Integrated Neuroregenerative Techniques for Plasticity of the Injured Spinal Cord
Abstract
1. Introduction
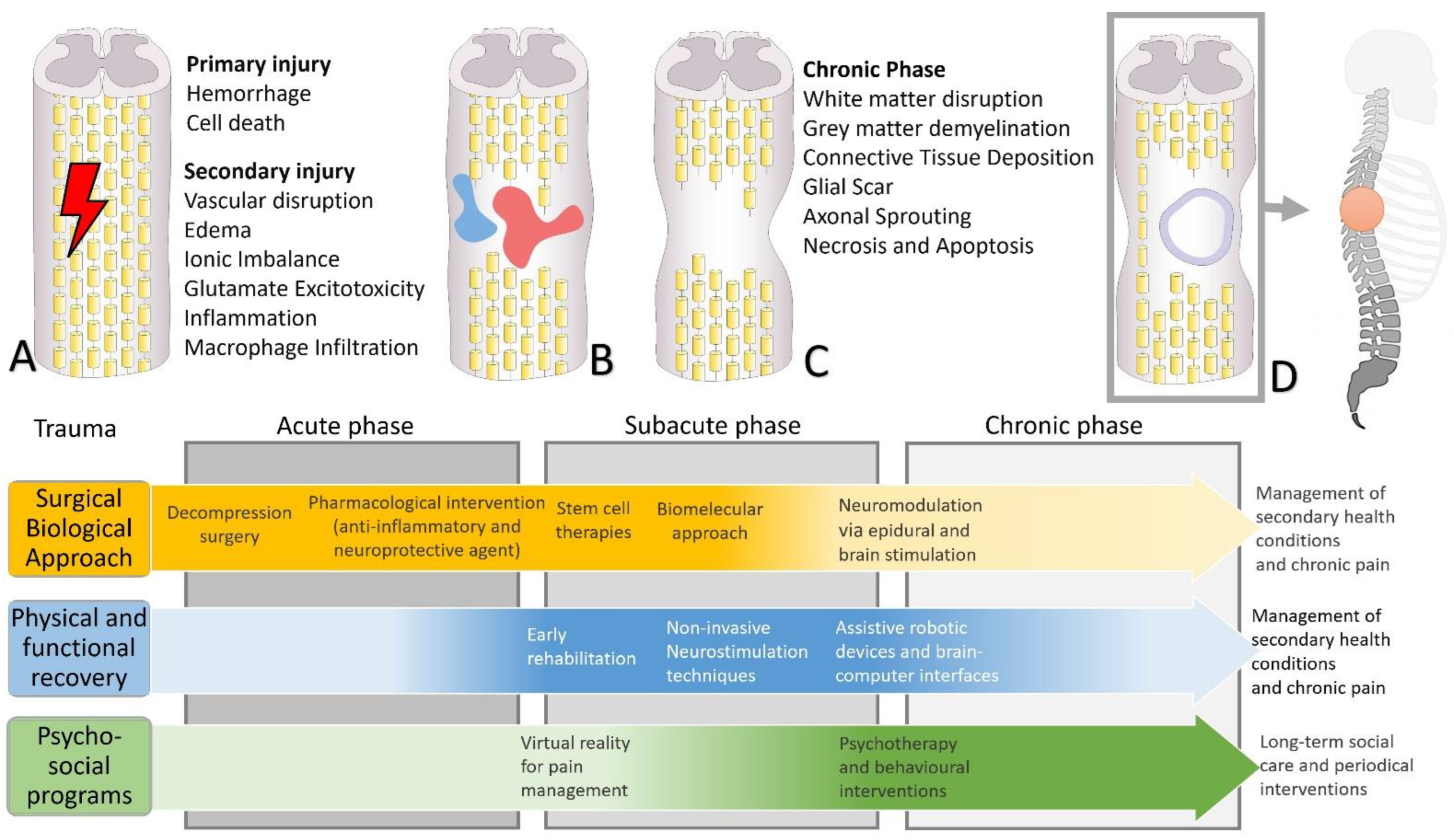
2. Pathophysiology and Functional Recovery
2.1. Spinal Cord Injury Pathophysiology
2.2. Functional Reorganization
3. From Pharmacological to Stem Cell Interventions
3.1. Pharmacological and Nanotechnological Approach
3.2. New Biomolecular Approaches
3.3. Stem Cells Approach
4. From Neuromodulation to Sensorimotor Rehabilitation Interventions
4.1. Neuromodulation Approach
4.2. Prosthetic and Neural Interfaces
5. Constructing an Integrative Behavioral Approach for a Better Quality of Life
6. Therapeutic Strategies and Innovative Biotechnological Opportunities
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Furlan, J.C.; Sakakibara, B.M.; Miller, W.C.; Krassioukov, A.V. Global incidence and prevalence of traumatic spinal cord injury. Can. J. Neurol. Sci. 2013, 40, 456–464. [Google Scholar] [CrossRef] [PubMed]
- WHO. International Perspectives on Spinal Cord Injury. In International Perspectives on Spinal Cord Injury; WHO: Geneva, Switzerland, 2013; pp. 1–231. [Google Scholar]
- Lee, B.B.; Cripps, R.A.; Fitzharris, M.; Wing, P.C. The global map for traumatic spinal cord injury epidemiology: Update 2011, global incidence rate. Spinal Cord 2014, 52, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Al Mamun, A.; Yuan, Y.; Lu, Q.; Xiong, J.; Yang, S.; Wu, C.; Wu, Y.; Wang, J. Acute spinal cord injury: Pathophysiology and pharmacological intervention (Review). Mol. Med. Rep. 2021, 23, 417. [Google Scholar] [CrossRef] [PubMed]
- Leemhuis, E.; Giuffrida, V.; De Martino, M.L.; Forte, G.; Pecchinenda, A.; De Gennaro, L.; Giannini, A.M.; Pazzaglia, M. Rethinking the Body in the Brain after Spinal Cord Injury. J. Clin. Med. 2022, 11, 388. [Google Scholar] [CrossRef] [PubMed]
- Waller, M.; Jorgensen, S.; Lexell, J. Changes over 6 years in secondary health conditions and activity limitations in older adults aging with long-term spinal cord injury. PMR 2022, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.; Weaver, L.C. The dark side of neuroplasticity. Exp. Neurol. 2012, 235, 133–141. [Google Scholar] [CrossRef]
- Muller, R.; Landmann, G.; Bechir, M.; Hinrichs, T.; Arnet, U.; Jordan, X.; Brinkhof, M.W.G. Chronic pain, depression and quality of life in individuals with spinal cord injury: Mediating role of participation. J. Rehabil. Med. 2017, 49, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, R.; Vieira Alves, Y.; Sperling, L.E.; Pranke, P. Nanotechnology for the Treatment of Spinal Cord Injury. Tissue Eng. Part B Rev. 2021, 27, 353–365. [Google Scholar] [CrossRef] [PubMed]
- Leemhuis, E.; De Gennaro, L.; Pazzaglia, A.M. Disconnected Body Representation: Neuroplasticity Following Spinal Cord Injury. J. Clin. Med. 2019, 8, 2144. [Google Scholar] [CrossRef]
- Alizadeh, A.; Dyck, S.M.; Karimi-Abdolrezaee, S. Traumatic Spinal Cord Injury: An Overview of Pathophysiology, Models and Acute Injury Mechanisms. Front. Neurol. 2019, 10, 282. [Google Scholar] [CrossRef] [PubMed]
- Guest, J.; Datta, N.; Jimsheleishvili, G.; Gater, D.R., Jr. Pathophysiology, Classification and Comorbidities after Traumatic Spinal Cord Injury. J. Pers. Med. 2022, 12, 1126. [Google Scholar] [CrossRef] [PubMed]
- Anjum, A.; Yazid, M.D.; Fauzi Daud, M.; Idris, J.; Ng, A.M.H.; Selvi Naicker, A.; Ismail, O.H.R.; Athi Kumar, R.K.; Lokanathan, Y. Spinal Cord Injury: Pathophysiology, Multimolecular Interactions, and Underlying Recovery Mechanisms. Int. J. Mol. Sci. 2020, 21, 7533. [Google Scholar] [CrossRef] [PubMed]
