Heat-Related Illness in Emergency and Critical Care: Recommendations for Recognition and Management with Medico-Legal Considerations
Abstract
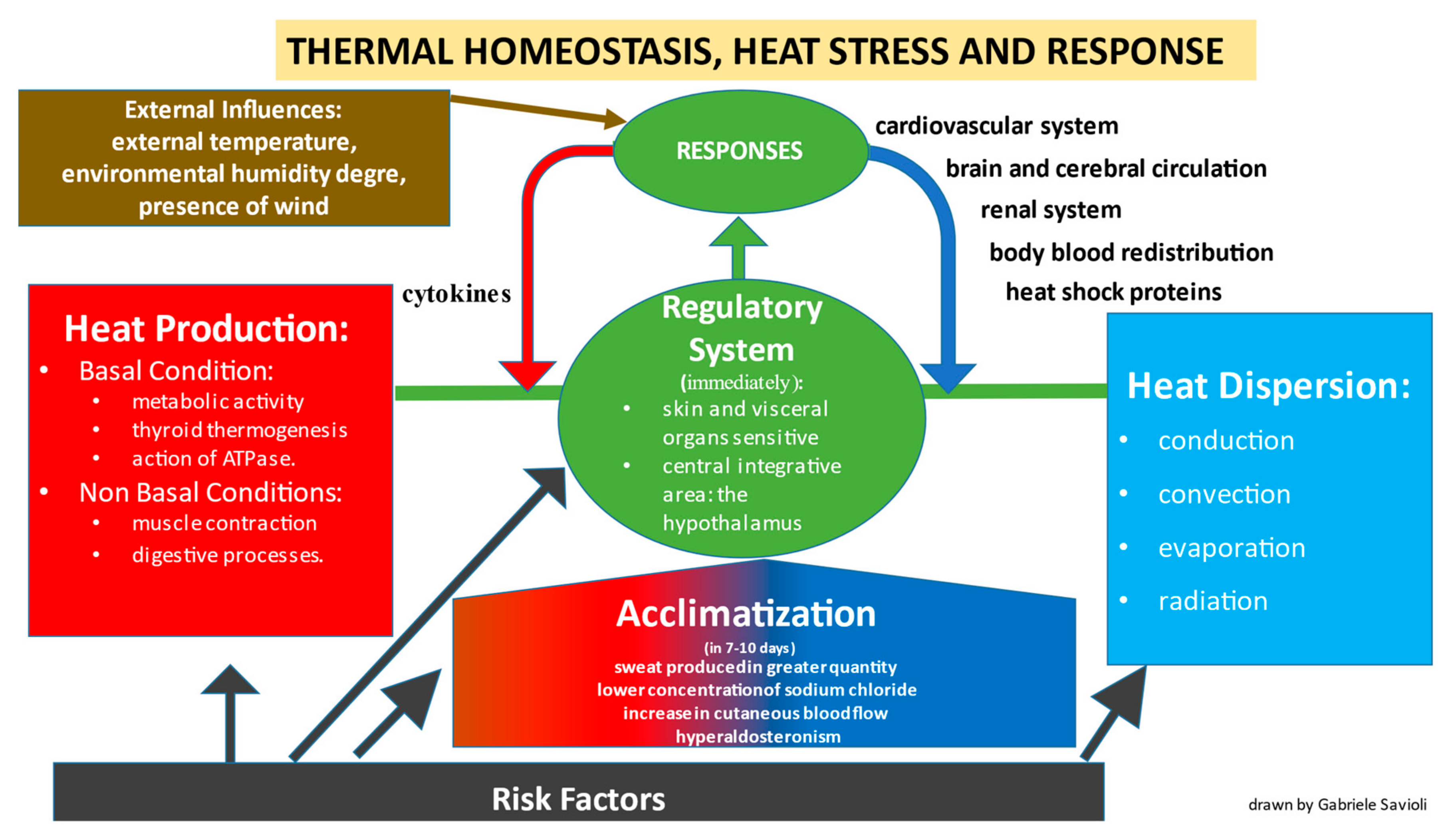
:1. Epidemiology
2. Risk Factors
2.1. Non-Modifiable Risk Factors
2.2. Modifiable Risk Factors
| Non-Modifiable Risk Factors | Modifiable Risk Factors |
|---|---|
| Age (geriatric patients or children) | Dehydration |
| Autonomic disorders that cause widespread anhidrosis (Ross syndrome, chronic idiopathic anhidrosis, Sjögren syndrome) | Prolonged exposure in a warm humid environment |
| Trauma with spine injuries | Occupational categories (military personnel, athletes, construction, field, mining, or well workers, etc.) |
| Endocrinological disorders (diabetes, hyperthyroidism) | Addictive behaviors (alcoholism, cocaine, amphetamine, heroin use, etc.) |
| Neurological disorders (epilepsy) | Drugs (anticholinergics, beta-blockers, diuretics, neuroleptics, anesthetics, topiramate) |
| Skin diseases (scleroderma, burns) | Infections |
| Hereditary disease (malignant hyperthermia) | Obesity |
3. Pathophysiology
3.1. Production of Heat
3.2. Heat Dispersion
- Conduction is the transfer of heat to a cooler object through direct contact.
- Convection is the transfer of heat at the body surface by air circulation.
- Evaporation cools the skin surfaces when sweat changes from a liquid to a vapor
- Radiation occurs through the transmission of electromagnetic waves.
