The Complex Roles of Adipokines in Polycystic Ovary Syndrome and Endometriosis
Abstract
1. Obesity and Adipokines
2. Polycystic Ovary Syndrome
2.1. Role of Adipokines in Polycystic Ovary Syndrome (PCOS)
2.1.1. Leptin in PCOS
2.1.2. Chemerin in PCOS
2.1.3. Adiponectin in PCOS
2.1.4. Omentin-1 in PCOS
2.1.5. Vaspin in PCOS
2.1.6. Apelin in PCOS
2.1.7. Follistatin in PCOS
2.1.8. Visfatin in PCOS
2.1.9. Brown Adipose Tissue and Batokines in PCOS
Batokine Adiponectin as Mediator of BAT Effects on PCOS
FGF21 in PCOS
3. Endometriosis
3.1. BMI and Endometriosis
3.2. Role of Adipokines in Endometriosis
3.2.1. Role of Leptin in Endometriosis
3.2.2. Role of Adiponectin in Endometriosis
3.2.3. Role of Chemerin in Endometriosis
3.2.4. Role of Follistatin in Endometriosis
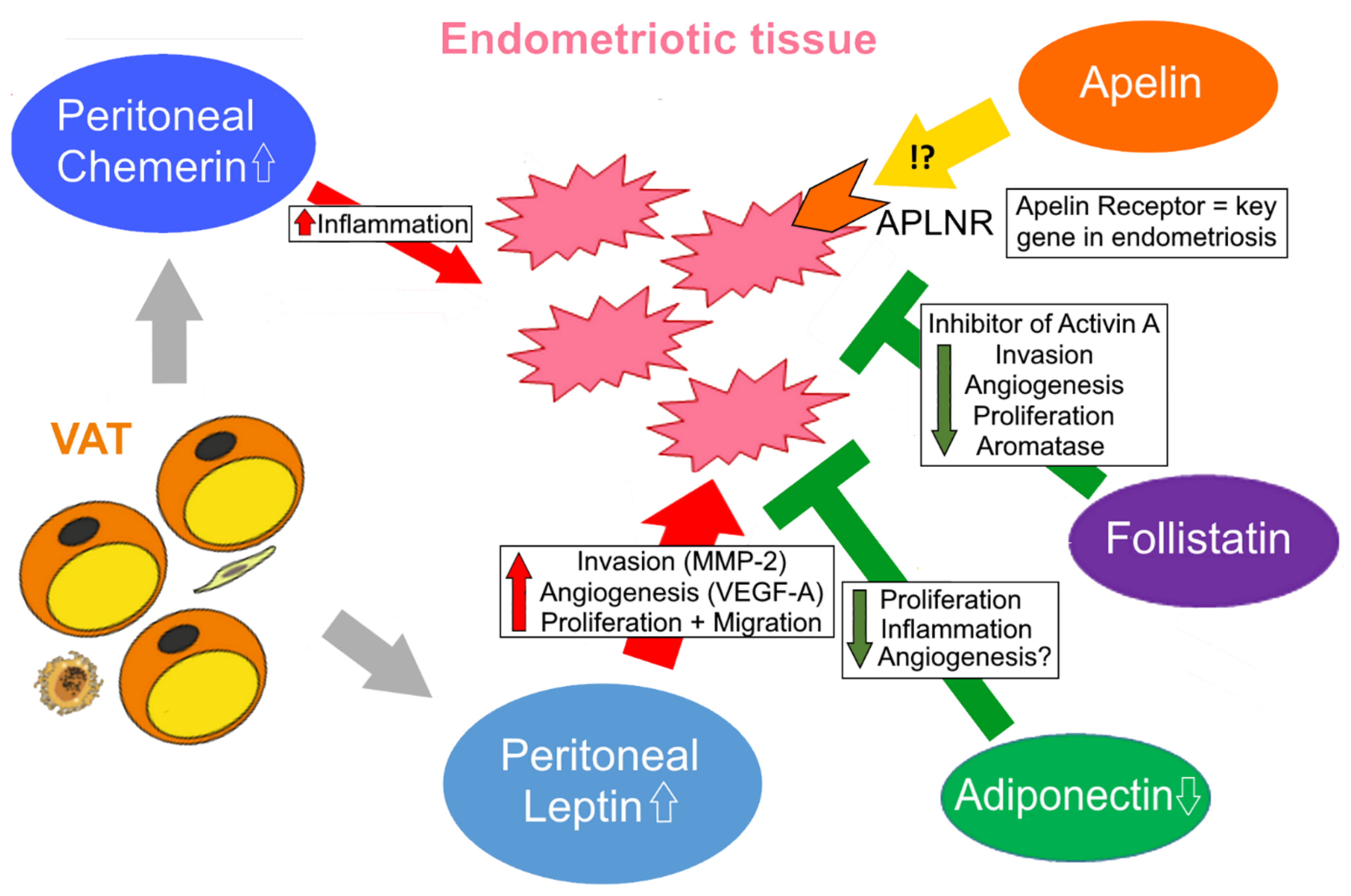
3.2.5. Apelin in Endometriosis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Purnell, J.Q. Endotext: Definitions, Classification, and Epidemiology of Obesity; Endotext: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Matafome, P.; Seiça, R. Function and Dysfunction of Adipose Tissue. Adv. Neurobiol. 2017, 19, 3–31. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Farias, M.; Fos-Domenech, J.; Serra, D.; Herrero, L.; Sánchez-Infantes, D. White adipose tissue dysfunction in obesity and aging. Biochem. Pharmacol. 2021, 192, 114723. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.H.; Solt, C.; Foster, M.T. Obesity associated disease risk: The role of inherent differences and location of adipose depots. Horm. Mol. Biol. Clin. Investig. 2018, 33. [Google Scholar] [CrossRef] [PubMed]
- Antonopoulos, A.S.; Tousoulis, D. The molecular mechanisms of obesity paradox. Cardiovasc. Res. 2017, 113, 1074–1086. [Google Scholar] [CrossRef]
- Schlecht, I.; Gronwald, W.; Behrens, G.; Baumeister, S.E.; Hertel, J.; Hochrein, J.; Zacharias, H.U.; Fischer, B.; Oefner, P.J.; Leitzmann, M.F. Visceral adipose tissue but not subcutaneous adipose tissue is associated with urine and serum metabolites. PLoS ONE 2017, 12, e0175133. [Google Scholar] [CrossRef]
- Shuster, A.; Patlas, M.; Pinthus, J.H.; Mourtzakis, M. The clinical importance of visceral adiposity: A critical review of methods for visceral adipose tissue analysis. Br. J. Radiol. 2012, 85, 1–10. [Google Scholar] [CrossRef]
- Philipsen, A.; Jørgensen, M.E.; Vistisen, D.; Sandbaek, A.; Almdal, T.P.; Christiansen, J.S.; Lauritzen, T.; Witte, D.R. Associations between ultrasound measures of abdominal fat distribution and indices of glucose metabolism in a population at high risk of type 2 diabetes: The ADDITION-PRO study. PLoS ONE 2015, 10, e0123062. [Google Scholar] [CrossRef]
- Ritchie, S.A.; Connell, J.M.C. The link between abdominal obesity, metabolic syndrome and cardiovascular disease. Nutr. Metab. Cardiovasc. Dis. 2007, 17, 319–326. [Google Scholar] [CrossRef]
- Cypess, A.M.; Lehman, S.; Williams, G.; Tal, I.; Rodman, D.; Goldfine, A.B.; Kuo, F.C.; Palmer, E.L.; Tseng, Y.-H.; Doria, A.; et al. Identification and importance of brown adipose tissue in adult humans. N. Engl. J. Med. 2009, 360, 1509–1517. [Google Scholar] [CrossRef]
- Frankl, J.; Sherwood, A.; Clegg, D.J.; Scherer, P.E.; Öz, O.K. Imaging Metabolically Active Fat: A Literature Review and Mechanistic Insights. Int. J. Mol. Sci. 2019, 20, 5509. [Google Scholar] [CrossRef]
- Omran, F.; Christian, M. Inflammatory Signaling and Brown Fat Activity. Front. Endocrinol. 2020, 11, 156. [Google Scholar] [CrossRef]
- Mancini, C.; Gohlke, S.; Garcia-Carrizo, F.; Zagoriy, V.; Stephanowitz, H.; Schulz, T.J. Identification of biomarkers of brown adipose tissue aging highlights the role of dysfunctional energy and nucleotide metabolism pathways. Sci. Rep. 2021, 11, 19928. [Google Scholar] [CrossRef]
- Kotzbeck, P.; Giordano, A.; Mondini, E.; Murano, I.; Severi, I.; Venema, W.; Cecchini, M.P.; Kershaw, E.E.; Barbatelli, G.; Haemmerle, G.; et al. Brown adipose tissue whitening leads to brown adipocyte death and adipose tissue inflammation. J. Lipid Res. 2018, 59, 784–794. [Google Scholar] [CrossRef]
- Betz, M.J.; Enerbäck, S. Targeting thermogenesis in brown fat and muscle to treat obesity and metabolic disease. Nat. Rev. Endocrinol. 2018, 14, 77–87. [Google Scholar] [CrossRef]
- Deshmukh, A.S.; Peijs, L.; Beaudry, J.L.; Jespersen, N.Z.; Nielsen, C.H.; Ma, T.; Brunner, A.D.; Larsen, T.J.; Bayarri-Olmos, R.; Prabhakar, B.S.; et al. Proteomics-Based Comparative Mapping of the Secretomes of Human Brown and White Adipocytes Reveals EPDR1 as a Novel Batokine. Cell Metab. 2019, 30, 963–975.e7. [Google Scholar] [CrossRef]
- Spann, R.A.; Morrison, C.D.; den Hartigh, L.J. The Nuanced Metabolic Functions of Endogenous FGF21 Depend on the Nature of the Stimulus, Tissue Source, and Experimental Model. Front. Endocrinol. 2021, 12, 802541. [Google Scholar] [CrossRef]
- Villarroya, J.; Cereijo, R.; Gavaldà-Navarro, A.; Peyrou, M.; Giralt, M.; Villarroya, F. New insights into the secretory functions of brown adipose tissue. J. Endocrinol. 2019, 243, R19–R27. [Google Scholar] [CrossRef]
- Zhang, Q.; Ye, R.; Zhang, Y.-Y.; Fan, C.-C.; Wang, J.; Wang, S.; Chen, S.; Liu, X. Brown Adipose Tissue and Novel Management Strategies for Polycystic Ovary Syndrome Therapy. Front. Endocrinol. 2022, 13, 847249. [Google Scholar] [CrossRef]
- Zhang, Y.; Proenca, R.; Maffei, M.; Barone, M.; Leopold, L.; Friedman, J.M. Positional cloning of the mouse obese gene and its human homologue. Nature 1994, 372, 425–432. [Google Scholar] [CrossRef]
- Nordström, V.; Willershäuser, M.; Herzer, S.; Rozman, J.; von Bohlen Und Halbach, O.; Meldner, S.; Rothermel, U.; Kaden, S.; Roth, F.C.; Waldeck, C.; et al. Neuronal expression of glucosylceramide synthase in central nervous system regulates body weight and energy homeostasis. PLoS Biol. 2013, 11, e1001506. [Google Scholar] [CrossRef]
- Obradovic, M.; Sudar-Milovanovic, E.; Soskic, S.; Essack, M.; Arya, S.; Stewart, A.J.; Gojobori, T.; Isenovic, E.R. Leptin and Obesity: Role and Clinical Implication. Front. Endocrinol. 2021, 12, 585887. [Google Scholar] [CrossRef]
- Misch, M.; Puthanveetil, P. The Head-to-Toe Hormone: Leptin as an Extensive Modulator of Physiologic Systems. Int. J. Mol. Sci. 2022, 23, 5439. [Google Scholar] [CrossRef]
- Izquierdo, A.G.; Crujeiras, A.B.; Casanueva, F.F.; Carreira, M.C. Leptin, Obesity, and Leptin Resistance: Where Are We 25 Years Later? Nutrients 2019, 11, 2704. [Google Scholar] [CrossRef]
- Funcke, J.-B.; Scherer, P.E. Beyond adiponectin and leptin: Adipose tissue-derived mediators of inter-organ communication. J. Lipid Res. 2019, 60, 1648–1684. [Google Scholar] [CrossRef]
- Schaab, M.; Kratzsch, J. The soluble leptin receptor. Best Pract. Res. Clin. Endocrinol. Metab. 2015, 29, 661–670. [Google Scholar] [CrossRef]
- Peng, Y.; Yang, H.; Song, J.; Di Feng; Na, Z.; Jiang, H.; Meng, Y.; Shi, B.; Da Li. Elevated Serum Leptin Levels as a Predictive Marker for Polycystic Ovary Syndrome. Front. Endocrinol. 2022, 13, 845165. [Google Scholar] [CrossRef]
- Childs, G.V.; Odle, A.K.; MacNicol, M.C.; MacNicol, A.M. The Importance of Leptin to Reproduction. Endocrinology 2021, 162, 458–464. [Google Scholar] [CrossRef]
- Arita, Y.; Kihara, S.; Ouchi, N.; Takahashi, M.; Maeda, K.; Miyagawa, J.; Hotta, K.; Shimomura, I.; Nakamura, T.; Miyaoka, K.; et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem. Biophys. Res. Commun. 1999, 257, 79–83. [Google Scholar] [CrossRef]
- Mori, T.; Koyama, Y.; Maeda, N.; Nakamura, Y.; Fujishima, Y.; Matsuda, K.; Funahashi, T.; Shimada, S.; Shimomura, I. Ultrastructural localization of adiponectin protein in vasculature of normal and atherosclerotic mice. Sci. Rep. 2014, 4, 4895. [Google Scholar] [CrossRef]
- Liu, M.; Liu, F. Regulation of adiponectin multimerization, signaling and function. Best Pract. Res. Clin. Endocrinol. Metab. 2014, 28, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, T.; Kadowaki, T. Adiponectin receptor as a key player in healthy longevity and obesity-related diseases. Cell Metab. 2013, 17, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Pascolutti, R.; Erlandson, S.C.; Burri, D.J.; Zheng, S.; Kruse, A.C. Mapping and engineering the interaction between adiponectin and T-cadherin. J. Biol. Chem. 2020, 295, 2749–2759. [Google Scholar] [CrossRef] [PubMed]
