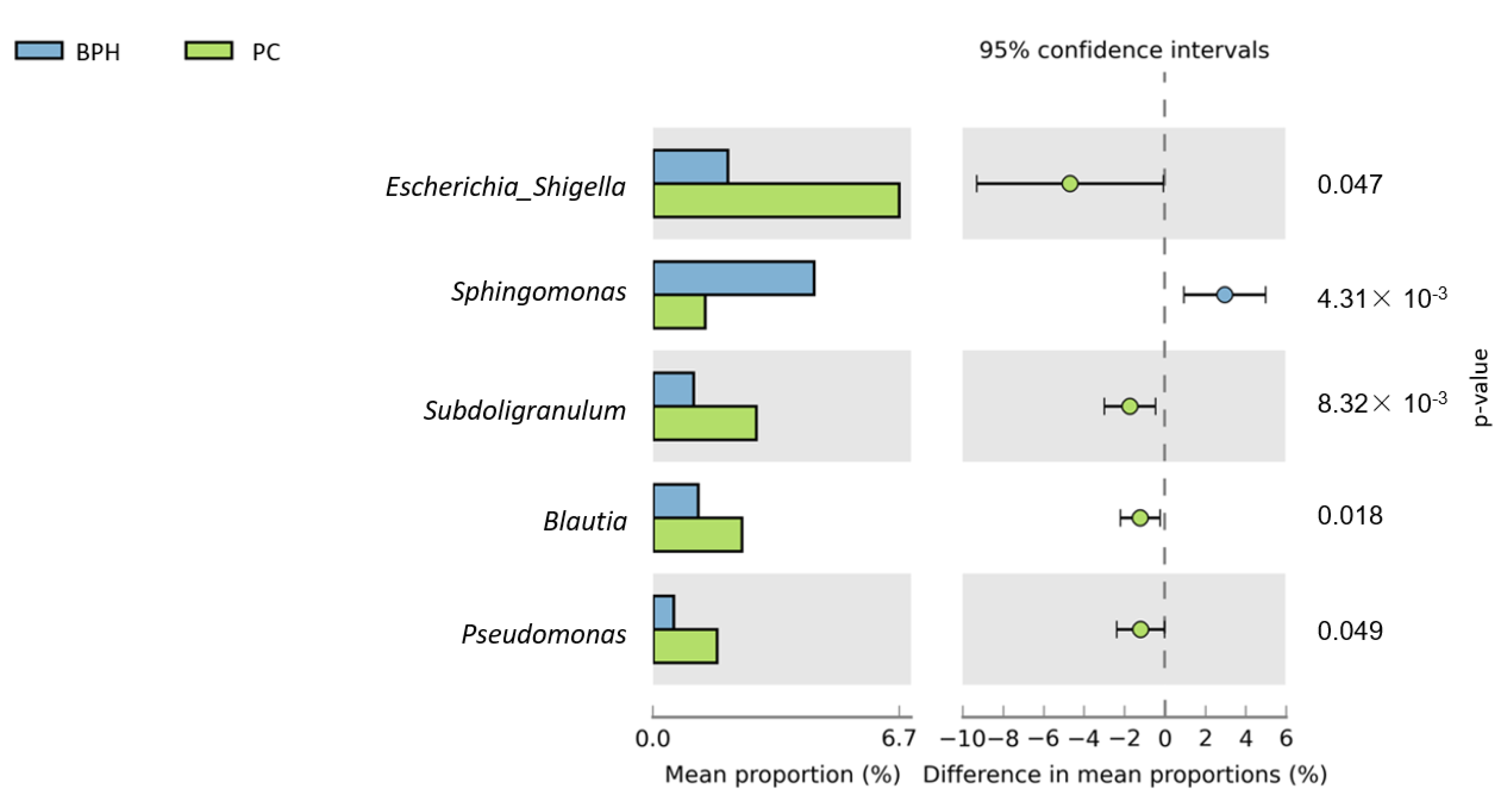
3.2. Bioinformatics Analysis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Picardo, S.L.; Coburn, B.; Hansen, A.R. The microbiome and cancer for clinicians. Crit. Rev. Oncol. Hematol. 2019, 141, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Aragón, I.M.; Herrera-Imbroda, B.; Queipo-Ortuño, M.I.; Castillo, E.; Del Moral, J.S.; Gómez-Millán, J.; Yucel, G.; Lara, M.F. The Urinary Tract Microbiome in Health and Disease. Eur. Urol. Focus. 2018, 4, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Claesson, M.J.; O’Sullivan, O.; Wang, Q.; Nikkilä, J.; Marchesi, J.R.; Smidt, H.; De Vos, W.M.; Paul Ross, R.; O’Toole, P.W. Comparative Analysis of Pyrosequencing and a Phylogenetic Microarray for Exploring Microbial Community Structures in the Human Distal Intestine. PLoS ONE 2009, 4, e6669. [Google Scholar] [CrossRef]
- Dai, Z.; Zhang, J.; Wu, Q.; Chen, J.; Liu, J.; Wang, L.; Chen, C.; Xu, J.; Zhang, H.; Shi, C.; et al. The role of microbiota in the development of colorectal cancer. Int. J. Cancer 2018, 145, 2032–2041. [Google Scholar] [CrossRef] [PubMed]
- Egan, K.B. The Epidemiology of Benign Prostatic Hyperplasia Associated with Lower Urinary Tract Symptoms: Prevalence and Incident Rates. Urol. Clin. N. Am. 2016, 43, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Vuichoud, C.; Loughlin, K.R. Benign prostatic hyperplasia: Epidemiology, economics and evaluation. Can. J. Urol. 2015, 22 (Suppl. 1), 1–6. [Google Scholar]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Daniyal, M.; Siddiqui, Z.A.; Akram, M.; Asif, H.M.; Sultana, S.; Khan, A. Epidemiology, etiology, diagnosis and treatment of prostate cancer. Asian Pac. J. Cancer Prev. 2014, 15, 9575–9578. [Google Scholar] [CrossRef]
- Modi, P.K.; Herrel, L.A.; Kaufman, S.R.; Yan, P.; Borza, T.; Skolarus, T.A.; Schroeck,, F.R.; Hollenbeck, B.K.; Shahinian, V.B. Urologist Practice Structure and Spending for Prostate Cancer Care. Urology 2019, 130, 65–71. [Google Scholar] [CrossRef]
- Yu, H.; Meng, H.; Zhou, F.; Ni, X.; Shen, S.; Das, U.N. Urinary microbiota in patients with prostate cancer and benign prostatic hyperplasia. Arch. Med. Sci. 2015, 11, 385–394. [Google Scholar] [CrossRef]