3.3. Regulatory System
3.3.1. Cutaneous System
3.3.2. Cardiovascular System
4. Left Ventricular Preload and Afterload
5. Blood Volume and Coagulation
6. Renal System
7. Brain and Cerebral Circulation
8. Cytokines
9. Heat Shock Proteins
Acclimatization
10. Diagnosis and Management
10.1. Mild Forms
10.2. Moderate Forms
10.3. Severe Forms
| Mild Form | Moderate Form | Severe Form |
|---|---|---|
| Heat edema | Exercise-associated collapse (or heat syncope) | Classic heatstroke |
| Muscle cramps from heat | Hypernatremic heat exhaustion | Exertional heatstroke |
| Heat rash | Hyponatremic heat exhaustion | |
| Tetany |
11. Prevention
12. Point of View of the Emergency Department
12.1. General Aspects
12.2. Inpatient Treatment of Heat Stroke
13. Differential Diagnosis
14. Post-Mortem Investigations in Cases of Death by Hyperthermia
14.1. Traditional Post-Mortem Examinations
14.2. Immunohistochemistry
14.3. Biochemical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jardine, D.S. Heat Illness and Heat Stroke. Pediatr. Rev. 2007, 28, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). Heat-Related Deaths after an Extreme Heat Event—Four States, 2012, and United States, 1999–2009. MMWR Morb. Mortal. Wkly. Rep. 2013, 62, 433–436. [Google Scholar]
- Hess, J.J.; Saha, S.; Luber, G. Summertime Acute Heat Illness in U.S. Emergency Departments from 2006 through 2010: Analysis of a Nationally Representative Sample. Environ. Health Perspect. 2014, 122, 1209–1215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choudhary, E.; Vaidyanathan, A. Heat Stress Illness Hospitalizations-Environmental Public Health Tracking Program, 20 States, 2001–2010. MMWR Surveill. Summ. 2014, 63, 1–10. [Google Scholar] [PubMed]
- Acharya, P.; Boggess, B.; Zhang, K. Assessing Heat Stress and Health among Construction Workers in a Changing Climate: A Review. Int. J. Environ. Res. Public Health 2018, 15, 247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kerr, Z.Y.; Casa, D.J.; Marshall, S.W.; Comstock, R.D. Epidemiology of Exertional Heat Illness Among U.S. High School Athletes. Am. J. Prev. Med. 2013, 44, 8–14. [Google Scholar] [CrossRef]
- Armed Forces Health Surveillance Branch. Update: Heat Illness, Active Component, U.S. Armed Forces, 2017. MSMR 2018, 25, 6–12. [Google Scholar]
- Binkley, H.M.; Beckett, J.; Casa, D.J.; Kleiner, D.M.; Plummer, P.E. National Athletic Trainers’ Association Position Statement: Exertional Heat Illnesses. J. Athl. Train. 2002, 37, 329–343. [Google Scholar]
- Casa, D.J.; Armstrong, L.E.; Ganio, M.S.; Yeargin, S.W. Exertional Heat Stroke in Competitive Athletes. Curr. Sports Med. Rep. 2005, 4, 309–317. [Google Scholar] [CrossRef]
- Howe, A.S.; Boden, B.P. Heat-Related Illness in Athletes. Am. J. Sports Med. 2007, 35, 1384–1395. [Google Scholar] [CrossRef]
- Stapleton, J.M.; Wright, H.E.; Hardcastle, S.G.; Kenny, G.P. Body Heat Storage during Intermittent Work in Hot–Dry and Warm–Wet Environments. Appl. Physiol. Nutr. Metab. 2012, 37, 840–849. [Google Scholar] [CrossRef]
- Kalkowsky, B.; Kampmann, B. Physiological Strain of Miners at Hot Working Places in German Coal Mines. Ind. Health 2006, 44, 465–473. [Google Scholar] [CrossRef] [Green Version]
- Meade, R.D.; Lauzon, M.; Poirier, M.P.; Flouris, A.D.; Kenny, G.P. An Evaluation of the Physiological Strain Experienced by Electrical Utility Workers in North America. J. Occup. Environ. Hyg. 2015, 12, 708–720. [Google Scholar] [CrossRef]
- Pryor, R.; Casa, D.; Holschen, J.; O’Connor, F.; Vandermark, L. Exertional Heat Stroke: Strategies for Prevention and Treatment from the Sports Field to the Emergency Department. Clin. Pediatr. Emerg. Med. 2013, 14, 267–278. [Google Scholar] [CrossRef]
- Pryor, R.R.; Bennett, B.L.; O’Connor, F.G.; Young, J.M.J.; Asplund, C.A. Medical Evaluation for Exposure Extremes: Heat. Wilderness Environ. Med. 2015, 26, 69–75. [Google Scholar] [CrossRef] [Green Version]
- Kenny, G.P.; Poirier, M.P.; Metsios, G.S.; Boulay, P.; Dervis, S.; Friesen, B.J.; Malcolm, J.; Sigal, R.J.; Seely, A.J.E.; Flouris, A.D. Hyperthermia and Cardiovascular Strain during an Extreme Heat Exposure in Young versus Older Adults. Temperature 2017, 4, 79–88. [Google Scholar] [CrossRef] [Green Version]
- Stapleton, J.M.; Poirier, M.P.; Flouris, A.D.; Boulay, P.; Sigal, R.J.; Malcolm, J.; Kenny, G.P. Aging Impairs Heat Loss, but When Does It Matter? J. Appl. Physiol. 2015, 118, 299–309. [Google Scholar] [CrossRef]