- Otvos, L. Potential Adiponectin Receptor Response Modifier Therapeutics. Front. Endocrinol. 2019, 10, 539. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Wu, Y.; Zhang, H.; Wang, X.; Tian, X.; Wang, Y.; Qiu, Z.; Zou, L.; Tang, Z.; Huang, M. Association of leptin and adiponectin levels with endometriosis: A systematic review and meta-analysis. Gynecol. Endocrinol. 2021, 37, 591–599. [Google Scholar] [CrossRef]
- Singh, A.; Choubey, M.; Bora, P.; Krishna, A. Adiponectin and Chemerin: Contrary Adipokines in Regulating Reproduction and Metabolic Disorders. Reprod. Sci. 2018, 25, 1462–1473. [Google Scholar] [CrossRef]
- Yang, R.-Z.; Lee, M.-J.; Hu, H.; Pray, J.; Wu, H.-B.; Hansen, B.C.; Shuldiner, A.R.; Fried, S.K.; McLenithan, J.C.; Gong, D.-W. Identification of omentin as a novel depot-specific adipokine in human adipose tissue: Possible role in modulating insulin action. Am. J. Physiol. Endocrinol. Metab. 2006, 290, E1253–E1261. [Google Scholar] [CrossRef]
- Zhao, A.; Xiao, H.; Zhu, Y.; Liu, S.; Zhang, S.; Yang, Z.; Du, L.; Li, X.; Niu, X.; Wang, C.; et al. Omentin-1: A newly discovered warrior against metabolic related diseases. Expert Opin. Ther. Targets 2022, 26, 275–289. [Google Scholar] [CrossRef]
- Lin, X.; Sun, Y.; Yang, S.; Yu, M.; Pan, L.; Yang, J.; Yang, J.; Shao, Q.; Liu, J.; Liu, Y.; et al. Omentin-1 Modulates Macrophage Function via Integrin Receptors αvβ3 and αvβ5 and Reverses Plaque Vulnerability in Animal Models of Atherosclerosis. Front. Cardiovasc. Med. 2021, 8, 757926. [Google Scholar] [CrossRef]
- Tang, Y.-L.; Yu, J.; Zeng, Z.-G.; Liu, Y.; Liu, J.-Y.; Xu, J.-X. Circulating omentin-1 levels in women with polycystic ovary syndrome: A meta-analysis. Gynecol. Endocrinol. 2017, 33, 244–249. [Google Scholar] [CrossRef]
- Krautbauer, S.; Wanninger, J.; Eisinger, K.; Hader, Y.; Beck, M.; Kopp, A.; Schmid, A.; Weiss, T.S.; Dorn, C.; Buechler, C. Chemerin is highly expressed in hepatocytes and is induced in non-alcoholic steatohepatitis liver. Exp. Mol. Pathol. 2013, 95, 199–205. [Google Scholar] [CrossRef]
- Villarroya, F.; Cereijo, R.; Gavaldà-Navarro, A.; Villarroya, J.; Giralt, M. Inflammation of brown/beige adipose tissues in obesity and metabolic disease. J. Intern. Med. 2018, 284, 492–504. [Google Scholar] [CrossRef]
- Ali Khan, A.; Hansson, J.; Weber, P.; Foehr, S.; Krijgsveld, J.; Herzig, S.; Scheideler, M. Comparative Secretome Analyses of Primary Murine White and Brown Adipocytes Reveal Novel Adipokines. Mol. Cell. Proteomics 2018, 17, 2358–2370. [Google Scholar] [CrossRef]
- Hansen, I.R.; Jansson, K.M.; Cannon, B.; Nedergaard, J. Contrasting effects of cold acclimation versus obesogenic diets on chemerin gene expression in brown and brite adipose tissues. Biochim. Biophys. Acta 2014, 1841, 1691–1699. [Google Scholar] [CrossRef]
- Goralski, K.B.; McCarthy, T.C.; Hanniman, E.A.; Zabel, B.A.; Butcher, E.C.; Parlee, S.D.; Muruganandan, S.; Sinal, C.J. Chemerin, a novel adipokine that regulates adipogenesis and adipocyte metabolism. J. Biol. Chem. 2007, 282, 28175–28188. [Google Scholar] [CrossRef]
- Mariani, F.; Roncucci, L. Chemerin/chemR23 axis in inflammation onset and resolution. Inflamm. Res. 2015, 64, 85–95. [Google Scholar] [CrossRef]
- Ferland, D.J.; Mullick, A.E.; Watts, S.W. Chemerin as a Driver of Hypertension: A Consideration. Am. J. Hypertens. 2020, 33, 975–986. [Google Scholar] [CrossRef]
- Léniz, A.; González, M.; Besné, I.; Carr-Ugarte, H.; Gómez-García, I.; Portillo, M.P. Role of chemerin in the control of glucose homeostasis. Mol. Cell. Endocrinol. 2022, 541, 111504. [Google Scholar] [CrossRef]
- De Henau, O.; Degroot, G.-N.; Imbault, V.; Robert, V.; de Poorter, C.; Mcheik, S.; Galés, C.; Parmentier, M.; Springael, J.-Y. Signaling Properties of Chemerin Receptors CMKLR1, GPR1 and CCRL2. PLoS ONE 2016, 11, e0164179. [Google Scholar] [CrossRef]
- Bufano, M.; Laffranchi, M.; Sozzani, S.; Raimondo, D.; Silvestri, R.; Coluccia, A. Exploring CCRL2 chemerin binding using accelerated molecular dynamics. Proteins 2022, 90, 1714–1720. [Google Scholar] [CrossRef]
- Bondue, B.; Wittamer, V.; Parmentier, M. Chemerin and its receptors in leukocyte trafficking, inflammation and metabolism. Cytokine Growth Factor Rev. 2011, 22, 331–338. [Google Scholar] [CrossRef]
- Toulany, J.; Parlee, S.D.; Sinal, C.J.; Slayter, K.; McNeil, S.; Goralski, K.B. CMKLR1 activation ex vivo does not increase proportionally to serum total chemerin in obese humans. Endocr. Connect. 2016, 5, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Quan, X.; Lan, Y.; Wei, Q.; Ye, J.; Yin, X.; Ji, Z.; Xing, H.; Yang, Y. Serum chemerin level in women with PCOS and its relation with the risk of spontaneous abortion. Gynecol. Endocrinol. 2018, 34, 864–867. [Google Scholar] [CrossRef] [PubMed]
- Klöting, N.; Berndt, J.; Kralisch, S.; Kovacs, P.; Fasshauer, M.; Schön, M.R.; Stumvoll, M.; Blüher, M. Vaspin gene expression in human adipose tissue: Association with obesity and type 2 diabetes. Biochem. Biophys. Res. Commun. 2006, 339, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.A.; Park, H.S.; Song, Y.S.; Jang, Y.J.; Kim, J.-H.; Lee, Y.J.; Heo, Y.-S. Relationship between vaspin gene expression and abdominal fat distribution of Korean women. Endocr. J. 2011, 58, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Blüher, M. Vaspin in obesity and diabetes: Pathophysiological and clinical significance. Endocrine 2012, 41, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Heiker, J.T.; Klöting, N.; Kovacs, P.; Kuettner, E.B.; Sträter, N.; Schultz, S.; Kern, M.; Stumvoll, M.; Blüher, M.; Beck-Sickinger, A.G. Vaspin inhibits kallikrein 7 by serpin mechanism. Cell. Mol. Life Sci. 2013, 70, 2569–2583. [Google Scholar] [CrossRef] [PubMed]
- Nakatsuka, A.; Wada, J.; Iseda, I.; Teshigawara, S.; Higashio, K.; Murakami, K.; Kanzaki, M.; Inoue, K.; Terami, T.; Katayama, A.; et al. Vaspin is an adipokine ameliorating ER stress in obesity as a ligand for cell-surface GRP78/MTJ-1 complex. Diabetes 2012, 61, 2823–2832. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, T.; Church, C.; Tsintzas, K.; Jones, R.; Breen, L.; Davis, E.T.; Baker, D.J.; Jones, S.W. Vaspin promotes insulin sensitivity of elderly muscle and is upregulated in obesity. J. Endocrinol. 2019, 241, 31–43. [Google Scholar] [CrossRef]
- Escoté, X.; Gómez-Zorita, S.; López-Yoldi, M.; Milton-Laskibar, I.; Fernández-Quintela, A.; Martínez, J.A.; Moreno-Aliaga, M.J.; Portillo, M.P. Role of Omentin, Vaspin, Cardiotrophin-1, TWEAK and NOV/CCN3 in Obesity and Diabetes Development. Int. J. Mol. Sci. 2017, 18, 1770. [Google Scholar] [CrossRef]
- Li, C.; Cheng, H.; Adhikari, B.K.; Wang, S.; Yang, N.; Liu, W.; Sun, J.; Wang, Y. The Role of Apelin-APJ System in Diabetes and Obesity. Front. Endocrinol. 2022, 13, 820002. [Google Scholar] [CrossRef]
- Yuzbashian, E.; Asghari, G.; Beheshti, N.; Hedayati, M.; Zarkesh, M.; Mirmiran, P.; Daneshafrooz, A.; Khalaj, A. Plasma Fatty Acid Composition Was Associated with Apelin Gene Expression in Human Adipose Tissues. Biomed Res. Int. 2021, 2021, 8846483. [Google Scholar] [CrossRef]
- Melgar-Lesmes, P.; Perramon, M.; Jiménez, W. Roles of the Hepatic Endocannabinoid and Apelin Systems in the Pathogenesis of Liver Fibrosis. Cells 2019, 8, 1311. [Google Scholar] [CrossRef]
- Lv, W.; Zhang, L.; Cheng, X.; Wang, H.; Qin, W.; Zhou, X.; Tang, B. Apelin Inhibits Angiotensin II-Induced Atrial Fibrosis and Atrial Fibrillation via TGF-β1/Smad2/α-SMA Pathway. Front. Physiol. 2020, 11, 583570. [Google Scholar] [CrossRef]
- Habchi, M.; Duvillard, L.; Cottet, V.; Brindisi, M.-C.; Bouillet, B.; Beacco, M.; Crevisy, E.; Buffier, P.; Baillot-Rudoni, S.; Verges, B.; et al. Circulating apelin is increased in patients with type 1 or type 2 diabetes and is associated with better glycaemic control. Clin. Endocrinol. 2014, 81, 696–701. [Google Scholar] [CrossRef]