- Craig, A.; Duff, J.; Middleton, J. Spinal Cord Injuries. In Comprehensive Clinical Psychology, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2022; Volume 8, pp. 301–328. [Google Scholar]
- Silvestro, S.; Bramanti, P.; Trubiani, O.; Mazzon, E. Stem cells therapy for spinal cord injury: An overview of clinical trials. Int. J. Mol. Sci. 2020, 21, 659. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, Y.; Zhou, G.; Hu, X.; Han, S.; Gao, J. The combination of nanoscaffolds and stem cell transplantation: Paving a promising road for spinal cord injury regeneration. Biomed Pharm. 2021, 143, 112233. [Google Scholar] [CrossRef] [PubMed]
- Cadel, L.; C. Everall, A.; Hitzig, S.L.; Packer, T.L.; Patel, T.; Lofters, A.; Guilcher, S.J.T. Spinal cord injury and polypharmacy: A scoping review. Disabil. Rehabil. 2020, 42, 3858–3870. [Google Scholar] [CrossRef] [PubMed]
- Curt, A.; Schwab, M.E.; Dietz, V. Providing the clinical basis for new interventional therapies: Refined diagnosis and assessment of recovery after spinal cord injury. Spinal Cord 2004, 42, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Curt, A.; Van Hedel, H.J.; Klaus, D.; Dietz, V.; Group, E.-S.S. Recovery from a spinal cord injury: Significance of compensation, neural plasticity, and repair. J. Neurotrauma 2008, 25, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Xiang, Z.; Yan, R.; Zhao, M.; Wu, Y.; Zhong, J.; Guo, L.; Li, H.; Wang, J.; Wu, J.; et al. Motor recovery at 6 months after admission is related to structural and functional reorganization of the spine and brain in patients with spinal cord injury. Hum. Brain Mapp. 2016, 37, 2195–2209. [Google Scholar] [CrossRef]
- Min, Y.S.; Park, J.W.; Jin, S.U.; Jang, K.E.; Nam, H.U.; Lee, Y.S.; Jung, T.D.; Chang, Y. Alteration of Resting-State Brain Sensorimotor Connectivity following Spinal Cord Injury: A Resting-State Functional Magnetic Resonance Imaging Study. J. Neurotrauma 2015, 32, 1422–1427. [Google Scholar] [CrossRef] [PubMed]
- Oni-Orisan, A.; Kaushal, M.; Li, W.; Leschke, J.; Ward, B.D.; Vedantam, A.; Kalinosky, B.; Budde, M.D.; Schmit, B.D.; Li, S.J.; et al. Alterations in Cortical Sensorimotor Connectivity following Complete Cervical Spinal Cord Injury: A Prospective Resting-State fMRI Study. PLoS ONE 2016, 11, e0150351. [Google Scholar] [CrossRef]
- Guo, Y.; Ge, Y.; Li, J.; Dou, W.; Pan, Y. Impact of injury duration on a sensorimotor functional network in complete spinal cord injury. J. Neurosci. Res. 2022, 100, 1765–1774. [Google Scholar] [CrossRef] [PubMed]
- Grabher, P.; Callaghan, M.F.; Ashburner, J.; Weiskopf, N.; Thompson, A.J.; Curt, A.; Freund, P. Tracking sensory system atrophy and outcome prediction in spinal cord injury. Ann. Neurol. 2015, 78, 751–761. [Google Scholar] [CrossRef] [PubMed]
- Karunakaran, K.D.; Yuan, R.; He, J.; Zhao, J.; Cui, J.L.; Zang, Y.F.; Zhang, Z.; Alvarez, T.L.; Biswal, B.B. Resting-State Functional Connectivity of the Thalamus in Complete Spinal Cord Injury. Neurorehabil. Neural Repair 2020, 34, 122–133. [Google Scholar] [CrossRef] [PubMed]
- Moxon, K.A.; Oliviero, A.; Aguilar, J.; Foffani, G. Cortical reorganization after spinal cord injury: Always for good? Neuroscience 2014, 283, 78–94. [Google Scholar] [CrossRef]
- Kikkert, S.; Pfyffer, D.; Verling, M.; Freund, P.; Wenderoth, N. Finger somatotopy is preserved after tetraplegia but deteriorates over time. Elife 2021, 10, e67713. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, G.; Grabher, P.; Thompson, A.; Altmann, D.; Hupp, M.; Ashburner, J.; Friston, K.; Weiskopf, N.; Curt, A.; Freund, P. Progressive neurodegeneration following spinal cord injury: Implications for clinical trials. Neurology 2018, 90, e1257–e1266. [Google Scholar] [CrossRef]