- Cheshire, W.P. Disorders of Thermal Regulation. In Cecil Essentials of Medicine, 8th ed.; Andreoli, T.E., Benjamin, I.J., Griggs, R.C., Wing, E.J., Eds.; Saunders Elsevier: Philadelphia, PA, USA, 2010; pp. 1083–1085. [Google Scholar]
- Low, P.A.; Hilz, M.J. Diabetic Autonomic Neuropathy. In Clinical Autonomic Research, 3rd ed.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 423–440. [Google Scholar]
- Cheshire, W.P.; Low, P.A. Disorders of Sweating and Thermoregulation. Continuum 2007, 13, 143–164. [Google Scholar] [CrossRef]
- Downey, J.A.; Huckaba, C.E.; Kelley, P.S.; Tam, H.S.; Darling, R.C.; Cheh, H.Y. Sweating Responses to Central and Peripheral Heating in Spinal Man. J. Appl. Physiol. 1976, 40, 701–706. [Google Scholar] [CrossRef]
- Seckendorf, R.; Randall, W.C. Thermal Reflex Sweating in Normal and Paraplegic Man. J. Appl. Physiol. 1961, 16, 796–800. [Google Scholar] [CrossRef] [Green Version]
- Rosenberg, H.; Davis, M.; James, D.; Pollock, N.; Stowell, K. Malignant Hyperthermia. Orphanet J. Rare Dis. 2007, 2, 21. [Google Scholar] [CrossRef] [Green Version]
- Betjemann, J.P.; Lowenstein, D.H. Status Epilepticus in Adults. Lancet Neurol. 2015, 14, 615–624. [Google Scholar] [CrossRef]
- De Carolis, P.; Magnifico, F.; Pierangeli, G.; Rinaldi, R.; Galeotti, M.; Cevoli, S.; Cortelli, P. Transient Hypohidrosis Induced by Topiramate. Epilepsia 2003, 44, 974–976. [Google Scholar] [CrossRef]
- Lee, C.-P.; Chen, P.-J.; Chang, C.-M. Heat Stroke during Treatment with Olanzapine, Trihexyphenidyl, and Trazodone in a Patient with Schizophrenia. Acta Neuropsychiatr. 2015, 27, 380–385. [Google Scholar] [CrossRef]
- Adubofour, K.O.; Kajiwara, G.T.; Goldberg, C.M.; King-Angell, J.L. Oxybutynin-Induced Heatstroke in an Elderly Patient. Ann. Pharmacother. 1996, 30, 144–147. [Google Scholar] [CrossRef]
- Clark, W.G.; Lipton, J.M. Drug-Related Heatstroke. Pharmacol. Ther. 1984, 26, 345–388. [Google Scholar] [CrossRef]
- Paden, M.S.; Franjic, L.; Halcomb, S.E. Hyperthermia Caused by Drug Interactions and Adverse Reactions. Emerg. Med. Clin. N. Am. 2013, 31, 1035–1044. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.-P.; Chang, C.-K.; Hayes, R.D.; Harrison, S.; Lee, W.; Broadbent, M.; Taylor, D.; Stewart, R. Retrospective Chart Review on Exposure to Psychotropic Medications Associated with Neuroleptic Malignant Syndrome. Acta Psychiatr. Scand. 2014, 130, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Atha, W.F. Heat-Related Illness. Emerg. Med. Clin. N. Am. 2013, 31, 1097–1108. [Google Scholar] [CrossRef] [PubMed]
- Wilson, T.E.; Crandall, C.G. Effect of Thermal Stress on Cardiac Function. Exerc. Sport Sci. Rev. 2011, 39, 12–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deschamps, A.; Magder, S. Skin Vascular Bed Is a Potential Blood Reservoir during Heat Stress. Am. J. Physiol.-Heart Circ. Physiol. 1990, 259, H1796–H1802. [Google Scholar] [CrossRef]
- Deschamps, A.; Magder, S. Effects of Heat Stress on Vascular Capacitance. Am. J. Physiol. Heart Circ. Physiol. 1994, 266, H2122–H2129. [Google Scholar] [CrossRef]
- Crandall, C.G.; Wilson, T.E. Human Cardiovascular Responses to Passive Heat Stress. In Comprehensive Physiology; Wiley: Hoboken, NJ, USA, 2014; pp. 17–43. [Google Scholar]
- Gorman, A.J.; Proppe, D.W. Mechanisms Producing Tachycardia in Conscious Baboons during Environmental Heat Stress. J. Appl. Physiol. 1984, 56, 441–446. [Google Scholar] [CrossRef]
- Crandall, C.G.; Wilson, T.E.; Marving, J.; Vogelsang, T.W.; Kjaer, A.; Hesse, B.; Secher, N.H. Effects of Passive Heating on Central Blood Volume and Ventricular Dimensions in Humans. J. Physiol. 2008, 586, 293–301. [Google Scholar] [CrossRef]
- Wilson, T.E.; Brothers, R.M.; Tollund, C.; Dawson, E.A.; Nissen, P.; Yoshiga, C.C.; Jons, C.; Secher, N.H.; Crandall, C.G. Effect of Thermal Stress on Frank-Starling Relations in Humans. J. Physiol. 2009, 587, 3383–3392. [Google Scholar] [CrossRef]
- Brothers, R.M.; Bhella, P.S.; Shibata, S.; Wingo, J.E.; Levine, B.D.; Crandall, C.G. Cardiac Systolic and Diastolic Function during Whole Body Heat Stress. Am. J. Physiol. Heart Circ. Physiol. 2009, 296, H1150–H1156. [Google Scholar] [CrossRef]