- Soriguer, F.; Garrido-Sanchez, L.; Garcia-Serrano, S.; Garcia-Almeida, J.M.; Garcia-Arnes, J.; Tinahones, F.J.; Garcia-Fuentes, E. Apelin levels are increased in morbidly obese subjects with type 2 diabetes mellitus. Obes. Surg. 2009, 19, 1574–1580. [Google Scholar] [CrossRef]
- Cano Martínez, L.J.; Coral Vázquez, R.M.; Méndez, J.P.; Trejo, S.; Pérez Razo, J.C.; Canto, P. Serum concentrations of apelin-17 isoform vary in accordance to blood pressure categories in individuals with obesity class 3. Clin. Exp. Hypertens. 2019, 41, 168–173. [Google Scholar] [CrossRef]
- Carbone, F.; Liberale, L.; Bonaventura, A.; Vecchiè, A.; Casula, M.; Cea, M.; Monacelli, F.; Caffa, I.; Bruzzone, S.; Montecucco, F.; et al. Regulation and Function of Extracellular Nicotinamide Phosphoribosyltransferase/Visfatin. Compr. Physiol. 2017, 7, 603–621. [Google Scholar] [CrossRef]
- Berndt, J.; Klöting, N.; Kralisch, S.; Kovacs, P.; Fasshauer, M.; Schön, M.R.; Stumvoll, M.; Blüher, M. Plasma visfatin concentrations and fat depot-specific mRNA expression in humans. Diabetes 2005, 54, 2911–2916. [Google Scholar] [CrossRef]
- Liang, N.-L.; Men, R.; Zhu, Y.; Yuan, C.; Wei, Y.; Liu, X.; Yang, L. Visfatin: An adipokine activator of rat hepatic stellate cells. Mol. Med. Rep. 2015, 11, 1073–1078. [Google Scholar] [CrossRef]
- Sawicka, K.; Michalska-Jakubus, M.; Potembska, E.; Kowal, M.; Pietrzak, A.; Krasowska, D. Visfatin and chemerin levels correspond with inflammation and might reflect the bridge between metabolism, inflammation and fibrosis in patients with systemic sclerosis. Postepy Dermatol. Alergol. 2019, 36, 551–565. [Google Scholar] [CrossRef]
- Yu, T.; Zhang, L.; Wang, Y.; Shen, X.; Lin, L.; Tang, Y. Effect of visfatin on KATP channel upregulation in colonic smooth muscle cells in diabetic colon dysmotility. Aging Albany N. Y. 2022, 14, 1292–1306. [Google Scholar] [CrossRef] [PubMed]
- Ezzati-Mobaser, S.; Malekpour-Dehkordi, Z.; Nourbakhsh, M.; Tavakoli-Yaraki, M.; Ahmadpour, F.; Golpour, P.; Nourbakhsh, M. The up-regulation of markers of adipose tissue fibrosis by visfatin in pre-adipocytes as well as obese children and adolescents. Cytokine 2020, 134, 155193. [Google Scholar] [CrossRef] [PubMed]
- Buechler, C.; Krautbauer, S.; Eisinger, K. Adipose tissue fibrosis. World J. Diabetes 2015, 6, 548–553. [Google Scholar] [CrossRef] [PubMed]
- Terra, X.; Auguet, T.; Quesada, I.; Aguilar, C.; Luna, A.M.; Hernández, M.; Sabench, F.; Porras, J.A.; Martínez, S.; Lucas, A.; et al. Increased levels and adipose tissue expression of visfatin in morbidly obese women: The relationship with pro-inflammatory cytokines. Clin. Endocrinol. 2012, 77, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-H.; Chang, D.-M.; Lin, K.-C.; Shin, S.-J.; Lee, Y.-J. Visfatin in overweight/obesity, type 2 diabetes mellitus, insulin resistance, metabolic syndrome and cardiovascular diseases: A meta-analysis and systemic review. Diabetes. Metab. Res. Rev. 2011, 27, 515–527. [Google Scholar] [CrossRef]
- Flanagan, J.N.; Linder, K.; Mejhert, N.; Dungner, E.; Wahlen, K.; Decaunes, P.; Rydén, M.; Björklund, P.; Arver, S.; Bhasin, S.; et al. Role of follistatin in promoting adipogenesis in women. J. Clin. Endocrinol. Metab. 2009, 94, 3003–3009. [Google Scholar] [CrossRef]
- Pervin, S.; Reddy, S.T.; Singh, R. Novel Roles of Follistatin/Myostatin in Transforming Growth Factor-β Signaling and Adipose Browning: Potential for Therapeutic Intervention in Obesity Related Metabolic Disorders. Front. Endocrinol. 2021, 12, 653179. [Google Scholar] [CrossRef]
- Raeisi, T.; Rezaie, H.; Darand, M.; Taheri, A.; Garousi, N.; Razi, B.; Roever, L.; Mohseni, R.; Hussien Mohammed, S.; Alizadeh, S. Circulating resistin and follistatin levels in obese and non-obese women with polycystic ovary syndrome: A systematic review and meta-analysis. PLoS ONE 2021, 16, e0246200. [Google Scholar] [CrossRef]
- Vannuccini, S.; Clemenza, S.; Rossi, M.; Petraglia, F. Hormonal treatments for endometriosis: The endocrine background. Rev. Endocr. Metab. Disord. 2022, 23, 333–355. [Google Scholar] [CrossRef]
- Tersigni, C.; Di Nicuolo, F.; D’Ippolito, S.; Veglia, M.; Castellucci, M.; Di Simone, N. Adipokines: New emerging roles in fertility and reproduction. Obstet. Gynecol. Surv. 2011, 66, 47–63. [Google Scholar] [CrossRef]
- Hart, R.J. Physiological Aspects of Female Fertility: Role of the Environment, Modern Lifestyle, and Genetics. Physiol. Rev. 2016, 96, 873–909. [Google Scholar] [CrossRef]
- Rushing, J.S.; Santoro, N. Fertility Issues in Polycystic Ovarian Disease: A Systematic Approach. Endocrinol. Metab. Clin. North Am. 2021, 50, 43–55. [Google Scholar] [CrossRef]
- Haydardedeoglu, B.; Zeyneloglu, H.B. The impact of endometriosis on fertility. Womens Health 2015, 11, 619–623. [Google Scholar] [CrossRef]
- March, W.A.; Moore, V.M.; Willson, K.J.; Phillips, D.I.W.; Norman, R.J.; Davies, M.J. The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum. Reprod. 2010, 25, 544–551. [Google Scholar] [CrossRef]
- Stener-Victorin, E.; Deng, Q. Epigenetic inheritance of polycystic ovary syndrome—Challenges and opportunities for treatment. Nat. Rev. Endocrinol. 2021, 17, 521–533. [Google Scholar] [CrossRef]
- Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil. Steril. 2004, 81, 19–25. [CrossRef]
- Collée, J.; Mawet, M.; Tebache, L.; Nisolle, M.; Brichant, G. Polycystic ovarian syndrome and infertility: Overview and insights of the putative treatments. Gynecol. Endocrinol. 2021, 37, 869–874. [Google Scholar] [CrossRef]
- Fauser, B.C.J.M.; Tarlatzis, B.C.; Rebar, R.W.; Legro, R.S.; Balen, A.H.; Lobo, R.; Carmina, E.; Chang, J.; Yildiz, B.O.; Laven, J.S.E.; et al. Consensus on women’s health aspects of polycystic ovary syndrome (PCOS): The Amsterdam ESHRE/ASRM-Sponsored 3rd PCOS Consensus Workshop Group. Fertil. Steril. 2012, 97, 28–38.e25. [Google Scholar] [CrossRef]
- Glintborg, D.; Kolster, N.D.; Ravn, P.; Andersen, M.S. Prospective Risk of Type 2 Diabetes in Normal Weight Women with Polycystic Ovary Syndrome. Biomedicines 2022, 10, 1455. [Google Scholar] [CrossRef]
- Guan, C.; Zahid, S.; Minhas, A.S.; Ouyang, P.; Vaught, A.; Baker, V.L.; Michos, E.D. Polycystic ovary syndrome: A “risk-enhancing” factor for cardiovascular disease. Fertil. Steril. 2022, 117, 924–935. [Google Scholar] [CrossRef]
- Rudnicka, E.; Suchta, K.; Grymowicz, M.; Calik-Ksepka, A.; Smolarczyk, K.; Duszewska, A.M.; Smolarczyk, R.; Meczekalski, B. Chronic Low Grade Inflammation in Pathogenesis of PCOS. Int. J. Mol. Sci. 2021, 22, 3789. [Google Scholar] [CrossRef] [PubMed]
- Joham, A.E.; Norman, R.J.; Stener-Victorin, E.; Legro, R.S.; Franks, S.; Moran, L.J.; Boyle, J.; Teede, H.J. Polycystic ovary syndrome. Lancet Diabetes Endocrinol. 2022, 10, 668–680. [Google Scholar] [CrossRef]
- Sanchez-Garrido, M.A.; Tena-Sempere, M. Metabolic dysfunction in polycystic ovary syndrome: Pathogenic role of androgen excess and potential therapeutic strategies. Mol. Metab. 2020, 35, 100937. [Google Scholar] [CrossRef] [PubMed]
- Gilling-Smith, C.; Story, H.; Rogers, V.; Franks, S. Evidence for a primary abnormality of thecal cell steroidogenesis in the polycystic ovary syndrome. Clin. Endocrinol. 1997, 47, 93–99. [Google Scholar] [CrossRef]
- Lizneva, D.; Suturina, L.; Walker, W.; Brakta, S.; Gavrilova-Jordan, L.; Azziz, R. Criteria, prevalence, and phenotypes of polycystic ovary syndrome. Fertil. Steril. 2016, 106, 6–15. [Google Scholar] [CrossRef]
- Broekmans, F.J.; Knauff, E.A.H.; Valkenburg, O.; Laven, J.S.; Eijkemans, M.J.; Fauser, B.C.J.M. PCOS according to the Rotterdam consensus criteria: Change in prevalence among WHO-II anovulation and association with metabolic factors. BJOG 2006, 113, 1210–1217. [Google Scholar] [CrossRef]
- National Institutes of Health. Evidence-Based Methodology Workshop on Polycystic Ovary Syndrome, December 3–5 2012. 2016. Available online: https://www.nichd.nih.gov/newsroom/resources/spotlight/112112-pcos (accessed on 30 August 2022).