- Chen, Q.; Zheng, W.; Chen, X.; Wan, L.; Qin, W.; Qi, Z.; Chen, N.; Li, K. Brain Gray Matter Atrophy after Spinal Cord Injury: A Voxel-Based Morphometry Study. Front. Hum. Neurosci. 2017, 11, 211. [Google Scholar] [CrossRef]
- Zhu, L.; Wu, G.; Zhou, X.; Li, J.; Wen, Z.; Lin, F. Altered spontaneous brain activity in patients with acute spinal cord injury revealed by resting-state functional MRI. PLoS ONE 2015, 10, e0118816. [Google Scholar] [CrossRef]
- Pascoal-Faria, P.; Yalcin, N.; Fregni, F. Neural markers of neuropathic pain associated with maladaptive plasticity in spinal cord injury. Pain Pract. 2015, 15, 371–377. [Google Scholar] [CrossRef]
- Siddall, P.J.; McClelland, J.M.; Rutkowski, S.B.; Cousins, M.J. A longitudinal study of the prevalence and characteristics of pain in the first 5 years following spinal cord injury. Pain 2003, 103, 249–257. [Google Scholar] [CrossRef]
- Wrigley, P.J.; Press, S.R.; Gustin, S.M.; Macefield, V.G.; Gandevia, S.C.; Cousins, M.J.; Middleton, J.W.; Henderson, L.A.; Siddall, P.J. Neuropathic pain and primary somatosensory cortex reorganization following spinal cord injury. Pain 2009, 141, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Hilton, B.J.; Tetzlaff, W. A brainstem bypass for spinal cord injury. Nat. Neurosci. 2018, 21, 457–458. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.; Peterson, C.; Yilmaz, E.; Halalmeh, D.R.; Moisi, M. Current advancements in the management of spinal cord injury: A comprehensive review of literature. Surg. Neurol. Int. 2020, 11, 2. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.Y.; Tsai, M.Y.; Juan, S.H.; Chang, S.F.; Yu, C.R.; Lin, J.C.; Johnson, K.R.; Lim, H.G.; Fann, Y.C.; Lee, Y.G. Exerting the Appropriate Application of Methylprednisolone in Acute Spinal Cord Injury Based on Time Course Transcriptomics Analysis. Int. J. Mol. Sci. 2021, 22, 13024. [Google Scholar] [CrossRef]
- Li, W.; Chen, J.; Zhao, S.; Huang, T.; Ying, H.; Trujillo, C.; Molinaro, G.; Zhou, Z.; Jiang, T.; Liu, W. High drug-loaded microspheres enabled by controlled in-droplet precipitation promote functional recovery after spinal cord injury. Nat. Commun. 2022, 13, 1262. [Google Scholar] [CrossRef]
- Forte, G.; Giuffrida, V.; Scuderi, A.; Pazzaglia, M. Future Treatment of Neuropathic Pain in Spinal Cord Injury: The Challenges of Nanomedicine, Supplements or Opportunities? Biomedicines 2022, 10, 1373. [Google Scholar] [CrossRef]
- Wilson, S.; Fredericks, D.; Safayi, S.; DeVries-Watson, N.; Holland, M.; Nagel, S.; Gillies, G.; Howard III, M. Ovine hemisection model of spinal cord injury. J. Investig. Surg. 2021, 34, 380–392. [Google Scholar] [CrossRef]
- Khan, A.S.; Livingstone, D.C.; Hurd, C.L.; Duchcherer, J.; Misiaszek, J.E.; Gorassini, M.A.; Manns, P.J.; Yang, J.F. Retraining walking over ground in a powered exoskeleton after spinal cord injury: A prospective cohort study to examine functional gains and neuroplasticity. J. Neuroeng. Rehabil. 2019, 16, 145. [Google Scholar] [CrossRef]
- Kim, H.; Choi, B.; Lim, H.; Min, H.; Oh, J.H.; Choi, S.; Cho, J.G.; Park, J.S.; Lee, S.J. Polyamidoamine dendrimer-conjugated triamcinolone acetonide attenuates nerve injury-induced spinal cord microglia activation and mechanical allodynia. Mol. Pain 2017, 13, 1744806917697006. [Google Scholar] [CrossRef]
- Reis, K.P.; Sperling, L.E.; Teixeira, C.; Sommer, L.; Colombo, M.; Koester, L.; Pranke, P. VPA/PLGA microfibers produced by coaxial electrospinning for the treatment of central nervous system injury. Braz. J. Med. Biol. Res. 2020, 53, e8993. [Google Scholar] [CrossRef]