- Nelson, M.D.; Haykowsky, M.J.; Petersen, S.R.; DeLorey, D.S.; Cheng-Baron, J.; Thompson, R.B. Increased Left Ventricular Twist, Untwisting Rates, and Suction Maintain Global Diastolic Function during Passive Heat Stress in Humans. Am. J. Physiol. Heart Circ. Physiol. 2010, 298, H930–H937. [Google Scholar] [CrossRef] [Green Version]
- Brothers, R.M.; Pecini, R.; Dalsgaard, M.; Bundgaard-Nielsen, M.; Wilson, T.E.; Secher, N.H.; Crandall, C.G. Beneficial Effects of Elevating Cardiac Preload on Left-Ventricular Diastolic Function and Volume during Heat Stress: Implications toward Tolerance during a Hemorrhagic Insult. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014, 307, R1036–R1041. [Google Scholar] [CrossRef] [Green Version]
- Vance Strother, S.; Bull, J.M.C.; Branham, S.A. Activation of Coagulation during Therapeutic Whole Body Hyperthermia. Thromb. Res. 1986, 43, 353–360. [Google Scholar] [CrossRef]
- Dodman, B.; Cunliffe, W.J.; Roberts, B.E.; Buchan, C.W. Effects of Changes in Temperature (Local and Central) on Plasma Fibrinolytic Activity. J. Clin. Pathol. 1973, 26, 248–249. [Google Scholar] [CrossRef] [Green Version]
- Wilson, T.E. Renal Sympathetic Nerve, Blood Flow, and Epithelial Transport Responses to Thermal Stress. Auton. Neurosci. 2017, 204, 25–34. [Google Scholar] [CrossRef]
- Bain, A.R.; Nybo, L.; Ainslie, P.N. Cerebral Vascular Control and Metabolism in Heat Stress. In Comprehensive Physiology; Wiley: Hoboken, NJ, USA, 2015; pp. 1345–1380. [Google Scholar]
- Wilson, T.E.; Cui, J.; Zhang, R.; Crandall, C.G. Heat Stress Reduces Cerebral Blood Velocity and Markedly Impairs Orthostatic Tolerance in Humans. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 291, R1443–R1448. [Google Scholar] [CrossRef] [Green Version]
- Nelson, N.G.; Collins, C.L.; Comstock, R.D.; McKenzie, L.B. Exertional Heat-Related Injuries Treated in Emergency Departments in the U.S., 1997–2006. Am. J. Prev. Med. 2011, 40, 54–60. [Google Scholar] [CrossRef]
- Bouchama, A.; Al-Sedairy, S.; Siddiqui, S.; Shail, E.; Bezeig, M. Elevated Pyrogenic Cytokines in Heatstroke. Chest 1993, 104, 1498–1502. [Google Scholar] [CrossRef]
- Bouchama, A.; Hammami, M.M.; al Shail, E.; DeVol, E. Differential Effects of in Vitro and in Vivo Hyperthermia on the Production of Interleukin-10. Intensive Care Med. 2000, 26, 1646–1651. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.; Lam, K.; Peng, Y.; Lee, Y.; Yang, C.; Tsai, Y.; Yen, M.; Cheng, P. Heat shock protein 70 and AMP-activated protein kinase contribute to 17-DMAG-dependent protection against heat stroke. J. Cell. Mol. Med. 2016, 20, 1889–1897. [Google Scholar] [CrossRef] [Green Version]
- Lugo-Amador, N.M.; Rothenhaus, T.; Moyer, P. Heat-Related Illness. Emerg. Med. Clin. N. Am. 2004, 22, 315–327. [Google Scholar] [CrossRef]
- Nelson, N.L.; Churilla, J.R. A Narrative Review of Exercise-Associated Muscle Cramps: Factors That Contribute to Neuromuscular Fatigue and Management Implications. Muscle Nerve 2016, 54, 177–185. [Google Scholar] [CrossRef]
- Miller, K.C. Rethinking the Cause of Exercise-Associated Muscle Cramping. Curr. Sports Med. Rep. 2015, 14, 353–354. [Google Scholar] [CrossRef]
- Krau, S.D. Heat-Related Illness. Crit. Care Nurs. Clin. N. Am. 2013, 25, 251–262. [Google Scholar] [CrossRef]
- Casa, D.J.; DeMartini, J.K.; Bergeron, M.F.; Csillan, D.; Eichner, E.R.; Lopez, R.M.; Ferrara, M.S.; Miller, K.C.; O’Connor, F.; Sawka, M.N.; et al. National Athletic Trainers’ Association Position Statement: Exertional Heat Illnesses. J. Athl. Train. 2015, 50, 986–1000. [Google Scholar] [CrossRef] [Green Version]
- O’Connor, F.G.; Casa, D.J.; Bergeron, M.F.; Carter, R.; Deuster, P.; Heled, Y.; Kark, J.; Leon, L.; MCDermott, B.; O’Brien, K.; et al. American College of Sports Medicine Roundtable on Exertional Heat Stroke—Return to Duty/Return to Play. Curr. Sports Med. Rep. 2010, 9, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Asplund, C.A.; O’Connor, F.G.; Noakes, T.D. Exercise-Associated Collapse: An Evidence-Based Review and Primer for Clinicians. Br. J. Sports Med. 2011, 45, 1157–1162. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, F.; Casa, D. Exertional Heat Illness in Adolescents and Adults: Management and Prevention. 2021. Available online: https://www.uptodate.com/contents/exertional-heat-illness-in-adolescents-and-adults-management-and-prevention (accessed on 1 September 2022).