- Dapas, M.; Lin, F.T.J.; Nadkarni, G.N.; Sisk, R.; Legro, R.S.; Urbanek, M.; Hayes, M.G.; Dunaif, A. Distinct subtypes of polycystic ovary syndrome with novel genetic associations: An unsupervised, phenotypic clustering analysis. PLoS Med. 2020, 17, e1003132. [Google Scholar] [CrossRef]
- Pei, C.-Z.; Jin, L.; Baek, K.-H. Pathogenetic analysis of polycystic ovary syndrome from the perspective of omics. Biomed. Pharmacother. 2021, 142, 112031. [Google Scholar] [CrossRef]
- Vázquez-Martínez, E.R.; Gómez-Viais, Y.I.; García-Gómez, E.; Reyes-Mayoral, C.; Reyes-Muñoz, E.; Camacho-Arroyo, I.; Cerbón, M. DNA methylation in the pathogenesis of polycystic ovary syndrome. Reproduction 2019, 158, R27–R40. [Google Scholar] [CrossRef]
- Liu, Y.-N.; Qin, Y.; Wu, B.; Peng, H.; Li, M.; Luo, H.; Liu, L.-L. DNA methylation in polycystic ovary syndrome: Emerging evidence and challenges. Reprod. Toxicol. 2022, 111, 11–19. [Google Scholar] [CrossRef]
- Ilie, I.R.; Georgescu, C.E. Polycystic Ovary Syndrome-Epigenetic Mechanisms and Aberrant MicroRNA. Adv. Clin. Chem. 2015, 71, 25–45. [Google Scholar] [CrossRef]
- Tu, M.; Wu, Y.; Mu, L.; Zhang, D. Long non-coding RNAs: Novel players in the pathogenesis of polycystic ovary syndrome. Ann. Transl. Med. 2021, 9, 173. [Google Scholar] [CrossRef]
- Echiburú, B.; Milagro, F.; Crisosto, N.; Pérez-Bravo, F.; Flores, C.; Arpón, A.; Salas-Pérez, F.; Recabarren, S.E.; Sir-Petermann, T.; Maliqueo, M. DNA methylation in promoter regions of genes involved in the reproductive and metabolic function of children born to women with PCOS. Epigenetics 2020, 15, 1178–1194. [Google Scholar] [CrossRef]
- Mehrabani, S.; Arab, A.; Karimi, E.; Nouri, M.; Mansourian, M. Blood Circulating Levels of Adipokines in Polycystic Ovary Syndrome Patients: A Systematic Review and Meta-analysis. Reprod. Sci. 2021, 28, 3032–3050. [Google Scholar] [CrossRef]
- Bongrani, A.; Mellouk, N.; Rame, C.; Cornuau, M.; Guérif, F.; Froment, P.; Dupont, J. Ovarian Expression of Adipokines in Polycystic Ovary Syndrome: A Role for Chemerin, Omentin, and Apelin in Follicular Growth Arrest and Ovulatory Dysfunction? Int. J. Mol. Sci. 2019, 20, 3778. [Google Scholar] [CrossRef]
- Kabil Kucur, S.; Kurek Eken, M.; Sanli, I.; Kutlu, T.; Bilgic, B.E.; Altuntas, Ş.L.; Cevik, O.; Ozkaya, E. Predictive value of serum and follicular fluid chemerin concentrations during assisted reproductive cycles in women with polycystic ovary syndrome. Gynecol. Endocrinol. 2021, 37, 814–818. [Google Scholar] [CrossRef]
- Mansoori, A.; Amoochi-Foroushani, G.; Zilaee, M.; Hosseini, S.A.; Azhdari, M. Serum and follicular fluid chemerin and chemerin mRNA expression in women with polycystic ovary syndrome: Systematic review and meta-analysis. Endocrinol. Diabetes Metab. 2022, 5, e00307. [Google Scholar] [CrossRef]
- Cloix, L.; Reverchon, M.; Cornuau, M.; Froment, P.; Ramé, C.; Costa, C.; Froment, G.; Lecomte, P.; Chen, W.; Royère, D.; et al. Expression and regulation of INTELECTIN1 in human granulosa-lutein cells: Role in IGF-1-induced steroidogenesis through NAMPT. Biol. Reprod. 2014, 91, 50. [Google Scholar] [CrossRef]
- Du, L.; Wang, Y.; Li, C.-R.; Chen, L.-J.; Cai, J.-Y.; Xia, Z.-R.; Zeng, W.-T.; Wang, Z.-B.; Chen, X.-C.; Hu, F.; et al. Rat BAT xenotransplantation recovers the fertility and metabolic health of PCOS mice. J. Endocrinol. 2021, 248, 249–264. [Google Scholar] [CrossRef]
- Zheng, S.-H.; Du, D.-F.; Li, X.-L. Leptin Levels in Women with Polycystic Ovary Syndrome: A Systematic Review and a Meta-Analysis. Reprod. Sci. 2017, 24, 656–670. [Google Scholar] [CrossRef]
- Chen, C.-I.; Hsu, M.-I.; Lin, S.-H.; Chang, Y.-C.I.; Hsu, C.-S.; Tzeng, C.-R. Adiponectin and leptin in overweight/obese and lean women with polycystic ovary syndrome. Gynecol. Endocrinol. 2015, 31, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Ozgokce, C.; Elci, E.; Yildizhan, R. C-Reactive Protein, Fibrinogen, Leptin, and Adiponectin Levels in Women with Polycystic Ovary Syndrome. J. Obstet. Gynaecol. India 2020, 70, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Hahn, S.; Haselhorst, U.; Quadbeck, B.; Tan, S.; Kimmig, R.; Mann, K.; Janssen, O.E. Decreased soluble leptin receptor levels in women with polycystic ovary syndrome. Eur. J. Endocrinol. 2006, 154, 287–294. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rizk, N.M.; Sharif, E. Leptin as well as Free Leptin Receptor Is Associated with Polycystic Ovary Syndrome in Young Women. Int. J. Endocrinol. 2015, 2015, 927805. [Google Scholar] [CrossRef]
- Guzmán, A.; Hernández-Coronado, C.G.; Rosales-Torres, A.M.; Hernández-Medrano, J.H. Leptin regulates neuropeptides associated with food intake and GnRH secretion. Ann. Endocrinol. 2019, 80, 38–46. [Google Scholar] [CrossRef]
- Kucera, R.; Babuska, V.; Ulcova-Gallova, Z.; Kulda, V.; Topolcan, O. Follicular fluid levels of anti-Müllerian hormone, insulin-like growth factor 1 and leptin in women with fertility disorders. Syst. Biol. Reprod. Med. 2018, 64, 220–223. [Google Scholar] [CrossRef]
- Boucsein, A.; Kamstra, K.; Tups, A. Central signalling cross-talk between insulin and leptin in glucose and energy homeostasis. J. Neuroendocrinol. 2021, 33, e12944. [Google Scholar] [CrossRef]
- Vilariño-García, T.; Pérez-Pérez, A.; Santamaría-López, E.; Prados, N.; Fernández-Sánchez, M.; Sánchez-Margalet, V. Sam68 mediates leptin signaling and action in human granulosa cells: Possible role in leptin resistance in PCOS. Endocr. Connect. 2020, 9, 479–488. [Google Scholar] [CrossRef]
- Zachow, R.J.; Magoffin, D.A. Direct intraovarian effects of leptin: Impairment of the synergistic action of insulin-like growth factor-I on follicle-stimulating hormone-dependent estradiol-17 beta production by rat ovarian granulosa cells. Endocrinology 1997, 138, 847–850. [Google Scholar] [CrossRef]
- Chen, P.; Jia, R.; Liu, Y.; Cao, M.; Zhou, L.; Zhao, Z. Progress of Adipokines in the Female Reproductive System: A Focus on Polycystic Ovary Syndrome. Front. Endocrinol. 2022, 13, 881684. [Google Scholar] [CrossRef]
- Nogues, P.; Dos Santos, E.; Jammes, H.; Berveiller, P.; Arnould, L.; Vialard, F.; Dieudonné, M.-N. Maternal obesity influences expression and DNA methylation of the adiponectin and leptin systems in human third-trimester placenta. Clin. Epigenet. 2019, 11, 20. [Google Scholar] [CrossRef]
- Stolzenbach, F.; Valdivia, S.; Ojeda-Provoste, P.; Toledo, F.; Sobrevia, L.; Kerr, B. DNA methylation changes in genes coding for leptin and insulin receptors during metabolic-altered pregnancies. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165465. [Google Scholar] [CrossRef]
- Lesseur, C.; Armstrong, D.A.; Paquette, A.G.; Koestler, D.C.; Padbury, J.F.; Marsit, C.J. Tissue-specific Leptin promoter DNA methylation is associated with maternal and infant perinatal factors. Mol. Cell. Endocrinol. 2013, 381, 160–167. [Google Scholar] [CrossRef]
- Catalano, P.M.; Presley, L.; Minium, J.; Hauguel-de Mouzon, S. Fetuses of obese mothers develop insulin resistance in utero. Diabetes Care 2009, 32, 1076–1080. [Google Scholar] [CrossRef]
- Josefson, J.L.; Zeiss, D.M.; Rademaker, A.W.; Metzger, B.E. Maternal leptin predicts adiposity of the neonate. Horm. Res. Paediatr. 2014, 81, 13–19. [Google Scholar] [CrossRef]
- Ghalandari, H.; Hosseini-Esfahani, F.; Mirmiran, P. The Association of Polymorphisms in Leptin/Leptin Receptor Genes and Ghrelin/Ghrelin Receptor Genes With Overweight/Obesity and the Related Metabolic Disturbances: A Review. Int. J. Endocrinol. Metab. 2015, 13, e19073. [Google Scholar] [CrossRef]
- Li, L.; Lee, K.-J.; Choi, B.-C.; Baek, K.-H. Relationship between leptin receptor and polycystic ovary syndrome. Gene 2013, 527, 71–74. [Google Scholar] [CrossRef]
- Tu, X.; Yu, C.; Gao, M.; Zhang, Y.; Zhang, Z.; He, Y.; Yao, L.; Du, J.; Sun, Y.; Sun, Z. LEPR gene polymorphism and plasma soluble leptin receptor levels are associated with polycystic ovary syndrome in Han Chinese women. Per. Med. 2017, 14, 299–307. [Google Scholar] [CrossRef]
- Liang, J.; Lan, J.; Li, M.; Wang, F. Associations of Leptin Receptor and Peroxisome Proliferator-Activated Receptor Gamma Polymorphisms with Polycystic Ovary Syndrome: A Meta-Analysis. Ann. Nutr. Metab. 2019, 75, 1–8. [Google Scholar] [CrossRef]
- Dallel, M.; Douma, Z.; Finan, R.R.; Hachani, F.; Letaifa, D.B.; Mahjoub, T.; Almawi, W.Y. Contrasting association of Leptin receptor polymorphisms and haplotypes with polycystic ovary syndrome in Bahraini and Tunisian women: A case-control study. Biosci. Rep. 2021, 41. [Google Scholar] [CrossRef]