- Ahmad, Z.; Salman, S.; Khan, S.A.; Amin, A.; Rahman, Z.U.; Al-Ghamdi, Y.O.; Akhtar, K.; Bakhsh, E.M.; Khan, S.B. Versatility of Hydrogels: From Synthetic Strategies, Classification, and Properties to Biomedical Applications. Gels 2022, 8, 167. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.; Dong, C.; Zhang, T.; Zhang, S. Hydrogels in Spinal Cord Injury Repair: A Review. Front. Bioeng. Biotechnol. 2022, 10, 931800. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wu, J.; Sun, R.; Zhao, Y.; Li, Y.; Pan, J.; Chen, Y.; Wang, X. Tubular scaffold with microchannels and an H-shaped lumen loaded with bone marrow stromal cells promotes neuroregeneration and inhibits apoptosis after spinal cord injury. J. Tissue Eng. Regen. Med. 2020, 14, 397–411. [Google Scholar] [CrossRef] [PubMed]
- Walsh, C.M.; Wychowaniec, J.K.; Brougham, D.F.; Dooley, D. Functional hydrogels as therapeutic tools for spinal cord injury: New perspectives on immunopharmacological interventions. Pharmacol. Ther. 2022, 234, 108043. [Google Scholar] [CrossRef]
- Ucar, B.; Kajtez, J.; Foidl, B.M.; Eigel, D.; Werner, C.; Long, K.R.; Emnéus, J.; Bizeau, J.; Lomora, M.; Pandit, A.; et al. Biomaterial based strategies to reconstruct the nigrostriatal pathway in organotypic slice co-cultures. Acta Biomater. 2021, 121, 250–262. [Google Scholar] [CrossRef]
- Yao, M.; Li, J.; Zhang, J.; Ma, S.; Wang, L.; Gao, F.; Guan, F. Dual-enzymatically cross-linked gelatin hydrogel enhances neural differentiation of human umbilical cord mesenchymal stem cells and functional recovery in experimental murine spinal cord injury. J. Mater. Chem. B 2021, 9, 440–452. [Google Scholar] [CrossRef]
- Yao, S.; He, F.; Cao, Z.; Sun, Z.; Chen, Y.; Zhao, H.; Yu, X.; Wang, X.; Yang, Y.; Rosei, F.; et al. Mesenchymal Stem Cell-Laden Hydrogel Microfibers for Promoting Nerve Fiber Regeneration in Long-Distance Spinal Cord Transection Injury. ACS Biomater. Sci. Eng. 2020, 6, 1165–1175. [Google Scholar] [CrossRef]
- Dalamagkas, K.; Tsintou, M.; Seifalian, A.M. Stem cells for spinal cord injuries bearing translational potential. Neural Regen. Res. 2018, 13, 35–42. [Google Scholar] [CrossRef]
- Jin, M.C.; Medress, Z.A.; Azad, T.D.; Doulames, V.M.; Veeravagu, A. Stem cell therapies for acute spinal cord injury in humans: A review. Neurosurg. Focus 2019, 46, E10. [Google Scholar] [CrossRef]
- Nagoshi, N.; Tsuji, O.; Nakamura, M.; Okano, H. Cell therapy for spinal cord injury using induced pluripotent stem cells. Regen. Ther. 2019, 11, 75–80. [Google Scholar] [CrossRef]
- Dasari, V.R.; Veeravalli, K.K.; Dinh, D.H. Mesenchymal stem cells in the treatment of spinal cord injuries: A review. World J. Stem Cells 2014, 6, 120–133. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Uezono, N.; Yasui, T.; Nakashima, K. Neural stem cell therapy aiming at better functional recovery after spinal cord injury. Dev. Dyn. 2018, 247, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Saberi, H.; Firouzi, M.; Habibi, Z.; Moshayedi, P.; Aghayan, H.R.; Arjmand, B.; Hosseini, K.; Razavi, H.E.; Yekaninejad, M.S. Safety of intramedullary Schwann cell transplantation for postrehabilitation spinal cord injuries: 2-year follow-up of 33 cases. J. Neurosurg. Spine 2011, 15, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Saberi, H.; Moshayedi, P.; Aghayan, H.-R.; Arjmand, B.; Hosseini, S.-K.; Emami-Razavi, S.-H.; Rahimi-Movaghar, V.; Raza, M.; Firouzi, M. Treatment of chronic thoracic spinal cord injury patients with autologous Schwann cell transplantation: An interim report on safety considerations and possible outcomes. Neurosci. Lett. 2008, 443, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.-H.; Ning, G.-Z.; Feng, S.-Q.; Kong, X.-H.; Chen, J.-T.; Zheng, Y.-F.; Ban, D.-X.; Liu, T.; Li, H.; Wang, P. Transplantation of autologous activated Schwann cells in the treatment of spinal cord injury: Six cases, more than five years of follow-up. Cell Transplant. 2012, 21, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Adnan, H.; Xu, B.; Wang, J.; Wang, C.; Li, F.; Tang, K. Effects of transplantation of olfactory ensheathing cells in chronic spinal cord injury: A systematic review and meta-analysis. Eur. Spine J. 2015, 24, 919–930. [Google Scholar] [CrossRef]