- Casa, D.J.; Armstrong, L.E.; Kenny, G.P.; O’Connor, F.G.; Huggins, R.A. Exertional Heat Stroke. Curr. Sports Med. Rep. 2012, 11, 115–123. [Google Scholar] [CrossRef]
- Sloan, B.K.; Kraft, E.M.; Clark, D.; Schmeissing, S.W.; Byrne, B.C.; Rusyniak, D.E. On-Site Treatment of Exertional Heat Stroke. Am.J. Sports Med. 2015, 43, 823–829. [Google Scholar] [CrossRef] [Green Version]
- McDermott, B.P.; Casa, D.J.; Ganio, M.S.; Lopez, R.M.; Yeargin, S.W.; Armstrong, L.E.; Maresh, C.M. Acute Whole-Body Cooling for Exercise-Induced Hyperthermia: A Systematic Review. J. Athl. Train. 2009, 44, 84–93. [Google Scholar] [CrossRef] [Green Version]
- Belval, L.N.; Casa, D.J.; Adams, W.M.; Chiampas, G.T.; Holschen, J.C.; Hosokawa, Y.; Jardine, J.; Kane, S.F.; Labotz, M.; Lemieux, R.S.; et al. Consensus Statement- Prehospital Care of Exertional Heat Stroke. Prehospital Emerg. Care 2018, 22, 392–397. [Google Scholar] [CrossRef]
- Gaudio, F.G.; Grissom, C.K. Cooling Methods in Heat Stroke. J. Emerg. Med. 2016, 50, 607–616. [Google Scholar] [CrossRef]
- Nichols, A.W. Heat-Related Illness in Sports and Exercise. Curr. Rev. Musculoskelet Med. 2014, 7, 355–365. [Google Scholar] [CrossRef] [Green Version]
- Armstrong, L.E.; Casa, D.J.; Millard-Stafford, M.; Moran, D.S.; Pyne, S.W.; Roberts, W.O. Exertional Heat Illness during Training and Competition. Med. Sci. Sports Exerc. 2007, 39, 556–572. [Google Scholar] [CrossRef]
- Savioli, G.; Ceresa, I.F.; Giordano, M.; Ferrari, I.; Varesi, A.; Floris, V.; Esposito, C.; Croesi, B.; Ricevuti, G.; Calvi, M.; et al. The Reliability of Anamnestic Data in the Management of Clostridium Tetani Infection in Elderly. Front. Med. 2021, 8, 684594. [Google Scholar] [CrossRef]
- Savioli, G.; Ceresa, I.F.; Gri, N.; Bavestrello Piccini, G.; Longhitano, Y.; Zanza, C.; Piccioni, A.; Esposito, C.; Ricevuti, G.; Bressan, M.A. Emergency Department Overcrowding: Understanding the Factors to Find Corresponding Solutions. J. Pers. Med. 2022, 12, 279. [Google Scholar] [CrossRef]
- Savioli, G.; Ceresa, I.; Guarnone, R.; Muzzi, A.; Novelli, V.; Ricevuti, G.; Iotti, G.; Bressan, M.; Oddone, E. Impact of Coronavirus Disease 2019 Pandemic on Crowding: A Call to Action for Effective Solutions to “Access Block”. Western J. Emerg. Med. 2021, 22, 860–870. [Google Scholar] [CrossRef]
- Savioli, G.; Ceresa, I.F.; Novelli, V.; Ricevuti, G.; Bressan, M.A.; Oddone, E. How the Coronavirus Disease 2019 Pandemic Changed the Patterns of Healthcare Utilization by Geriatric Patients and the Crowding: A Call to Action for Effective Solutions to the Access Block. Intern. Emerg. Med. 2022, 17, 503–514. [Google Scholar] [CrossRef]
- Savioli, G.; Ceresa, I.F.; Macedonio, S.; Gerosa, S.; Belliato, M.; Luzzi, S.; Lucifero, A.G.; Manzoni, F.; Ricevuti, G.; Bressan, M.A. Major Trauma in Elderly Patients: Worse Mortality and Outcomes in an Italian Trauma Center. J. Emerg. Trauma Shock. 2021, 14, 98–103. [Google Scholar] [CrossRef]
- Savioli, G.; Ceresa, I.F.; Luzzi, S.; Giotta Lucifero, A.; Cambiè, G.; Manzoni, F.; Preda, L.; Ricevuti, G.; Bressan, M.A. Mild Head Trauma (MHT) and Antiplatelet Therapy. Reply to dLorenzati et al. Comment on “Savioli et al. Mild Head Trauma: Is Antiplatelet Therapy a Risk Factor for Hemorrhagic Complications? Medicina 2021, 57, 889. [Google Scholar] [CrossRef]
- Savioli, G.; Ceresa, I.F.; Luzzi, S.; Gragnaniello, C.; Giotta Lucifero, A.; del Maestro, M.; Marasco, S.; Manzoni, F.; Ciceri, L.; Gelfi, E.; et al. Rates of Intracranial Hemorrhage in Mild Head Trauma Patients Presenting to Emergency Department and Their Management: A Comparison of Direct Oral Anticoagulant Drugs with Vitamin K Antagonists. Medicina 2020, 56, 308. [Google Scholar] [CrossRef]
- Savioli, G.; Ceresa, I.F.; Luzzi, S.; Giotta Lucifero, A.; Pioli Di Marco, M.S.; Manzoni, F.; Preda, L.; Ricevuti, G.; Bressan, M.A. Mild Head Trauma: Is Antiplatelet Therapy a Risk Factor for Hemorrhagic Complications? Medicina 2021, 57, 357. [Google Scholar] [CrossRef]