- Estienne, A.; Mellouk, N.; Bongrani, A.; Plotton, I.; Langer, I.; Ramé, C.; Petit, C.; Guérif, F.; Froment, P.; Dupont, J. Involvement of chemerin and CMKLR1 in the progesterone decrease by PCOS granulosa cells. Reproduction 2021, 162, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.K.; Chen, J.; Farhatullah, S.; Adya, R.; Kaur, J.; Heutling, D.; Lewandowski, K.C.; O’Hare, J.P.; Lehnert, H.; Randeva, H.S. Insulin and metformin regulate circulating and adipose tissue chemerin. Diabetes 2009, 58, 1971–1977. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhu, Q.; Wang, W.; Qi, J.; He, Y.; Wang, Y.; Lu, Y.; Wu, H.; Ding, Y.; Sun, Y. Elevated chemerin induces insulin resistance in human granulosa-lutein cells from polycystic ovary syndrome patients. FASEB J. 2019, 33, 11303–11313. [Google Scholar] [CrossRef] [PubMed]
- Lima, P.D.A.; Nivet, A.-L.; Wang, Q.; Chen, Y.-A.; Leader, A.; Cheung, A.; Tzeng, C.-R.; Tsang, B.K. Polycystic ovary syndrome: Possible involvement of androgen-induced, chemerin-mediated ovarian recruitment of monocytes/macrophages. Biol. Reprod. 2018, 99, 838–852. [Google Scholar] [CrossRef] [PubMed]
- Kumariya, S.; Ubba, V.; Jha, R.K.; Gayen, J.R. Autophagy in ovary and polycystic ovary syndrome: Role, dispute and future perspective. Autophagy 2021, 17, 2706–2733. [Google Scholar] [CrossRef] [PubMed]
- Da Li; You, Y.; Bi, F.-F.; Zhang, T.-N.; Jiao, J.; Wang, T.-R.; Zhou, Y.-M.; Shen, Z.-Q.; Wang, X.-X.; Yang, Q. Autophagy is activated in the ovarian tissue of polycystic ovary syndrome. Reproduction 2018, 155, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Gong, Y.; Cai, L.; Zhang, L.; Dong, X. Chemerin regulates autophagy to participate in polycystic ovary syndrome. J. Int. Med. Res. 2021, 49. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, Q.; Zhang, L.; Wei, W.; Liu, L.; Li, B.; Zhang, L.; Zhang, Y.; Hui, Y.; Lei, Y. Circulating chemerin levels in women with polycystic ovary syndrome: A meta-analysis. Gynecol. Endocrinol. 2022, 38, 22–27. [Google Scholar] [CrossRef]
- Lin, K.; Sun, X.; Wang, X.; Wang, H.; Chen, X. Circulating Adipokine Levels in Nonobese Women with Polycystic Ovary Syndrome and in Nonobese Control Women: A Systematic Review and Meta-Analysis. Front. Endocrinol. 2020, 11, 537809. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, R.; Li, X.; Zhu, Q.; Liao, Y.; Tao, T.; Kang, X.; Liu, W.; Li, S.; Sun, Y. High concentration of chemerin caused by ovarian hyperandrogenism may lead to poor IVF outcome in polycystic ovary syndrome: A pilot study. Gynecol. Endocrinol. 2019, 35, 1072–1077. [Google Scholar] [CrossRef]
- Bongrani, A.; Plotton, I.; Mellouk, N.; Ramé, C.; Guerif, F.; Froment, P.; Dupont, J. High androgen concentrations in follicular fluid of polycystic ovary syndrome women. Reprod. Biol. Endocrinol. 2022, 20, 88. [Google Scholar] [CrossRef]
- Bozaoglu, K.; Bolton, K.; McMillan, J.; Zimmet, P.; Jowett, J.; Collier, G.; Walder, K.; Segal, D. Chemerin is a novel adipokine associated with obesity and metabolic syndrome. Endocrinology 2007, 148, 4687–4694. [Google Scholar] [CrossRef]
- Wang, C.; Mao, X.; Wang, L.; Liu, M.; Wetzel, M.D.; Guan, K.-L.; Dong, L.Q.; Liu, F. Adiponectin sensitizes insulin signaling by reducing p70 S6 kinase-mediated serine phosphorylation of IRS-1. J. Biol. Chem. 2007, 282, 7991–7996. [Google Scholar] [CrossRef]
- Khoramipour, K.; Chamari, K.; Hekmatikar, A.A.; Ziyaiyan, A.; Taherkhani, S.; Elguindy, N.M.; Bragazzi, N.L. Adiponectin: Structure, Physiological Functions, Role in Diseases, and Effects of Nutrition. Nutrients 2021, 13, 1180. [Google Scholar] [CrossRef]
- Van Stijn, C.M.W.; Kim, J.; Lusis, A.J.; Barish, G.D.; Tangirala, R.K. Macrophage polarization phenotype regulates adiponectin receptor expression and adiponectin anti-inflammatory response. FASEB J. 2015, 29, 636–649. [Google Scholar] [CrossRef]
- Williams, K.J.; Wu, X. Imbalanced insulin action in chronic over nutrition: Clinical harm, molecular mechanisms, and a way forward. Atherosclerosis 2016, 247, 225–282. [Google Scholar] [CrossRef]
- Højlund, K. Metabolism and insulin signaling in common metabolic disorders and inherited insulin resistance. Dan. Med. J. 2014, 61, B4890. [Google Scholar]
- Straub, L.G.; Scherer, P.E. Metabolic Messengers: Adiponectin. Nat. Metab. 2019, 1, 334–339. [Google Scholar] [CrossRef]
- Comim, F.V.; Hardy, K.; Franks, S. Adiponectin and its receptors in the ovary: Further evidence for a link between obesity and hyperandrogenism in polycystic ovary syndrome. PLoS ONE 2013, 8, e80416. [Google Scholar] [CrossRef]
- Barbe, A.; Bongrani, A.; Mellouk, N.; Estienne, A.; Kurowska, P.; Grandhaye, J.; Elfassy, Y.; Levy, R.; Rak, A.; Froment, P.; et al. Mechanisms of Adiponectin Action in Fertility: An Overview from Gametogenesis to Gestation in Humans and Animal Models in Normal and Pathological Conditions. Int. J. Mol. Sci. 2019, 20, 1526. [Google Scholar] [CrossRef]
- Lagaly, D.V.; Aad, P.Y.; Grado-Ahuir, J.A.; Hulsey, L.B.; Spicer, L.J. Role of adiponectin in regulating ovarian theca and granulosa cell function. Mol. Cell. Endocrinol. 2008, 284, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Artimani, T.; Saidijam, M.; Aflatoonian, R.; Ashrafi, M.; Amiri, I.; Yavangi, M.; SoleimaniAsl, S.; Shabab, N.; Karimi, J.; Mehdizadeh, M. Downregulation of adiponectin system in granulosa cells and low levels of HMW adiponectin in PCOS. J. Assist. Reprod. Genet. 2016, 33, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Deepa, S.S.; Dong, L.Q. APPL1: Role in adiponectin signaling and beyond. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E22–E36. [Google Scholar] [CrossRef] [PubMed]
- Dehghan, R.; Saidijam, M.; Mehdizadeh, M.; Shabab, N.; Yavangi, M.; Artimani, T. Evidence for decreased expression of APPL1 associated with reduced insulin and adiponectin receptors expression in PCOS patients. J. Endocrinol. Investig. 2016, 39, 1075–1082. [Google Scholar] [CrossRef]
- Li, S.; Huang, X.; Zhong, H.; Peng, Q.; Chen, S.; Xie, Y.; Qin, X.; Qin, A. Low circulating adiponectin levels in women with polycystic ovary syndrome: An updated meta-analysis. Tumour Biol. 2014, 35, 3961–3973. [Google Scholar] [CrossRef]
- Mirza, S.S.; Shafique, K.; Shaikh, A.R.; Khan, N.A.; Anwar Qureshi, M. Association between circulating adiponectin levels and polycystic ovarian syndrome. J. Ovarian Res. 2014, 7, 18. [Google Scholar] [CrossRef]
- Arroyo-Jousse, V.; Jaramillo, A.; Castaño-Moreno, E.; Lépez, M.; Carrasco-Negüe, K.; Casanello, P. Adipokines underlie the early origins of obesity and associated metabolic comorbidities in the offspring of women with pregestational obesity. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165558. [Google Scholar] [CrossRef]
- Pekcan, M.K.; Tokmak, A.; Akkaya, H.; Pekcan, G.; Onur, A.; Kısa, Ü.; Çil, A.P. Assessment of the Relationship Between Serum High Molecular Weight Adiponectin Hormone Levels and Insulin Resistance in Patients with Polycystic Ovary Syndrome. Horm. Metab. Res. 2019, 51, 261–266. [Google Scholar] [CrossRef]
- Shorakae, S.; Ranasinha, S.; Abell, S.; Lambert, G.; Lambert, E.; de Courten, B.; Teede, H. Inter-related effects of insulin resistance, hyperandrogenism, sympathetic dysfunction and chronic inflammation in PCOS. Clin. Endocrinol. 2018, 89, 628–633. [Google Scholar] [CrossRef]
- Al-Awadi, A.M.; Sarray, S.; Arekat, M.R.; Saleh, L.R.; Mahmood, N.; Almawi, W.Y. The high-molecular weight multimer form of adiponectin is a useful marker of polycystic ovary syndrome in Bahraini Arab women. Clin. Nutr. ESPEN 2016, 13, e33–e38. [Google Scholar] [CrossRef]
- Duan, X.; Zhou, M.; Zhou, G.; Zhu, Q.; Li, W. Effect of metformin on adiponectin in PCOS: A meta-analysis and a systematic review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 267, 61–67. [Google Scholar] [CrossRef]
- Yang, K.; Zeng, L.; Bao, T.; Ge, J. Effectiveness of Omega-3 fatty acid for polycystic ovary syndrome: A systematic review and meta-analysis. Reprod. Biol. Endocrinol. 2018, 16, 27. [Google Scholar] [CrossRef]
- Tosatti, J.A.G.; Alves, M.T.; Cândido, A.L.; Reis, F.M.; Araújo, V.E.; Gomes, K.B. Influence of n-3 fatty acid supplementation on inflammatory and oxidative stress markers in patients with polycystic ovary syndrome: A systematic review and meta-analysis. Br. J. Nutr. 2021, 125, 657–668. [Google Scholar] [CrossRef]
- Alfaqih, M.A.; Khader, Y.S.; Al-Dwairi, A.N.; Alzoubi, A.; Al-Shboul, O.; Hatim, A. Correction: Lower Levels of Serum Adiponectin and the T Allele of rs1501299 of the ADIPOQ Gene Are Protective against Polycystic Ovarian Syndrome in Jordan. Korean J. Fam. Med. 2018, 39, 207. [Google Scholar] [CrossRef]