- Shrestha, E.; White, J.R.; Yu, S.H.; Kulac, I.; Ertunc, O.; De Marzo, A.M.; Yegnasubramanian, S.; Mangold, L.A.; Partin, A.W.; Sfanos, K.S. Profiling the Urinary Microbiome in Men with Positive versus Negative Biopsies for Prostate Cancer. J Urol. 2018, 199, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Jandhyala, S.M.; Talukdar, R.; Subramanyam, C.; Vuyyuru, H.; Sasikala, M.; Nageshwar Reddy, D. Role of the normal gut microbiota. World J. Gastroenterol. 2015, 21, 8787–8803. [Google Scholar] [CrossRef] [PubMed]
- Kalliomäki, M.; Collado, M.C.; Salminen, S.; Isolauri, E. Early differences in fecal microbiota composition in children may predict overweight. Am. J. Clin. Nutr. 2008, 87, 534–538. [Google Scholar] [CrossRef]
- Pascale, A.; Marchesi, N.; Marelli, C.; Coppola, A.; Luzi, L.; Govoni, S.; Giustina, A.; Gazzaruso, C. Microbiota and metabolic diseases. Endocrine 2018, 61, 357–371. [Google Scholar] [CrossRef] [PubMed]
- Holland, B.; Karr, M.; Delfino, K.; Dynda, D.; El-Zawahry, A.; Braundmeier-Fleming, A.; McVary, K.; Alanee, S. The effect of the urinary and faecal microbiota on lower urinary tract symptoms measured by the International Prostate Symptom Score: Analysis utilising next-generation sequencing. BJU Int. 2020, 125, 905–910. [Google Scholar]
- Amirian, E.S.; Petrosino, J.F.; Ajami, N.J.; Liu, Y.; Mims, M.P.; Scheurer, M.E. Potential role of gastrointestinal microbiota composition in prostate cancer risk. Infect. Agent Cancer 2013, 8, 42. [Google Scholar] [CrossRef]
- Bajic, P.; Van Kuiken, M.E.; Burge, B.K.; Kirshenbaum, E.J.; Joyce, C.; Wolfe, A.J.; Branch, J.D.; Bresler, L.; Farooq, A.V. Male Bladder Microbiome Relates to Lower Urinary Tract Symptoms. Eur. Urol. Focus 2020, 6, 376–382. [Google Scholar] [CrossRef]
- Untergasser, G.; Madersbacher, S.; Berger, P. Benign prostatic hyperplasia: Age-related tissue-remodeling. Exp. Gerontol. 2005, 40, 121–128. [Google Scholar] [CrossRef]
- Gandaglia, G.; Briganti, A.; Gontero, P.; Mondaini, N.; Novara, G.; Salonia, A.; Sciarra, A.; Montorsi, F. The role of chronic prostatic inflammation in the pathogenesis and progression of benign prostatic hyperplasia (BPH). BJU Int. 2013, 112, 432–441. [Google Scholar] [CrossRef]
- Sfanos, K.S.; De Marzo, A.M. Prostate cancer and inflammation: The evidence. Histopathology 2012, 60, 199–215. [Google Scholar] [CrossRef]
- Yin, S.; Xu, D.; Zhang, M.; Zhang, P.; Guan, Y.; Kzhyshkowska, J.; Liang, C. Urine flora imbalance and new biomarkers in prostate cancer and benign prostatic hyperplasia. Arch. Med. Sci. 2021. [Google Scholar] [CrossRef]