- Tabakow, P.; Raisman, G.; Fortuna, W.; Czyz, M.; Huber, J.; Li, D.; Szewczyk, P.; Okurowski, S.; Miedzybrodzki, R.; Czapiga, B. Functional regeneration of supraspinal connections in a patient with transected spinal cord following transplantation of bulbar olfactory ensheathing cells with peripheral nerve bridging. Cell Transplant. 2014, 23, 1631–1655. [Google Scholar] [CrossRef] [PubMed]
- Amemori, T.; Jendelova, P.; Ruzickova, K.; Arboleda, D.; Sykova, E. Co-transplantation of olfactory ensheathing glia and mesenchymal stromal cells does not have synergistic effects after spinal cord injury in the rat. Cytotherapy 2010, 12, 212–225. [Google Scholar] [CrossRef]
- Torres-Espin, A.; Redondo-Castro, E.; Hernandez, J.; Navarro, X. Bone marrow mesenchymal stromal cells and olfactory ensheathing cells transplantation after spinal cord injury—A morphological and functional comparison in rats. Eur. J. Neurosci. 2014, 39, 1704–1717. [Google Scholar] [CrossRef] [PubMed]
- Zarepour, A.; Bal Ozturk, A.; Koyuncu Irmak, D.; Yasayan, G.; Gokmen, A.; Karaoz, E.; Zarepour, A.; Zarrabi, A.; Mostafavi, E. Combination therapy using nanomaterials and stem cells to treat spinal cord injuries. Eur. J. Pharm. Biopharm. 2022, 177, 224–240. [Google Scholar] [CrossRef]
- James, N.D.; McMahon, S.B.; Field-Fote, E.C.; Bradbury, E.J. Neuromodulation in the restoration of function after spinal cord injury. Lancet Neurol. 2018, 17, 905–917. [Google Scholar] [CrossRef]
- Angeli, C.A.; Edgerton, V.R.; Gerasimenko, Y.P.; Harkema, S.J. Altering spinal cord excitability enables voluntary movements after chronic complete paralysis in humans. Brain 2014, 137, 1394–1409. [Google Scholar] [CrossRef] [PubMed]
- Harkema, S.; Gerasimenko, Y.; Hodes, J.; Burdick, J.; Angeli, C.; Chen, Y.; Ferreira, C.; Willhite, A.; Rejc, E.; Grossman, R.G.; et al. Effect of epidural stimulation of the lumbosacral spinal cord on voluntary movement, standing, and assisted stepping after motor complete paraplegia: A case study. Lancet 2011, 377, 1938–1947. [Google Scholar] [CrossRef]
- Grahn, P.J.; Lavrov, I.A.; Sayenko, D.G.; Van Straaten, M.G.; Gill, M.L.; Strommen, J.A.; Calvert, J.S.; Drubach, D.I.; Beck, L.A.; Linde, M.B.; et al. Enabling Task-Specific Volitional Motor Functions via Spinal Cord Neuromodulation in a Human With Paraplegia. Mayo Clin. Proc. 2017, 92, 544–554. [Google Scholar] [CrossRef] [PubMed]
- Song, G.Q.; Sun, Y.; Foreman, R.; Chen, J. Therapeutic potential of spinal cord stimulation for gastrointestinal motility disorders: A preliminary rodent study. Neurogastroenterol. Motil. 2014, 26, 377–384. [Google Scholar] [CrossRef]
- Harkema, S.J.; Legg Ditterline, B.; Wang, S.; Aslan, S.; Angeli, C.A.; Ovechkin, A.; Hirsch, G.A. Epidural Spinal Cord Stimulation Training and Sustained Recovery of Cardiovascular Function in Individuals With Chronic Cervical Spinal Cord Injury. JAMA Neurol. 2018, 75, 1569–1571. [Google Scholar] [CrossRef]
- Gad, P.N.; Kreydin, E.; Zhong, H.; Latack, K.; Edgerton, V.R. Non-invasive neuromodulation of spinal cord restores lower urinary tract function after paralysis. Front. Neurosci. 2018, 12, 432. [Google Scholar] [CrossRef] [PubMed]