- Sun, X.; Wu, X.; Duan, Y.; Liu, G.; Yu, X.; Zhang, W. Family-Based Association Study of rs17300539 and rs12495941 Polymorphism in Adiponectin Gene and Polycystic Ovary Syndrome in a Chinese Population. Med. Sci. Monit. 2017, 23, 78–84. [Google Scholar] [CrossRef][Green Version]
- Liu, Z.; Wang, Z.; Hao, C.; Tian, Y.; Fu, J. Effects of ADIPOQ polymorphisms on PCOS risk: A meta-analysis. Reprod. Biol. Endocrinol. 2018, 16, 120. [Google Scholar] [CrossRef]
- Nowak, I.; Ciećwież, S.; Łój, B.; Brodowski, J.; Brodowska, A. Adiponectin Gene Polymorphism (rs17300539) Has No Influence on the Occurrence of Metabolic Syndrome in Women with Polycystic Ovary Syndrome. Genes 2021, 12, 1902. [Google Scholar] [CrossRef]
- Choi, J.-H.; Rhee, E.-J.; Kim, K.-H.; Woo, H.-Y.; Lee, W.-Y.; Sung, K.-C. Plasma omentin-1 levels are reduced in non-obese women with normal glucose tolerance and polycystic ovary syndrome. Eur. J. Endocrinol. 2011, 165, 789–796. [Google Scholar] [CrossRef][Green Version]
- Yang, H.-Y.; Ma, Y.; Lu, X.-H.; Liang, X.-H.; Suo, Y.-J.; Huang, Z.-X.; Lu, D.-C.; Qin, Y.-F.; Luo, Z.-J. The correlation of plasma omentin-1 with insulin resistance in non-obese polycystic ovary syndrome. Ann. Endocrinol. 2015, 76, 620–627. [Google Scholar] [CrossRef]
- Tan, B.K.; Adya, R.; Farhatullah, S.; Lewandowski, K.C.; O’Hare, P.; Lehnert, H.; Randeva, H.S. Omentin-1, a novel adipokine, is decreased in overweight insulin-resistant women with polycystic ovary syndrome: Ex vivo and in vivo regulation of omentin-1 by insulin and glucose. Diabetes 2008, 57, 801–808. [Google Scholar] [CrossRef]
- Franik, G.; Sadlocha, M.; Madej, P.; Owczarek, A.; Skrzypulec-Plinta, V.; Plinta, R.; Chudek, J.; Olszanecka-Glinianowicz, M. Circulating omentin-1 levels and inflammation in polycystic ovary syndrome. Ginekol. Pol. 2020, 91, 308–312. [Google Scholar] [CrossRef] [PubMed]
- Scheller, J.; Chalaris, A.; Schmidt-Arras, D.; Rose-John, S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim. Biophys. Acta 2011, 1813, 878–888. [Google Scholar] [CrossRef] [PubMed]
- Feng, R.; Li, Y.; Wang, C.; Luo, C.; Liu, L.; Chuo, F.; Li, Q.; Sun, C. Higher vaspin levels in subjects with obesity and type 2 diabetes mellitus: A meta-analysis. Diabetes Res. Clin. Pract. 2014, 106, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Hida, K.; Wada, J.; Eguchi, J.; Zhang, H.; Baba, M.; Seida, A.; Hashimoto, I.; Okada, T.; Yasuhara, A.; Nakatsuka, A.; et al. Visceral adipose tissue-derived serine protease inhibitor: A unique insulin-sensitizing adipocytokine in obesity. Proc. Natl. Acad. Sci. USA 2005, 102, 10610–10615. [Google Scholar] [CrossRef]
- Tan, B.K.; Heutling, D.; Chen, J.; Farhatullah, S.; Adya, R.; Keay, S.D.; Kennedy, C.R.; Lehnert, H.; Randeva, H.S. Metformin decreases the adipokine vaspin in overweight women with polycystic ovary syndrome concomitant with improvement in insulin sensitivity and a decrease in insulin resistance. Diabetes 2008, 57, 1501–1507. [Google Scholar] [CrossRef]
- Bongrani, A.; Mellouk, N.; Ramé, C.; Cornuau, M.; Guerif, F.; Froment, P.; Dupont, J. Vaspin, a novel adipokine in woman granulosa cells physiology and PCOS pathogenesis? J. Endocrinol. 2021, 249, 57–70. [Google Scholar] [CrossRef]
- Breitfeld, J.; Tönjes, A.; Böttcher, Y.; Schleinitz, D.; Wiele, N.; Marzi, C.; Brockhaus, C.; Rathmann, W.; Huth, C.; Grallert, H.; et al. Genetic variation in the vaspin gene affects circulating serum vaspin concentrations. Int. J. Obes. 2013, 37, 861–866. [Google Scholar] [CrossRef]
- Tang, Y.; Huang, T.; Pan, Y. Correlation Analysis of Vaspin Gene Polymorphisms and Polycystic Ovary Syndrome Based on Intelligent Medicine. Comput. Intell. Neurosci. 2022, 2022, 6154233. [Google Scholar] [CrossRef]
- Kohan, L.; Zarei, A.; Fallahi, S.; Tabiee, O. Association between vaspin rs2236242 gene polymorphism and polycystic ovary syndrome risk. Gene 2014, 539, 209–212. [Google Scholar] [CrossRef]
- Estienne, A.; Bongrani, A.; Reverchon, M.; Ramé, C.; Ducluzeau, P.-H.; Froment, P.; Dupont, J. Involvement of Novel Adipokines, Chemerin, Visfatin, Resistin and Apelin in Reproductive Functions in Normal and Pathological Conditions in Humans and Animal Models. Int. J. Mol. Sci. 2019, 20, 4431. [Google Scholar] [CrossRef]
- Pope, G.R.; Roberts, E.M.; Lolait, S.J.; O’Carroll, A.-M. Central and peripheral apelin receptor distribution in the mouse: Species differences with rat. Peptides 2012, 33, 139–148. [Google Scholar] [CrossRef]
- Shuang, L.; Jidong, W.; Hongjuan, P.; Zhenwei, Y. Effects of apelin on proliferation and apoptosis in rat ovarian granulosa cells. Clin. Exp. Obstet. Gynecol. 2016, 43, 409–413. [Google Scholar] [CrossRef]
- Rak, A.; Drwal, E.; Rame, C.; Knapczyk-Stwora, K.; Słomczyńska, M.; Dupont, J.; Gregoraszczuk, E.L. Expression of apelin and apelin receptor (APJ) in porcine ovarian follicles and in vitro effect of apelin on steroidogenesis and proliferation through APJ activation and different signaling pathways. Theriogenology 2017, 96, 126–135. [Google Scholar] [CrossRef]
- Schilffarth, S.; Antoni, B.; Schams, D.; Meyer, H.H.D.; Berisha, B. The expression of apelin and its receptor APJ during different physiological stages in the bovine ovary. Int. J. Biol. Sci. 2009, 5, 344–350. [Google Scholar] [CrossRef]
- Roche, J.; Ramé, C.; Reverchon, M.; Mellouk, N.; Cornuau, M.; Guerif, F.; Froment, P.; Dupont, J. Apelin (APLN) and Apelin Receptor (APLNR) in Human Ovary: Expression, Signaling, and Regulation of Steroidogenesis in Primary Human Luteinized Granulosa Cells. Biol. Reprod. 2016, 95, 104. [Google Scholar] [CrossRef]
- Abramovich, D.; Irusta, G.; Bas, D.; Cataldi, N.I.; Parborell, F.; Tesone, M. Angiopoietins/TIE2 system and VEGF are involved in ovarian function in a DHEA rat model of polycystic ovary syndrome. Endocrinology 2012, 153, 3446–3456. [Google Scholar] [CrossRef]
- Basini, G.; Bussolati, S.; Santini, S.E.; Bianchi, F.; Careri, M.; Mangia, A.; Musci, M.; Grasselli, F. Antiangiogenesis in swine ovarian follicle: A potential role for 2-methoxyestradiol. Steroids 2007, 72, 660–665. [Google Scholar] [CrossRef]
- Chang, C.-Y.; Tsai, Y.-C.; Lee, C.-H.; Chan, T.-F.; Wang, S.-H.; Su, J.-H. Lower serum apelin levels in women with polycystic ovary syndrome. Fertil. Steril. 2011, 95, 2520–2523.e2. [Google Scholar] [CrossRef]
- Ueno, N.; Ling, N.; Ying, S.Y.; Esch, F.; Shimasaki, S.; Guillemin, R. Isolation and partial characterization of follistatin: A single-chain Mr 35,000 monomeric protein that inhibits the release of follicle-stimulating hormone. Proc. Natl. Acad. Sci. USA 1987, 84, 8282–8286. [Google Scholar] [CrossRef]
- Welt, C.; Sidis, Y.; Keutmann, H.; Schneyer, A. Activins, inhibins, and follistatins: From endocrinology to signaling. A paradigm for the new millennium. Exp. Biol. Med. 2002, 227, 724–752. [Google Scholar] [CrossRef]
- Singh, R.; Braga, M.; Pervin, S. Regulation of brown adipocyte metabolism by myostatin/follistatin signaling. Front. Cell Dev. Biol. 2014, 2, 60. [Google Scholar] [CrossRef]
- Ye, R.; Yan, C.; Zhou, H.; Huang, Y.; Dong, M.; Zhang, H.; Jiang, X.; Yuan, S.; Chen, L.; Jiang, R.; et al. Brown Adipose Tissue Activation by Cold Treatment Ameliorates Polycystic Ovary Syndrome in Rat. Front. Endocrinol. 2021, 12, 744628. [Google Scholar] [CrossRef]
- Zhang, X.-H.; Chen, Y.; Li, B.; Liu, J.-Y.; Yang, C.-M.; Ma, M.-Z. Blocking follistatin-like 1 attenuates liver fibrosis in mice by regulating transforming growth factor-beta signaling. Int. J. Clin. Exp. Pathol. 2018, 11, 1112–1122. [Google Scholar]
- Fang, D.; Shi, X.; Lu, T.; Ruan, H.; Gao, Y. The glycoprotein follistatin-like 1 promotes brown adipose thermogenesis. Metabolism 2019, 98, 16–26. [Google Scholar] [CrossRef]
- Cheng, J.-X.; Yu, K. New Discovered Adipokines Associated with the Pathogenesis of Obesity and Type 2 Diabetes. Diabetes Metab. Syndr. Obes. 2022, 15, 2381–2389. [Google Scholar] [CrossRef]
- Reverchon, M.; Cornuau, M.; Cloix, L.; Ramé, C.; Guerif, F.; Royère, D.; Dupont, J. Visfatin is expressed in human granulosa cells: Regulation by metformin through AMPK/SIRT1 pathways and its role in steroidogenesis. Mol. Hum. Reprod. 2013, 19, 313–326. [Google Scholar] [CrossRef]
- Shen, C.-J.; Tsai, E.-M.; Lee, J.-N.; Chen, Y.-L.; Lee, C.-H.; Chan, T.-F. The concentrations of visfatin in the follicular fluids of women undergoing controlled ovarian stimulation are correlated to the number of oocytes retrieved. Fertil. Steril. 2010, 93, 1844–1850. [Google Scholar] [CrossRef]
- Reverchon, M.; Rame, C.; Bunel, A.; Chen, W.; Froment, P.; Dupont, J. VISFATIN (NAMPT) Improves In Vitro IGF1-Induced Steroidogenesis and IGF1 Receptor Signaling Through SIRT1 in Bovine Granulosa Cells. Biol. Reprod. 2016, 94, 54. [Google Scholar] [CrossRef]