- Mändar, R.; Punab, M.; Korrovits, P.; Türk, S.; Ausmees, K.; Lapp, E.; Preem, J.K.; Oopkaup, K.; Salumets, A.; Truu, J. Seminal microbiome in men with and without prostatitis. Int. J. Urol. 2017, 24, 211–216. [Google Scholar] [CrossRef]
- Takeda, K.; Okumura, K. Effects of a Fermented Milk Drink Containing Lactobacillus casei Strain Shirota on the Human NK-Cell Activity. J. Nutr. 2007, 137 (Suppl. 2), 791S–793S. [Google Scholar] [CrossRef] [PubMed]
- Horinaka, M.; Yoshida, T.; Kishi, A.; Akatani, K.; Yasuda, T.; Kouhara, J.; Wakada, M.; Sakai, T. Lactobacillus strains induce TRAIL production and facilitate natural killer activity against cancer cells. FEBS Lett. 2010, 584, 577–582. [Google Scholar] [CrossRef]
- Divella, R.; DE Palma, G.; Tufaro, A.; Pelagio, G.; Gadaleta-Caldarola, G.; Bringiotti, R.; Paradiso, A. Diet, Probiotics and Physical Activity: The Right Allies for a Healthy Microbiota. Anticancer Res. 2021, 41, 2759–2772. [Google Scholar] [CrossRef] [PubMed]
- Li, J.K.M.; Wang, L.L.; Wong, C.Y.P.; Chiu, P.K.F.; Teoh, J.Y.C.; Kwok, H.S.W.; Leung, S.C.H.; Wong, S.H.; Tsui, S.K.W.; Ng, C.-F. A cross-sectional study on gut microbiota in prostate cancer patients with prostatectomy or androgen deprivation therapy. Prostate Cancer Prostatic Dis. 2021, 24, 1063–1072. [Google Scholar] [CrossRef] [PubMed]

| Control | BPH | PC | p Value | |
|---|---|---|---|---|
| Number of Urine Sample | 46 | 77 | 62 | |
| Mean ± SD | Mean ± SD | Mean ± SD | ||
| Age | 62.84 ± 7.70 | 69.44 ± 8.23 | 71.15 ± 7.35 | <0.01 |
| Height (m) | 1.67 ± 0.06 | 1.67 ± 0.07 | 1.66 ± 0.06 | 0.24 |
| Weight (kg) | 69.08 ± 8.61 | 67.91 ± 11.67 | 67.36 ± 8.94 | 0.68 |
| BMI | 24.77 ± 2.89 | 24.14 ± 3.29 | 24.50 ± 2.68 | 0.51 |
| Prostate size (gm) | N/A | 42.29 ± 20.64 | 47.43 ± 22.35 | 0.19 |
| IPSS | 2.29 ± 2.07 | 8.74 ± 6.88 | 6.66 ± 6.51 | <0.01 |
| Voiding symptoms | 0.44 ± 1.12 | 4.26 ± 4.21 | 2.75 ± 4.05 | <0.01 |
| Storage symptoms | 1.84 ± 1.26 | 4.48 ± 3.11 | 3.90 ± 3.00 | <0.01 |
| QOLS | 1.18 ± 0.44 | 2.56 ± 1.21 | 2.23 ± 1.01 | <0.01 |
| Ac sugar | 109.77 ± 26.59 | 110.54 ± 17.22 | 115.48 ± 21.48 | 0.29 |
| HbAlc | 5.95 ± 0.53 | 5.81 ± 0.67 | 6.02 ± 0.85 | 0.19 |
| Chol | 185.36 ± 31.92 | 181.47 ± 42.32 | 186.68 ± 37.91 | 0.71 |