- Savioli, G.; Ceresa, I.F.; Novara, E.; Persiano, T.; Grulli, F.; Ricevuti, G.; Bressan, M.A.; Oddone, E. Brief Intensive Observation Areas in the Management of Acute Heart Failure in Elderly Patients Leading to High Stabilisation Rate and Less Admissions. J. Gerontol. Geriatr. 2021, 69, 87–97. [Google Scholar] [CrossRef]
- Barbier, P.; Cucco, C.; Guglielmo, M.; Simioniuc, A.; Fabiani, I.; Pugliese, N.R.; Savioli, G.; Dini, F.L. Estimation of Increased Pulmonary Wedge Pressure by an Algorithm Based on Noninvasively Measured Pulmonary Diastolic Pressure in Cardiac Patients Independent of Left Ventricular Ejection Fraction. Echocardiography 2020, 37, 215–222. [Google Scholar] [CrossRef]
- Tavazzi, G.; Boffi, A.; Savioli, G.; Greco, A.; Pavesi, C.; Klersy, C.; Guida, S.; Iotti, G.; Mojoli, F.; Ghio, S.; et al. Right Ventricular Total Isovolumic Time: Reference Value Study. Echocardiography 2019, 36, 1234–1240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbier, P.; Mirea, O.; Cefalù, C.; Maltagliati, A.; Savioli, G.; Guglielmo, M. Reliability and Feasibility of Longitudinal AFI Global and Segmental Strain Compared with 2D Left Ventricular Volumes and Ejection Fraction: Intra- and Inter-Operator, Test–Retest, and Inter-Cycle Reproducibility. Eur. Heart. J. Cardiovasc. Imaging 2015, 16, 642–652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Savioli, G.; Ceresa, I.F.; Maggioni, P.; Lava, M.; Ricevuti, G.; Manzoni, F.; Oddone, E.; Bressan, M.A. Impact of ED Organization with a Holding Area and a Dedicated Team on the Adherence to International Guidelines for Patients with Acute Pulmonary Embolism: Experience of an Emergency Department Organized in Areas of Intensity of Care. Medicines 2020, 7, 60. [Google Scholar] [CrossRef] [PubMed]
- Savioli, G.; Ceresa, I.F.; Manzoni, F.; Ricevuti, G.; Bressan, M.A.; Oddone, E. Role of a Brief Intensive Observation Area with a Dedicated Team of Doctors in the Management of Acute Heart Failure Patients: A Retrospective Observational Study. Medicina 2020, 56, 251. [Google Scholar] [CrossRef]
- Ceresa, I.F.; Savioli, G.; Angeli, V.; Novelli, V.; Muzzi, A.; Grugnetti, G.; Cobianchi, L.; Manzoni, F.; Klersy, C.; Lago, P.; et al. Preparing for the Maximum Emergency with a Simulation: A Table-Top Test to Evaluate Bed Surge Capacity and Staff Compliance with Training. Open Access Emerg. Med. 2020, 12, 377–387. [Google Scholar] [CrossRef]
- Costrini, A. Emergency Treatment of Exertional Heatstroke and Comparison of Whole Body Cooling Techniques. Med. Sci. Sports Exerc. 1990, 22, 15–18. [Google Scholar] [CrossRef]
- Graham, B.S. Nonexertional Heatstroke. Physiologic Management and Cooling in 14 Patients. Arch. Intern. Med. 1986, 146, 87–90. [Google Scholar] [CrossRef]
- Calvello, E.J.; Hu, K.; Khoujah, D. Management of the Hyperthermic Patient. Br. J. Hosp. Med. 2011, 72, 571–575. [Google Scholar] [CrossRef]
- Mok, G.; DeGroot, D.; Hathaway, N.E.; Bigley, D.P.; McGuire, C.S. Exertional Heat Injury: Effects of Adding Cold (4 °C) Intravenous Saline to Prehospital Protocol. Curr. Sports Med. Rep. 2017, 16, 103–108. [Google Scholar] [CrossRef]
- Lipman, G.S.; Eifling, K.P.; Ellis, M.A.; Gaudio, F.G.; Otten, E.M.; Grissom, C.K. Wilderness Medical Society Practice Guidelines for the Prevention and Treatment of Heat-Related Illness. Wilderness Environ. Med. 2013, 24, 351–361. [Google Scholar] [CrossRef] [Green Version]
- Wexler, R.K. Evaluation and Treatment of Heat-Related Illnesses. Am. Fam. Physician 2002, 65, 2307. [Google Scholar]
- Leon, L.R.; Helwig, B.G. Heat Stroke: Role of the Systemic Inflammatory Response. J. Appl. Physiol. 2010, 109, 1980–1988. [Google Scholar] [CrossRef] [Green Version]
- Rimmelé, T.; Kellum, J.A.; Warner, D.S. High-Volume Hemofiltration in the Intensive Care Unit. Anesthesiology 2012, 116, 1377–1387. [Google Scholar] [CrossRef] [Green Version]
- Joannes-Boyau, O.; Honoré, P.M.; Perez, P.; Bagshaw, S.M.; Grand, H.; Canivet, J.-L.; Dewitte, A.; Flamens, C.; Pujol, W.; Grandoulier, A.-S.; et al. High-Volume versus Standard-Volume Haemofiltration for Septic Shock Patients with Acute Kidney Injury (IVOIRE Study): A Multicentre Randomized Controlled Trial. Int. Care Med. 2013, 39, 1536–1546. [Google Scholar] [CrossRef]