- Sartori, A.M.; Salemi, S.; Hofer, A.-S.; Baumgartner, V.; Eberli, D.; Liechti, M.D.; Schwab, M.E.; Kessler, T.M. Early Transcutaneous Tibial Nerve Stimulation Acutely Improves Lower Urinary Tract Function in Spinal Cord Injured Rats. Neurotrauma Rep. 2022, 3, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Huang, H.; Yu, Y.; Jia, Y.; Liu, Z.; Shi, X.; Wang, F.; Zhang, T. Non-invasive Brain Stimulation for Neuropathic Pain after Spinal Cord Injury: A Systematic Review and Network Meta-Analysis. Front. Neurosci. 2021, 15, 800560. [Google Scholar] [CrossRef] [PubMed]
- Bockbrader, M.; Annetta, N.; Friedenberg, D.; Schwemmer, M.; Skomrock, N.; Colachis, S.t.; Zhang, M.; Bouton, C.; Rezai, A.; Sharma, G.; et al. Clinically Significant Gains in Skillful Grasp Coordination by an Individual with Tetraplegia Using an Implanted Brain-Computer Interface with Forearm Transcutaneous Muscle Stimulation. Arch Phys. Med. Rehabil. 2019, 100, 1201–1217. [Google Scholar] [CrossRef] [PubMed]
- Osuagwu, B.C.; Wallace, L.; Fraser, M.; Vuckovic, A. Rehabilitation of hand in subacute tetraplegic patients based on brain computer interface and functional electrical stimulation: A randomised pilot study. J. Neural Eng. 2016, 13, 065002. [Google Scholar] [CrossRef] [PubMed]
- King, C.E.; Wang, P.T.; McCrimmon, C.M.; Chou, C.C.; Do, A.H.; Nenadic, Z. Brain-computer interface driven functional electrical stimulation system for overground walking in spinal cord injury participant. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2014, 2014, 1238–1242. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Zhang, R.; Wang, Q.; Chen, Y.; Yang, T.; Feng, Z.; Zhang, Y.; Shao, M.; Li, Y. A P300-Based Threshold-Free Brain Switch and Its Application in Wheelchair Control. IEEE Trans. Neural Syst. Rehabil. Eng. 2017, 25, 715–725. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Liu, Y.; Hu, D.; Zhou, Z. Towards BCI-actuated smart wheelchair system. Biomed Eng. Online 2018, 17, 111. [Google Scholar] [CrossRef] [PubMed]
- Mazor, Y.; Jones, M.; Andrews, A.; Kellow, J.E.; Malcolm, A. Anorectal biofeedback for neurogenic bowel dysfunction in incomplete spinal cord injury. Spinal Cord 2016, 54, 1132–1138. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ganzer, P.D.; Colachis, S.C.t.; Schwemmer, M.A.; Friedenberg, D.A.; Dunlap, C.F.; Swiftney, C.E.; Jacobowitz, A.F.; Weber, D.J.; Bockbrader, M.A.; Sharma, G. Restoring the Sense of Touch Using a Sensorimotor Demultiplexing Neural Interface. Cell 2020, 181, 763–773.e712. [Google Scholar] [CrossRef]
- Donati, A.R.; Shokur, S.; Morya, E.; Campos, D.S.; Moioli, R.C.; Gitti, C.M.; Augusto, P.B.; Tripodi, S.; Pires, C.G.; Pereira, G.A.; et al. Long-Term Training with a Brain-Machine Interface-Based Gait Protocol Induces Partial Neurological Recovery in Paraplegic Patients. Sci. Rep. 2016, 6, 30383. [Google Scholar] [CrossRef] [PubMed]
- Wen, D.; Fan, Y.; Hsu, S.-H.; Xu, J.; Zhou, Y.; Tao, J.; Lan, X.; Li, F. Combining brain–computer interface and virtual reality for rehabilitation in neurological diseases: A narrative review. Ann. Phys. Rehabil. Med. 2021, 64, 101404. [Google Scholar] [CrossRef] [PubMed]
- Coogan, C.G.; He, B. Brain-computer interface control in a virtual reality environment and applications for the internet of things. IEEE Access 2018, 6, 10840–10849. [Google Scholar] [CrossRef] [PubMed]
- Benabid, A.L.; Costecalde, T.; Eliseyev, A.; Charvet, G.; Verney, A.; Karakas, S.; Foerster, M.; Lambert, A.; Moriniere, B.; Abroug, N.; et al. An exoskeleton controlled by an epidural wireless brain-machine interface in a tetraplegic patient: A proof-of-concept demonstration. Lancet Neurol. 2019, 18, 1112–1122. [Google Scholar] [CrossRef]