| TG | 104.59 ± 47.76 | 108.32 ± 66.26 | 113.80 ± 63.12 | 0.74 |
| LDL | 115.87 ± 28.52 | 110.49 ± 33.61 | 113.63 ± 35.21 | 0.68 |
| HDL | 48.77 ± 12.81 | 54.06 ± 24.29 | 50.17 ± 19.87 | 0.34 |
| GOT | 27.44 ± 7.69 | 26.00 ± 7.59 | 29.68 ± 18.47 | 0.23 |
| GPT | 31.07 ± 18.95 | 26.08 ± 12.78 | 29.90 ± 23.12 | 0.28 |
| BUN | 13.32 ± 3.07 | 15.37 ± 4.95 | 14.73 ± 4.21 | 0.08 |
| Cr | 0.96 ± 0.18 | 0.98 ± 0.21 | 1.02 ± 0.24 | 0.41 |
| PSA | 2.07 ± 3.21 | 2.99 ± 3.20 | 2.66 ± 8.86 | 0.62 |
| Free PSA | 0.60 ± 1.07 | 0.60 ± 0.44 | 0.29 ± 1.07 | 0.60 |
| Testosterone (ng/dL) | 565.06 ± 213.58 | 551.67 ± 246.50 | 307.82 ± 258.03 | <0.01 |
| Urine Sample | R-Value | p-Value |
| Control vs. PC | 0.0491 | 0.0170 |
| Control vs. BPH | 0.1086 | 0.0010 |
| BPH vs. PC | 0.0646 | 0.0010 |
| Stool Sample | R-Value | p-Value |
| Control vs. PC | −0.0176 | 0.7430 |
| Control vs. BPH | 0.0239 | 0.2170 |
| BPH vs. PC | 0.0179 | 0.1030 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, K.-Y.; Wu, D.-C.; Wu, W.-J.; Wang, J.-W.; Juan, Y.-S.; Li, C.-C.; Liu, C.-J.; Lee, H.-Y. Exploring the Association between Gut and Urine Microbiota and Prostatic Disease including Benign Prostatic Hyperplasia and Prostate Cancer Using 16S rRNA Sequencing. Biomedicines 2022, 10, 2676. https://doi.org/10.3390/biomedicines10112676
Tsai K-Y, Wu D-C, Wu W-J, Wang J-W, Juan Y-S, Li C-C, Liu C-J, Lee H-Y. Exploring the Association between Gut and Urine Microbiota and Prostatic Disease including Benign Prostatic Hyperplasia and Prostate Cancer Using 16S rRNA Sequencing. Biomedicines. 2022; 10(11):2676. https://doi.org/10.3390/biomedicines10112676
Chicago/Turabian StyleTsai, Kai-Yen, Deng-Chyang Wu, Wen-Jeng Wu, Jiunn-Wei Wang, Yung-Shun Juan, Ching-Chia Li, Chung-Jung Liu, and Hsiang-Ying Lee. 2022. "Exploring the Association between Gut and Urine Microbiota and Prostatic Disease including Benign Prostatic Hyperplasia and Prostate Cancer Using 16S rRNA Sequencing" Biomedicines 10, no. 11: 2676. https://doi.org/10.3390/biomedicines10112676
APA StyleTsai, K.-Y., Wu, D.-C., Wu, W.-J., Wang, J.-W., Juan, Y.-S., Li, C.-C., Liu, C.-J., & Lee, H.-Y. (2022). Exploring the Association between Gut and Urine Microbiota and Prostatic Disease including Benign Prostatic Hyperplasia and Prostate Cancer Using 16S rRNA Sequencing. Biomedicines, 10(11), 2676. https://doi.org/10.3390/biomedicines10112676