- Clark, E.; Molnar, A.O.; Joannes-Boyau, O.; Honoré, P.M.; Sikora, L.; Bagshaw, S.M. High-Volume Hemofiltration for Septic Acute Kidney Injury: A Systematic Review and Meta-Analysis. Crit. Care. 2014, 18, R7. [Google Scholar] [CrossRef] [Green Version]
- Expert Consensus on Standardized Diagnosis and Treatment for Heat Stroke. Mil. Med. Res. 2016, 3, 4. [CrossRef] [Green Version]
- Sorensen, C.; Hess, J. Treatment and Prevention of Heat-Related Illness. N. Engl. J. Med. 2022. [Google Scholar] [CrossRef]
- Barbour, S.J.; Canney, M.; Coppo, R.; Zhang, H.; Liu, Z.-H.; Suzuki, Y.; Matsuzaki, K.; Katafuchi, R.; Induruwage, D.; Er, L.; et al. Improving Treatment Decisions Using Personalized Risk Assessment from the International IgA Nephropathy Prediction Tool. Kidney Int. 2020, 98, 1009–1019. [Google Scholar] [CrossRef]
- Esposito, V.; Mazzon, G.; Baiardi, P.; Torreggiani, M.; Semeraro, L.; Catucci, D.; Colucci, M.; Mariotto, A.; Grosjean, F.; Bovio, G.; et al. Safety and Adequacy of Percutaneous Kidney Biopsy Performed by Nephrology Trainees. BMC Nephrol. 2018, 19, 14. [Google Scholar] [CrossRef] [Green Version]
- Santoro, D.; Torreggiani, M.; Pellicanò, V.; Cernaro, V.; Messina, R.M.; Longhitano, E.; Siligato, R.; Gembillo, G.; Esposito, C.; Piccoli, G.B. Kidney Biopsy in Type 2 Diabetic Patients: Critical Reflections on Present Indications and Diagnostic Alternatives. Int. J. Mol. Sci. 2021, 22, 5425. [Google Scholar] [CrossRef]
- Burt, A. Diagnosis and Management of Heat Stroke. Intensive Care; WFSA: Dallas, TX, USA, 2016. [Google Scholar]
- Horseman, M.A.; Rather-Conally, J.; Saavedra, C.; Surani, S. A Case of Severe Heatstroke and Review of Pathophysiology, Clinical Presentation, and Treatment. J. Int. Care Med. 2013, 28, 334–340. [Google Scholar] [CrossRef]
- Chan, Y.K.; Mamat, M. Management of Heat Stroke. Trends Anaesth. Crit. Care 2015, 5, 65–69. [Google Scholar] [CrossRef]
- Mørch, S.S.; Andersen, J.D.H.; Bestle, M.H. Heat Stroke: A Medical Emergency Appearing in New Regions. Case. Rep. Crit. Care 2017, 2017, 6219236. [Google Scholar] [CrossRef] [Green Version]
- Mattis, J.G.; Yates, A.M. Heat Stroke. Nurse Pract. 2011, 36, 48–52. [Google Scholar] [CrossRef]
- Leon, L.R.; Bouchama, A. Heat Stroke. In Comprehensive Physiology; Wiley: Hoboken, NJ, USA, 2015. [Google Scholar]
- Al Mahri, S.; Bouchama, A. Heatstroke. Handb. Clin. Neurol. 2018, 157, 531–545. [Google Scholar]
- Bunai, Y.; Akaza, K.; Jiang, W.-X.; Nagai, A. Fatal Hyperthermia Associated with Excited Delirium during an Arrest. Leg. Med. 2008, 10, 306–309. [Google Scholar] [CrossRef]
- Zhu, B.-L.; Ishikawa, T.; Michiue, T.; Li, D.-R.; Zhao, D.; Quan, L.; Oritani, S.; Bessho, Y.; Maeda, H. Postmortem Serum Catecholamine Levels in Relation to the Cause of Death. Forensic Sci. Int. 2007, 173, 122–129. [Google Scholar] [CrossRef]
- Nixdorf-Miller, A.; Hunsaker, D.M.; Hunsaker, J.C., III. Hypothermia and Hyperthermia Medicolegal Investigation of Morbidity and Mortality From Exposure to Environmental Temperature Extremes. Arch. Pathol. Lab. Med. 2006, 130, 1297–1304. [Google Scholar] [CrossRef]
- Green, H.; Gilbert, J.; James, R.; Byard, R.W. An Analysis of Factors Contributing to a Series of Deaths Caused by Exposure to High Environmental Temperatures. Am. J. Forensic Med. Pathol. 2001, 22, 196–199. [Google Scholar] [CrossRef]
- Sweeney, K.G. Heat-Related Deaths. J. Insur. Med 2002, 34, 114–119. [Google Scholar]