- Forte, G.; Leemhuis, E.; Favieri, F.; Casagrande, M.; Giannini, A.M.; De Gennaro, L.; Pazzaglia, M. Exoskeletons for Mobility after Spinal Cord Injury: A Personalized Embodied Approach. J. Pers. Med. 2022, 12, 380. [Google Scholar] [CrossRef] [PubMed]
- Hill, M.R.; Noonan, V.K.; Sakakibara, B.M.; Miller, W.C.; Team, S.R. Quality of life instruments and definitions in individuals with spinal cord injury: A systematic review. Spinal Cord 2010, 48, 438–450. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.R.; Hashimoto, R.E.; Dettori, J.R.; Fehlings, M.G. Spinal cord injury and quality of life: A systematic review of outcome measures. Evid. Based Spine Care J. 2011, 2, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Scivoletto, G.; Galli, G.; Torre, M.; Molinari, M.; Pazzaglia, M. The Overlooked Outcome Measure for Spinal Cord Injury: Use of Assistive Devices. Front. Neurol. 2019, 10, 272. [Google Scholar] [CrossRef] [PubMed]
- Dijkers, M. Quality of life after spinal cord injury: A meta analysis of the effects of disablement components. Spinal Cord 1997, 35, 829–840. [Google Scholar] [CrossRef] [PubMed]
- Clayton, K.S.; Chubon, R.A. Factors associated with the quality of life of long-term spinal cord injured persons. Arch. Phys. Med. Rehabil. 1994, 75, 633–638. [Google Scholar] [CrossRef]
- Krause, J.S.; Crewe, N.M. Chronologic age, time since injury, and time of measurement: Effect on adjustment after spinal cord injury. Arch. Phys. Med. Rehabil. 1991, 72, 91–100. [Google Scholar] [PubMed]
- Lam, C.Y.; Koljonen, P.A.; Yip, C.C.; Su, I.Y.; Hu, Y.; Wong, Y.W.; Cheung, K.M. Functional recovery priorities and community rehabilitation service preferences of spinal cord injury individuals and caregivers of Chinese ethnicity and cultural background. Front. Neurol. 2022, 13, 941256. [Google Scholar] [CrossRef] [PubMed]
- Pecchinenda, A.; Gonzalez Pizzio, A.P.; Salera, C.; Pazzaglia, M. The role of arousal and motivation in emotional conflict resolution: Implications for spinal cord injury. Front. Hum. Neurosci.-Cogn. Neurosci. 2022, 16, 927622. [Google Scholar] [CrossRef]
- Kazim, S.F.; Bowers, C.A.; Cole, C.D.; Varela, S.; Karimov, Z.; Martinez, E.; Ogulnick, J.V.; Schmidt, M.H. Corticospinal Motor Circuit Plasticity After Spinal Cord Injury: Harnessing Neuroplasticity to Improve Functional Outcomes. Mol. Neurobiol. 2021, 58, 5494–5516. [Google Scholar] [CrossRef] [PubMed]
- Pazzaglia, M.; Galli, G. Translating novel findings of perceptual-motor codes into the neuro-rehabilitation of movement disorders. Front. Behav. Neurosci. 2015, 9, 222. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Leemhuis, E.; Esposito, R.M.; De Gennaro, L.; Pazzaglia, M. Go Virtual to Get Real: Virtual Reality as a Resource for Spinal Cord Treatment. Int. J. Environ. Res. Public Health 2021, 18, 1819. [Google Scholar] [CrossRef] [PubMed]
- Leemhuis, E.; Giuffrida, V.; Giannini, A.M.; Pazzaglia, M. A Therapeutic Matrix: Virtual Reality as a Clinical Tool for Spinal Cord Injury-Induced Neuropathic Pain. Brain Sci. 2021, 11, 1201. [Google Scholar] [CrossRef] [PubMed]
- Austin, P.D.; Siddall, P.J. Virtual reality for the treatment of neuropathic pain in people with spinal cord injuries: A scoping review. J. Spinal Cord Med. 2021, 44, 8–18. [Google Scholar] [CrossRef]
- Pais-Vieira, C.; Gaspar, P.; Matos, D.; Alves, L.P.; da Cruz, B.M.; Azevedo, M.J.; Gago, M.; Poleri, T.; Perrotta, A.; Pais-Vieira, M. Embodiment Comfort Levels during Motor Imagery Training Combined with Immersive Virtual Reality in a Spinal Cord Injury Patient. Front. Hum. Neurosci. 2022, 16, 909112. [Google Scholar] [CrossRef] [PubMed]