- Saddick, S.Y. Identifying genes associated with the development of human polycystic ovary syndrome. Saudi J. Biol. Sci. 2020, 27, 1271–1279. [Google Scholar] [CrossRef]
- Annie, L.; Gurusubramanian, G.; Roy, V.K. Inhibition of visfatin by FK866 mitigates pathogenesis of cystic ovary in letrozole-induced hyperandrogenised mice. Life Sci. 2021, 276, 119409. [Google Scholar] [CrossRef]
- Ali, A.I.; Nori, W. Correlation of Serum Visfatin Level in Non-obese Women with Polycystic Ovary Syndrome and Matched Control. Reprod. Sci. 2022. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Maksoud, R.S.; Zidan, H.E.; Saleh, H.S.; Amer, S.A. Visfatin and SREBP-1c mRNA Expressions and Serum Levels among Egyptian Women with Polycystic Ovary Syndrome. Genet. Test. Mol. Biomark. 2020, 24, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Bargut, T.C.L.; Aguila, M.B.; Mandarim-de-Lacerda, C.A. Brown adipose tissue: Updates in cellular and molecular biology. Tissue Cell 2016, 48, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Villarroya, F.; Cereijo, R.; Villarroya, J.; Giralt, M. Brown adipose tissue as a secretory organ. Nat. Rev. Endocrinol. 2017, 13, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Stanford, K.I.; Middelbeek, R.J.W.; Townsend, K.L.; An, D.; Nygaard, E.B.; Hitchcox, K.M.; Markan, K.R.; Nakano, K.; Hirshman, M.F.; Tseng, Y.-H.; et al. Brown adipose tissue regulates glucose homeostasis and insulin sensitivity. J. Clin. Investig. 2013, 123, 215–223. [Google Scholar] [CrossRef]
- Lee, P.; Linderman, J.D.; Smith, S.; Brychta, R.J.; Wang, J.; Idelson, C.; Perron, R.M.; Werner, C.D.; Phan, G.Q.; Kammula, U.S.; et al. Irisin and FGF21 are cold-induced endocrine activators of brown fat function in humans. Cell Metab. 2014, 19, 302–309. [Google Scholar] [CrossRef]
- Oliveira, F.R.; Mamede, M.; Bizzi, M.F.; Rocha, A.L.L.; Ferreira, C.N.; Gomes, K.B.; Cândido, A.L.; Reis, F.M. Brown adipose tissue activity is reduced in women with polycystic ovary syndrome. Eur. J. Endocrinol. 2019, 181, 473–480. [Google Scholar] [CrossRef]
- Barber, T.M.; Dimitriadis, G.K.; Andreou, A.; Franks, S. Polycystic ovary syndrome: Insight into pathogenesis and a common association with insulin resistance. Clin. Med. 2016, 16, 262–266. [Google Scholar] [CrossRef]
- Carey, A.L.; Kingwell, B.A. Brown adipose tissue in humans: Therapeutic potential to combat obesity. Pharmacol. Ther. 2013, 140, 26–33. [Google Scholar] [CrossRef]
- Harms, M.; Seale, P. Brown and beige fat: Development, function and therapeutic potential. Nat. Med. 2013, 19, 1252–1263. [Google Scholar] [CrossRef]
- Yuan, X.; Hu, T.; Zhao, H.; Huang, Y.; Ye, R.; Lin, J.; Zhang, C.; Zhang, H.; Wei, G.; Zhou, H.; et al. Brown adipose tissue transplantation ameliorates polycystic ovary syndrome. Proc. Natl. Acad. Sci. USA 2016, 113, 2708–2713. [Google Scholar] [CrossRef]
- Hu, T.; Yuan, X.; Ye, R.; Zhou, H.; Lin, J.; Zhang, C.; Zhang, H.; Wei, G.; Dong, M.; Huang, Y.; et al. Brown adipose tissue activation by rutin ameliorates polycystic ovary syndrome in rat. J. Nutr. Biochem. 2017, 47, 21–28. [Google Scholar] [CrossRef]
- Hui, X.; Gu, P.; Zhang, J.; Nie, T.; Pan, Y.; Wu, D.; Feng, T.; Zhong, C.; Wang, Y.; Lam, K.S.L.; et al. Adiponectin Enhances Cold-Induced Browning of Subcutaneous Adipose Tissue via Promoting M2 Macrophage Proliferation. Cell Metab. 2015, 22, 279–290. [Google Scholar] [CrossRef]
- Liu, X.; Wang, S.; You, Y.; Meng, M.; Zheng, Z.; Dong, M.; Lin, J.; Zhao, Q.; Zhang, C.; Yuan, X.; et al. Brown Adipose Tissue Transplantation Reverses Obesity in Ob/Ob Mice. Endocrinology 2015, 156, 2461–2469. [Google Scholar] [CrossRef]
- Szczepańska, E.; Gietka-Czernel, M. FGF21: A Novel Regulator of Glucose and Lipid Metabolism and Whole-Body Energy Balance. Horm. Metab. Res. 2022, 54, 203–211. [Google Scholar] [CrossRef]
- Bednarska, S.; Fryczak, J.; Siejka, A. Serum β-Klotho concentrations are increased in women with polycystic ovary syndrome. Cytokine 2020, 134, 155188. [Google Scholar] [CrossRef]
- Gorar, S.; Culha, C.; Uc, Z.A.; Dellal, F.D.; Serter, R.; Aral, S.; Aral, Y. Serum fibroblast growth factor 21 levels in polycystic ovary syndrome. Gynecol. Endocrinol. 2010, 26, 819–826. [Google Scholar] [CrossRef]
- Olszanecka-Glinianowicz, M.; Madej, P.; Wdowczyk, M.; Owczarek, A.; Chudek, J. Circulating FGF21 levels are related to nutritional status and metabolic but not hormonal disturbances in polycystic ovary syndrome. Eur. J. Endocrinol. 2015, 172, 173–179. [Google Scholar] [CrossRef]
- Kapoor, R.; Stratopoulou, C.A.; Dolmans, M.-M. Pathogenesis of Endometriosis: New Insights into Prospective Therapies. Int. J. Mol. Sci. 2021, 22, 1700. [Google Scholar] [CrossRef]
- Sampson, J.A. Metastatic or Embolic Endometriosis, due to the Menstrual Dissemination of Endometrial Tissue into the Venous Circulation. Am. J. Pathol. 1927, 3, 93–110.43. [Google Scholar]
- Giudice, L.C.; Kao, L.C. Endometriosis. Lancet 2004, 364, 1789–1799. [Google Scholar] [CrossRef]
- Taylor, H.S.; Kotlyar, A.M.; Flores, V.A. Endometriosis is a chronic systemic disease: Clinical challenges and novel innovations. Lancet 2021, 397, 839–852. [Google Scholar] [CrossRef]
- Nisolle, M.; Donnez, J. Peritoneal endometriosis, ovarian endometriosis, and adenomyotic nodules of the rectovaginal septum are three different entities. Fertil. Steril. 1997, 68, 585–596. [Google Scholar] [CrossRef]
- Mohammed Rasheed, H.A.; Hamid, P. Inflammation to Infertility: Panoramic View on Endometriosis. Cureus 2020, 12, e11516. [Google Scholar] [CrossRef]
- Giacomini, E.; Minetto, S.; Li Piani, L.; Pagliardini, L.; Somigliana, E.; Viganò, P. Genetics and Inflammation in Endometriosis: Improving Knowledge for Development of New Pharmacological Strategies. Int. J. Mol. Sci. 2021, 22, 9033. [Google Scholar] [CrossRef] [PubMed]
- García-Gómez, E.; Vázquez-Martínez, E.R.; Reyes-Mayoral, C.; Cruz-Orozco, O.P.; Camacho-Arroyo, I.; Cerbón, M. Regulation of Inflammation Pathways and Inflammasome by Sex Steroid Hormones in Endometriosis. Front. Endocrinol. 2019, 10, 935. [Google Scholar] [CrossRef]
- Donnez, J.; Dolmans, M.-M. Endometriosis and Medical Therapy: From Progestogens to Progesterone Resistance to GnRH Antagonists: A Review. J. Clin. Med. 2021, 10, 1085. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, B.D.; Bulun, S.E. Endometriosis and nuclear receptors. Hum. Reprod. Update 2019, 25, 473–485. [Google Scholar] [CrossRef]
- Pantelis, A.; Machairiotis, N.; Lapatsanis, D.P. The Formidable yet Unresolved Interplay between Endometriosis and Obesity. Sci. World J. 2021, 2021, 6653677. [Google Scholar] [CrossRef]
- Backonja, U.; Buck Louis, G.M.; Lauver, D.R. Overall Adiposity, Adipose Tissue Distribution, and Endometriosis: A Systematic Review. Nurs. Res. 2016, 65, 151–166. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, S.S.; Ferreira, T.; Benonisdottir, S.; Rahmioglu, N.; Becker, C.M.; Granne, I.; Zondervan, K.T.; Holmes, M.V.; Lindgren, C.M.; Wittemans, L.B.L. Obesity and risk of female reproductive conditions: A Mendelian randomisation study. PLoS Med. 2022, 19, e1003679. [Google Scholar] [CrossRef]
- Zolbin, M.M.; Mamillapalli, R.; Nematian, S.E.; Goetz, T.G.; Taylor, H.S. Adipocyte alterations in endometriosis: Reduced numbers of stem cells and microRNA induced alterations in adipocyte metabolic gene expression. Reprod. Biol. Endocrinol. 2019, 17, 36. [Google Scholar] [CrossRef]
- Meligy, F.Y.; Elgamal, D.A.; Abdelzaher, L.A.; Khashbah, M.Y.; El-Mokhtar, M.A.; Sayed, A.A.; Refaiy, A.M.; Othman, E.R. Adipose tissue-derived mesenchymal stem cells reduce endometriosis cellular proliferation through their anti-inflammatory effects. Clin. Exp. Reprod. Med. 2021, 48, 322–336. [Google Scholar] [CrossRef]
- Hong, J.; Yi, K.W. What is the link between endometriosis and adiposity? Obstet. Gynecol. Sci. 2022, 65, 227–233. [Google Scholar] [CrossRef]
- Matarese, G.; Alviggi, C.; Sanna, V.; Howard, J.K.; Lord, G.M.; Carravetta, C.; Fontana, S.; Lechler, R.I.; Bloom, S.R.; de Placido, G. Increased leptin levels in serum and peritoneal fluid of patients with pelvic endometriosis. J. Clin. Endocrinol. Metab. 2000, 85, 2483–2487. [Google Scholar] [CrossRef]
- Shah, D.K.; Correia, K.F.; Harris, H.R.; Missmer, S.A. Plasma adipokines and endometriosis risk: A prospective nested case-control investigation from the Nurses’ Health Study II. Hum. Reprod. 2013, 28, 315–321. [Google Scholar] [CrossRef]
- Kalaitzopoulos, D.R.; Lempesis, I.G.; Samartzis, N.; Kolovos, G.; Dedes, I.; Daniilidis, A.; Nirgianakis, K.; Leeners, B.; Goulis, D.G.; Samartzis, E.P. Leptin concentrations in endometriosis: A systematic review and meta-analysis. J. Reprod. Immunol. 2021, 146, 103338. [Google Scholar] [CrossRef]