- Palmiere, C.; Mangin, P. Hyperthermia and Postmortem Biochemical Investigations. Int. J. Legal. Med. 2013, 127, 93–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fineschi, V.; D’Errico, S.; Neri, M.; Panarese, F.; Ricci, P.A.; Turillazzi, E. Heat Stroke in an Incubator: An Immunohistochemical Study in a Fatal Case. Int. J. Legal. Med. 2005, 119, 94–97. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, T.; Zhu, B.-L.; Li, D.-R.; Zhao, D.; Michiue, T.; Maeda, H. Immunohistochemical Investigation of Ubiquitin and Myoglobin in the Kidney in Medicolegal Autopsy Cases. Forensic Sci. Int. 2007, 171, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, T.; Quan, L.; Li, D.-R.; Zhao, D.; Michiue, T.; Hamel, M.; Maeda, H. Postmortem Biochemistry and Immunohistochemistry of Adrenocorticotropic Hormone with Special Regard to Fatal Hypothermia. Forensic Sci. Int. 2008, 179, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, T.; Yoshida, C.; Michiue, T.; Perdekamp, M.G.; Pollak, S.; Maeda, H. Immunohistochemistry of Catecholamines in the Hypothalamic–Pituitary–Adrenal System with Special Regard to Fatal Hypothermia and Hyperthermia. Leg. Med. 2010, 12, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Quan, L.; Ishikawa, T.; Michiue, T.; Li, D.-R.; Zhao, D.; Zhu, B.-L.; Maeda, H. Quantitative Analysis of Ubiquitin-Immunoreactivity in the Midbrain Periaqueductal Gray Matter with Regard to the Causes of Death in Forensic Autopsy. Leg. Med. 2005, 7, 151–156. [Google Scholar] [CrossRef]
- Zhu, B.-L.; Ishikawa, T.; Michiue, T.; Li, D.-R.; Zhao, D.; Quan, L.; Maeda, H. Evaluation of Postmortem Urea Nitrogen, Creatinine and Uric Acid Levels in Pericardial Fluid in Forensic Autopsy. Leg. Med. 2005, 7, 287–292. [Google Scholar] [CrossRef]
- Zhu, B.-L.; Ishikawa, T.; Michiue, T.; Tanaka, S.; Zhao, D.; Li, D.-R.; Quan, L.; Oritani, S.; Maeda, H. Differences in Postmortem Urea Nitrogen, Creatinine and Uric Acid Levels between Blood and Pericardial Fluid in Acute Death. Leg. Med. 2007, 9, 115–122. [Google Scholar] [CrossRef]
- Fujita, M.Q.; Zhu, B.-L.; Ishida, K.; Quan, L.; Oritani, S.; Maeda, H. Serum C-Reactive Protein Levels in Postmortem Blood—An Analysis with Special Reference to the Cause of Death and Survival Time. Forensic Sci. Int. 2002, 130, 160–166. [Google Scholar] [CrossRef]
- Maeda, H.; Zhu, B.-L.; Bessho, Y.; Ishikawa, T.; Quan, L.; Michiue, T.; Zhao, D.; Li, D.-R.; Komatsu, A. Postmortem Serum Nitrogen Compounds and C-Reactive Protein Levels with Special Regard to Investigation of Fatal Hyperthermia. Forensic Sci. Med. Pathol. 2008, 4, 175–180. [Google Scholar] [CrossRef]
- Zhu, B.-L.; Ishikawa, T.; Michiue, T.; Li, D.-R.; Zhao, D.; Oritani, S.; Kamikodai, Y.; Tsuda, K.; Okazaki, S.; Maeda, H. Postmortem Cardiac Troponin T Levels in the Blood and Pericardial Fluid. Part 1. Analysis with Special Regard to Traumatic Causes of Death. Leg. Med. 2006, 8, 86–93. [Google Scholar] [CrossRef]
- Wang, Q.; Michiue, T.; Ishikawa, T.; Zhu, B.-L.; Maeda, H. Combined Analyses of Creatine Kinase MB, Cardiac Troponin I and Myoglobin in Pericardial and Cerebrospinal Fluids to Investigate Myocardial and Skeletal Muscle Injury in Medicolegal Autopsy Cases. Leg. Med. 2011, 13, 226–232. [Google Scholar] [CrossRef]
- Kortelainen, M.-L.; Huttunen, P.; Lapinlampi, T. Urinary Catecholamines in Hyperthermia-Related Deaths. Forensic Sci. Int. 1990, 48, 103–110. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Savioli, G.; Zanza, C.; Longhitano, Y.; Nardone, A.; Varesi, A.; Ceresa, I.F.; Manetti, A.C.; Volonnino, G.; Maiese, A.; La Russa, R. Heat-Related Illness in Emergency and Critical Care: Recommendations for Recognition and Management with Medico-Legal Considerations. Biomedicines 2022, 10, 2542. https://doi.org/10.3390/biomedicines10102542
Savioli G, Zanza C, Longhitano Y, Nardone A, Varesi A, Ceresa IF, Manetti AC, Volonnino G, Maiese A, La Russa R. Heat-Related Illness in Emergency and Critical Care: Recommendations for Recognition and Management with Medico-Legal Considerations. Biomedicines. 2022; 10(10):2542. https://doi.org/10.3390/biomedicines10102542
Chicago/Turabian StyleSavioli, Gabriele, Christian Zanza, Yaroslava Longhitano, Alba Nardone, Angelica Varesi, Iride Francesca Ceresa, Alice Chiara Manetti, Gianpietro Volonnino, Aniello Maiese, and Raffaele La Russa. 2022. "Heat-Related Illness in Emergency and Critical Care: Recommendations for Recognition and Management with Medico-Legal Considerations" Biomedicines 10, no. 10: 2542. https://doi.org/10.3390/biomedicines10102542