- Pazzaglia, M.; Galli, G.; Lewis, J.W.; Scivoletto, G.; Giannini, A.M.; Molinari, M. Embodying functionally relevant action sounds in patients with spinal cord injury. Sci. Rep. 2018, 8, 15641. [Google Scholar] [CrossRef]
- Tang, Y.Y.; Holzel, B.K.; Posner, M.I. The neuroscience of mindfulness meditation. Nat. Rev. Neurosci. 2015, 16, 213–225. [Google Scholar] [CrossRef]
- Tang, R.; Friston, K.J.; Tang, Y.Y. Brief Mindfulness Meditation Induces Gray Matter Changes in a Brain Hub. Neural Plast. 2020, 2020, 8830005. [Google Scholar] [CrossRef]
- Hearn, J.H.; Cross, A. Mindfulness for pain, depression, anxiety, and quality of life in people with spinal cord injury: A systematic review. BMC Neurol. 2020, 20, 32. [Google Scholar] [CrossRef]
- Xi, M.; Shen, X.W.; Guliyeva, K.; Hancock-Howard, R.; Coyte, P.C.; Chan, B.C.F. Cost-utility analysis of transcranial direct current stimulation therapy with and without virtual illusion for neuropathic pain for adults with spinal cord injury in Canada. J. Spinal Cord Med. 2021, 44, S159–S172. [Google Scholar] [CrossRef]
- Pazzaglia, M.; Molinari, M. The re-embodiment of bodies, tools, and worlds after spinal cord injury: An intricate picture: Reply to comments on “The embodiment of assistive devices-From wheelchair to exoskeleton”. Phys. Life Rev. 2016, 16, 191–194. [Google Scholar] [CrossRef] [PubMed]
- Pazzaglia, M.; Molinari, M. The embodiment of assistive devices-from wheelchair to exoskeleton. Phys. Life Rev. 2016, 16, 163–175. [Google Scholar] [CrossRef] [PubMed]
- van Nes, I.J.W.; van Dijsseldonk, R.B.; van Herpen, F.H.M.; Rijken, H.; Geurts, A.C.H.; Keijsers, N.L.W. Improvement of quality of life after 2-month exoskeleton training in patients with chronic spinal cord injury. J. Spinal Cord Med. 2022, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Hutson, T.H.; Di Giovanni, S. The translational landscape in spinal cord injury: Focus on neuroplasticity and regeneration. Nat. Rev. Neurol. 2019, 15, 732–745. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.R.; Andrews, M.R.; Wang, D.; Warren, P.; Gullo, M.; Schnell, L.; Schwab, M.E.; Fawcett, J.W. Combination treatment with anti-Nogo-A and chondroitinase ABC is more effective than single treatments at enhancing functional recovery after spinal cord injury. Eur. J. Neurosci. 2013, 38, 2946–2961. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Alias, G.; Barkhuysen, S.; Buckle, M.; Fawcett, J.W. Chondroitinase ABC treatment opens a window of opportunity for task-specific rehabilitation. Nat. Neurosci. 2009, 12, 1145–1151. [Google Scholar] [CrossRef]
- Nagappan, P.G.; Chen, H.; Wang, D.Y. Neuroregeneration and plasticity: A review of the physiological mechanisms for achieving functional recovery postinjury. Mil. Med. Res. 2020, 7, 30. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leemhuis, E.; Favieri, F.; Forte, G.; Pazzaglia, M. Integrated Neuroregenerative Techniques for Plasticity of the Injured Spinal Cord. Biomedicines 2022, 10, 2563. https://doi.org/10.3390/biomedicines10102563
Leemhuis E, Favieri F, Forte G, Pazzaglia M. Integrated Neuroregenerative Techniques for Plasticity of the Injured Spinal Cord. Biomedicines. 2022; 10(10):2563. https://doi.org/10.3390/biomedicines10102563
Chicago/Turabian StyleLeemhuis, Erik, Francesca Favieri, Giuseppe Forte, and Mariella Pazzaglia. 2022. "Integrated Neuroregenerative Techniques for Plasticity of the Injured Spinal Cord" Biomedicines 10, no. 10: 2563. https://doi.org/10.3390/biomedicines10102563
APA StyleLeemhuis, E., Favieri, F., Forte, G., & Pazzaglia, M. (2022). Integrated Neuroregenerative Techniques for Plasticity of the Injured Spinal Cord. Biomedicines, 10(10), 2563. https://doi.org/10.3390/biomedicines10102563