- Tian, Z.; Wang, Y.; Zhao, Y.; Chang, X.-H.; Zhu, H.-L. Serum and peritoneal fluid leptin levels in endometriosis: A systematic review and meta-analysis. Gynecol. Endocrinol. 2021, 37, 689–693. [Google Scholar] [CrossRef]
- Casabiell, X.; Piñeiro, V.; Peino, R.; Lage, M.; Camiña, J.; Gallego, R.; Vallejo, L.G.; Dieguez, C.; Casanueva, F.F. Gender differences in both spontaneous and stimulated leptin secretion by human omental adipose tissue in vitro: Dexamethasone and estradiol stimulate leptin release in women, but not in men. J. Clin. Endocrinol. Metab. 1998, 83, 2149–2155. [Google Scholar] [CrossRef][Green Version]
- Choi, Y.S.; Oh, H.K.; Choi, J.-H. Expression of adiponectin, leptin, and their receptors in ovarian endometrioma. Fertil. Steril. 2013, 100, 135–141.e2. [Google Scholar] [CrossRef]
- Lima-Couy, I.; Cervero, A.; Bonilla-Musoles, F.; Pellicer, A.; Simón, C. Endometrial leptin and leptin receptor expression in women with severe/moderate endometriosis. Mol. Hum. Reprod. 2004, 10, 777–782. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.-H.; Chuang, P.-C.; Chen, H.-M.; Lin, C.-C.; Tsai, S.-J. Increased leptin expression in endometriosis cells is associated with endometrial stromal cell proliferation and leptin gene up-regulation. Mol. Hum. Reprod. 2002, 8, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.-H.; Choi, Y.S.; Choi, J.-H. Leptin promotes human endometriotic cell migration and invasion by up-regulating MMP-2 through the JAK2/STAT3 signaling pathway. Mol. Hum. Reprod. 2015, 21, 792–802. [Google Scholar] [CrossRef] [PubMed]
- Manjunathan, R.; Devarajan, N.; Ragunathan, M. Possible Mechanism of Human Recombinant Leptin-Induced VEGF A Synthesis via PI3K/Akt/mTOR/S6 Kinase Signaling Pathway while Inducing Angiogenesis: An Analysis Using Chicken Chorioallantoic Membrane Model. J. Vasc. Res. 2021, 58, 343–360. [Google Scholar] [CrossRef]
- Styer, A.K.; Sullivan, B.T.; Puder, M.; Arsenault, D.; Petrozza, J.C.; Serikawa, T.; Chang, S.; Hasan, T.; Gonzalez, R.R.; Rueda, B.R. Ablation of leptin signaling disrupts the establishment, development, and maintenance of endometriosis-like lesions in a murine model. Endocrinology 2008, 149, 506–514. [Google Scholar] [CrossRef]
- Cheng, J.; Li, C.; Ying, Y.; Lv, J.; Qu, X.; McGowan, E.; Lin, Y.; Zhu, X. Metformin Alleviates Endometriosis and Potentiates Endometrial Receptivity via Decreasing VEGF and MMP9 and Increasing Leukemia Inhibitor Factor and HOXA10. Front. Pharmacol. 2022, 13, 750208. [Google Scholar] [CrossRef]
- Bohlouli, S.; Rabzia, A.; Sadeghi, E.; Chobsaz, F.; Khazaei, M. in vitro Anti-Proliferative Effect of Adiponectin on Human Endometriotic Stromal Cells through AdipoR1 and AdipoR2 Gene Receptor Expression. Iran. Biomed. J. 2016, 20, 12–17. [Google Scholar] [CrossRef]
- Bohlouli, S.; Khazaei, M.; Teshfam, M.; Hassanpour, H. Adiponectin effect on the viability of human endometrial stromal cells and mRNA expression of adiponectin receptors. Int. J. Fertil. Steril. 2013, 7, 43–48. [Google Scholar]
- Takemura, Y.; Osuga, Y.; Harada, M.; Hirata, T.; Koga, K.; Yoshino, O.; Hirota, Y.; Morimoto, C.; Yano, T.; Taketani, Y. Concentration of adiponectin in peritoneal fluid is decreased in women with endometriosis. Am. J. Reprod. Immunol. 2005, 54, 217–221. [Google Scholar] [CrossRef]
- Takemura, Y.; Osuga, Y.; Harada, M.; Hirata, T.; Koga, K.; Morimoto, C.; Hirota, Y.; Yoshino, O.; Yano, T.; Taketani, Y. Serum adiponectin concentrations are decreased in women with endometriosis. Hum. Reprod. 2005, 20, 3510–3513. [Google Scholar] [CrossRef]
- Ernst, M.C.; Issa, M.; Goralski, K.B.; Sinal, C.J. Chemerin exacerbates glucose intolerance in mouse models of obesity and diabetes. Endocrinology 2010, 151, 1998–2007. [Google Scholar] [CrossRef]
- Jin, C.H.; Yi, K.W.; Ha, Y.R.; Shin, J.-H.; Park, H.T.; Kim, T.; Hur, J.-Y. Chemerin Expression in the Peritoneal Fluid, Serum, and Ovarian Endometrioma of Women with Endometriosis. Am. J. Reprod. Immunol. 2015, 74, 379–386. [Google Scholar] [CrossRef]
- Kasai, K.; Kato, T.; Kadota, Y.; Erdenebayar, O.; Keyama, K.; Kawakita, T.; Yoshida, K.; Kuwahara, A.; Matsuzaki, T.; Irahara, M. Intraperitoneal administration of activin A promotes development of endometriotic lesions in a mouse model of endometriosis. J. Med. Invest. 2019, 66, 123–127. [Google Scholar] [CrossRef]
- Ferreira, M.C.; Witz, C.A.; Hammes, L.S.; Kirma, N.; Petraglia, F.; Schenken, R.S.; Reis, F.M. Activin A increases invasiveness of endometrial cells in an in vitro model of human peritoneum. Mol. Hum. Reprod. 2008, 14, 301–307. [Google Scholar] [CrossRef][Green Version]
- Rocha, A.L.L.; Carrarelli, P.; Novembri, R.; de Pascalis, F.; Luisi, S.; Reis, F.M.; Petraglia, F. Activin A stimulates interleukin 8 and vascular endothelial growth factor release from cultured human endometrial stromal cells: Possible implications for the pathogenesis of endometriosis. Reprod. Sci. 2012, 19, 832–838. [Google Scholar] [CrossRef]
- Akiyama, I.; Yoshino, O.; Osuga, Y.; Izumi, G.; Urata, Y.; Hirota, Y.; Hirata, T.; Harada, M.; Koga, K.; Ogawa, K.; et al. Follistatin is induced by IL-1β and TNF-α in stromal cells from endometrioma. Reprod. Sci. 2013, 20, 675–679. [Google Scholar] [CrossRef]
- Florio, P.; Reis, F.M.; Torres, P.B.; Calonaci, F.; Abrao, M.S.; Nascimento, L.L.; Franchini, M.; Cianferoni, L.; Petraglia, F. High serum follistatin levels in women with ovarian endometriosis. Hum. Reprod. 2009, 24, 2600–2606. [Google Scholar] [CrossRef]
- Peng, Y.; Peng, C.; Fang, Z.; Chen, G. Bioinformatics Analysis Identifies Molecular Markers Regulating Development and Progression of Endometriosis and Potential Therapeutic Drugs. Front. Genet. 2021, 12, 622683. [Google Scholar] [CrossRef]

| Adipokine | Serum Levels in Obese Individuals | Primary Functions |
|---|---|---|
| Leptin | + | Suppresses food uptake and regulates energy homeostasis, induces fibrosis |
| Adiponectin | − | Promoter of insulin sensitivity + hepatocyte fatty acid oxidation, reduces gluconeogensis + inflammation |
| Omentin-1 | − | Reduction in oxidative stress, inflammation + apoptosis |
| Chemerin | + | Leukocyte chemotattractant, involved in regulation of insulin response, blood pressure and adipogenesis |
| Vaspin | + | Anti-inflammatory promoter of glucose deposition and of insulin sensitivity |
| Apelin | + | Promoter of glucose tolerance, insulin sensitivity, fatty acid oxidation and of mitochondrial biogenesis |
| Visfatin | + | Activation of inflammatory pathways, promoter of vascular remodeling and fibrogenesis |
| Follistatin | = | Antagonist of members of the TGF-β superfamily, including activins, and protects from metabolic disease |
| Adipokine | Serum Levels in PCOS | Main Effects in PCOS |
|---|---|---|
| Leptin | + | Insulin sensitivity ↓, inflammation ↑, fibrosis ↑, GnRH secretion ↑, P4 synthesis ↑, apoptosis ↑ |
| Chemerin | + | Insulin sensitivity ↓, ovarian E2 and P4 synthesis ↓, inflammation ↑, PCOM ↑ |
| Adiponectin | − | Insulin sensitivity ↑, ovulation ↑, inflammation ↓, fibrosis ↓ |
| Omentin-1 | − | Insulin sensitivity ↑, inflammation ↓, IGF-1-induced steroidogenesis in GC ↑ |
| Vaspin | + | Insulin sensitivity ↑, inflammation ↓, GC viability and steroidogenesis ↑ |
| Apelin | = | Insulin sensitivity ↑, ovarian E2 and P4 synthesis ↑, follicular angiogenesis ↑ |
| Follistatin | + | Activin-triggered FSH release ↓, folliculogenesis ↓, fibrosis ↓ |
| Visfatin | + | Oocyte maturation ↑, androgen level ↓, ovarian E2 and P4 synthesis ↑, inflammation ↑ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schüler-Toprak, S.; Ortmann, O.; Buechler, C.; Treeck, O. The Complex Roles of Adipokines in Polycystic Ovary Syndrome and Endometriosis. Biomedicines 2022, 10, 2503. https://doi.org/10.3390/biomedicines10102503
Schüler-Toprak S, Ortmann O, Buechler C, Treeck O. The Complex Roles of Adipokines in Polycystic Ovary Syndrome and Endometriosis. Biomedicines. 2022; 10(10):2503. https://doi.org/10.3390/biomedicines10102503
Chicago/Turabian StyleSchüler-Toprak, Susanne, Olaf Ortmann, Christa Buechler, and Oliver Treeck. 2022. "The Complex Roles of Adipokines in Polycystic Ovary Syndrome and Endometriosis" Biomedicines 10, no. 10: 2503. https://doi.org/10.3390/biomedicines10102503
APA StyleSchüler-Toprak, S., Ortmann, O., Buechler, C., & Treeck, O. (2022). The Complex Roles of Adipokines in Polycystic Ovary Syndrome and Endometriosis. Biomedicines, 10(10), 2503. https://doi.org/10.3390/biomedicines10102503







