Model of Acute Liver Failure in an Isolated Perfused Porcine Liver—Challenges and Lessons Learned
Abstract
:1. Introduction
2. Materials and Methods
2.1. Liver Procurement
2.2. Normothermic Machine Perfusion (NMP)
2.3. Experimental Protocol
2.4. Perfusate Biochemistry
2.5. Histology
2.6. Chromogenic and Enzyme-Linked Immunosorbent Assays
2.7. In Vitro Testing of Acetaminophen Metabolites and CCl4
2.8. Statistical Analysis
3. Results
3.1. Perfusion Parameters
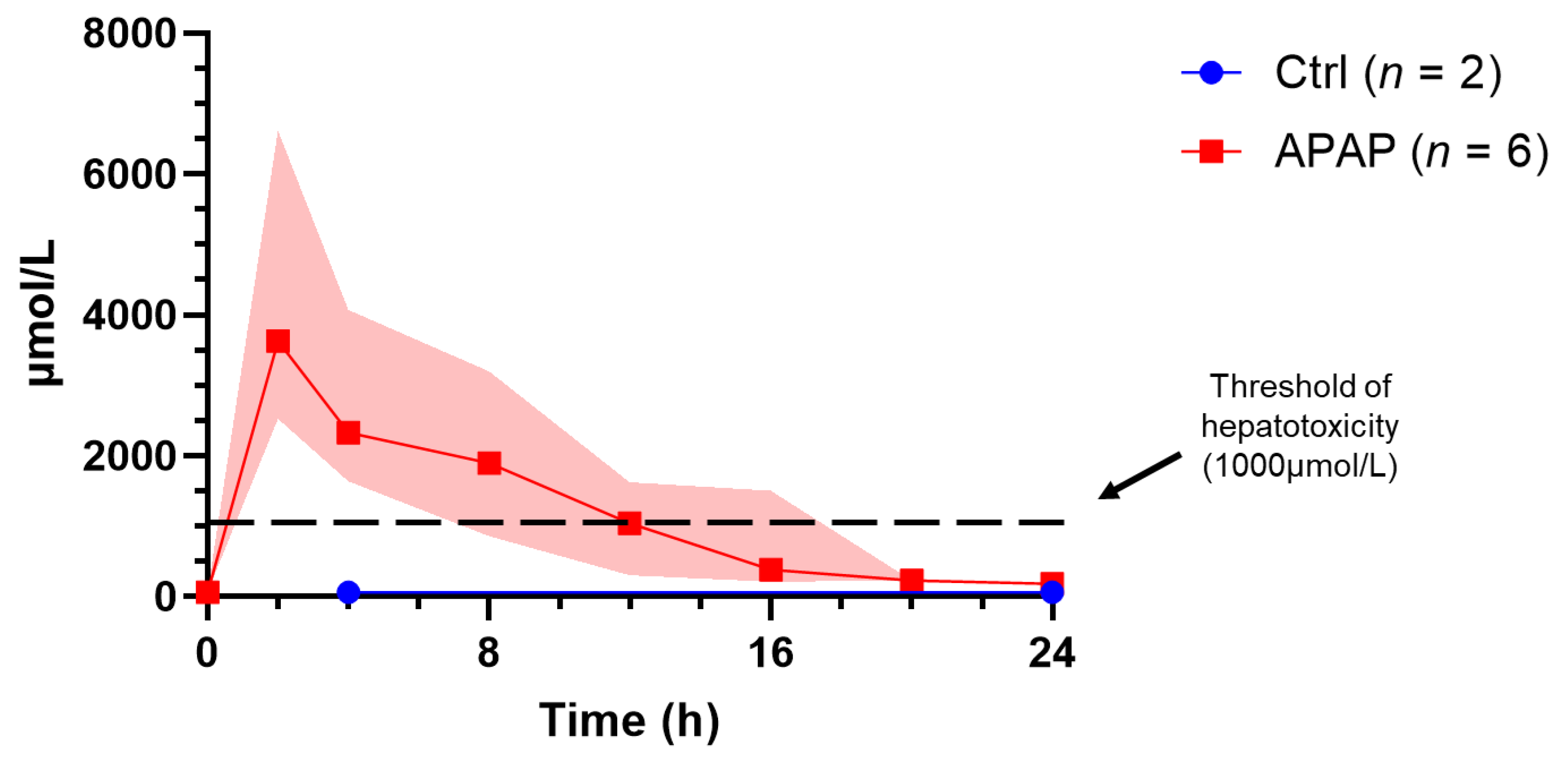
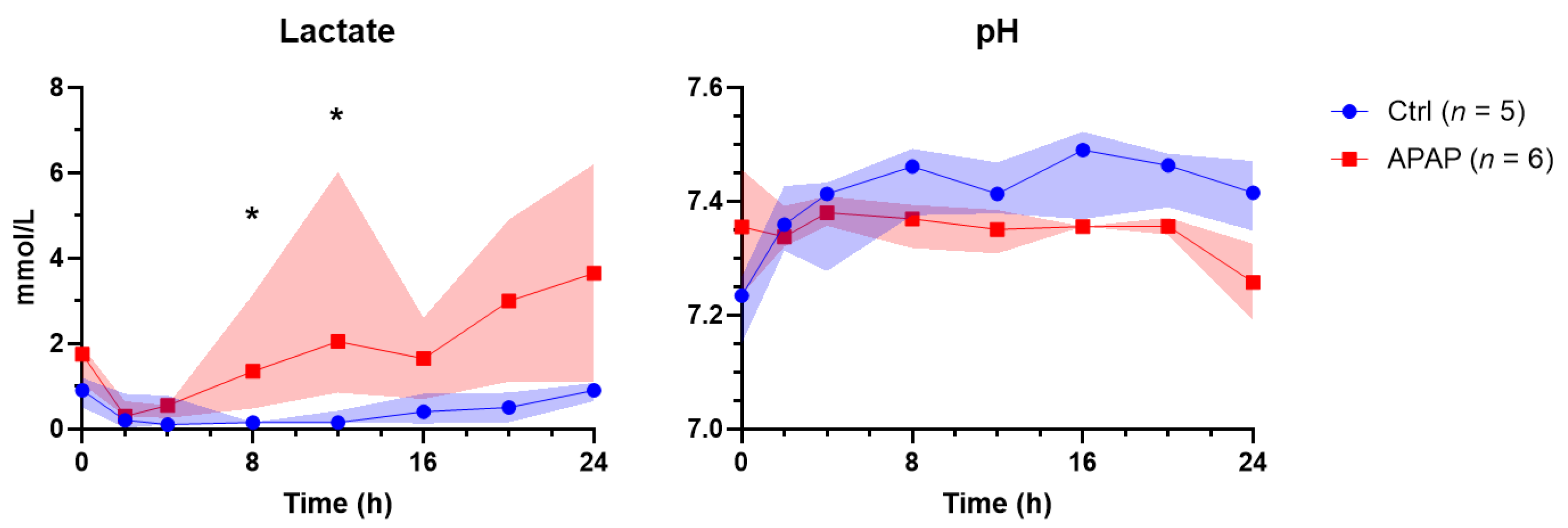
3.2. Perfusate Biochemistry
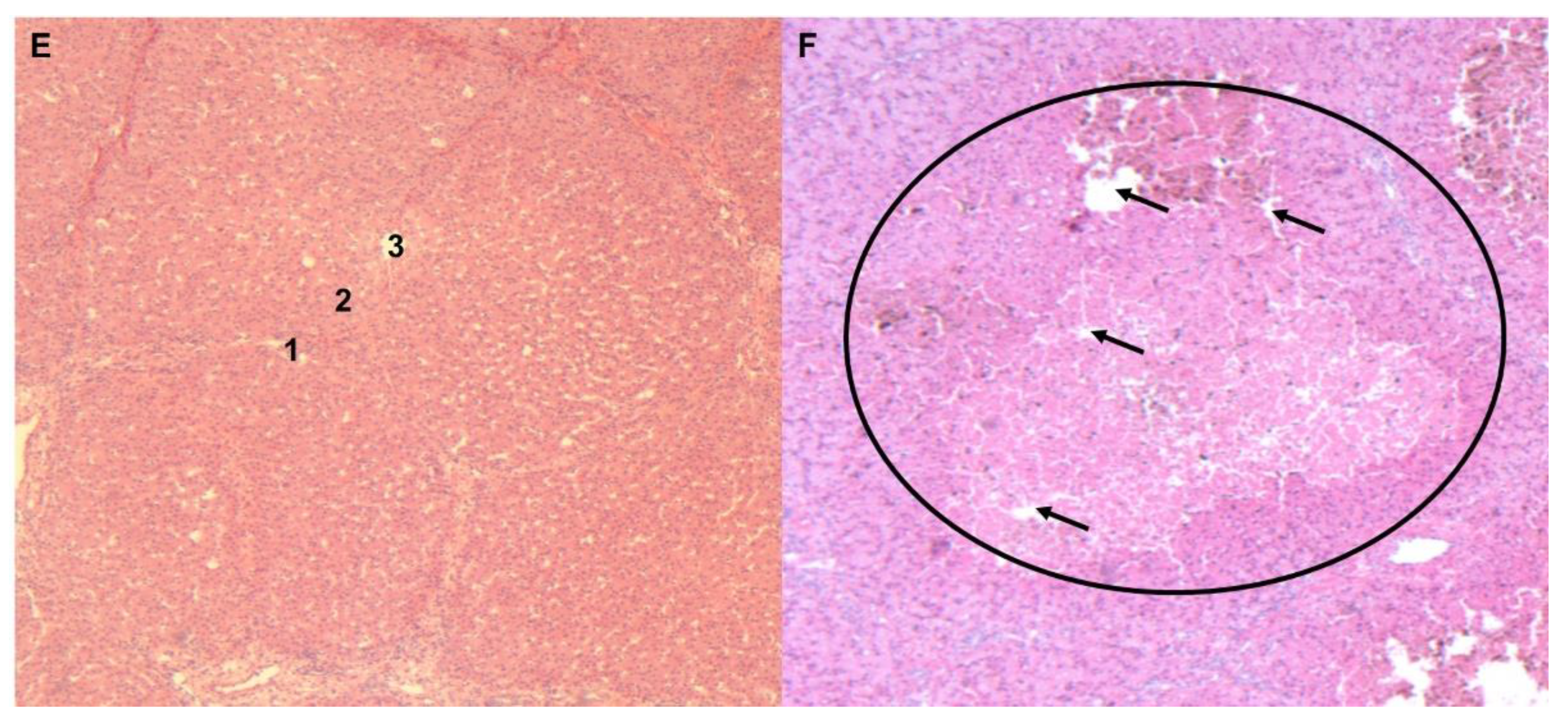
3.3. Histology
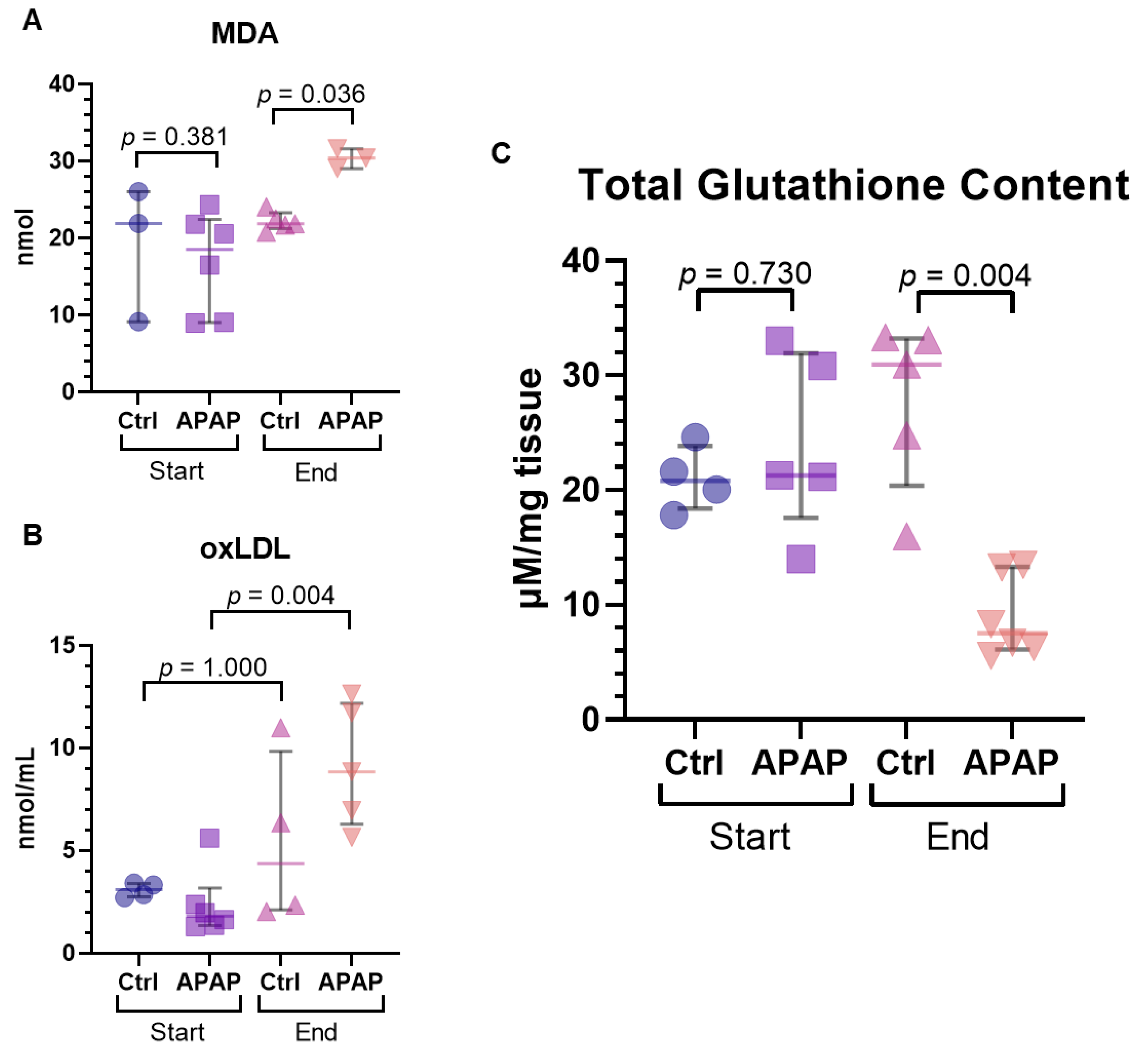
3.4. Measures of Oxidative Stress
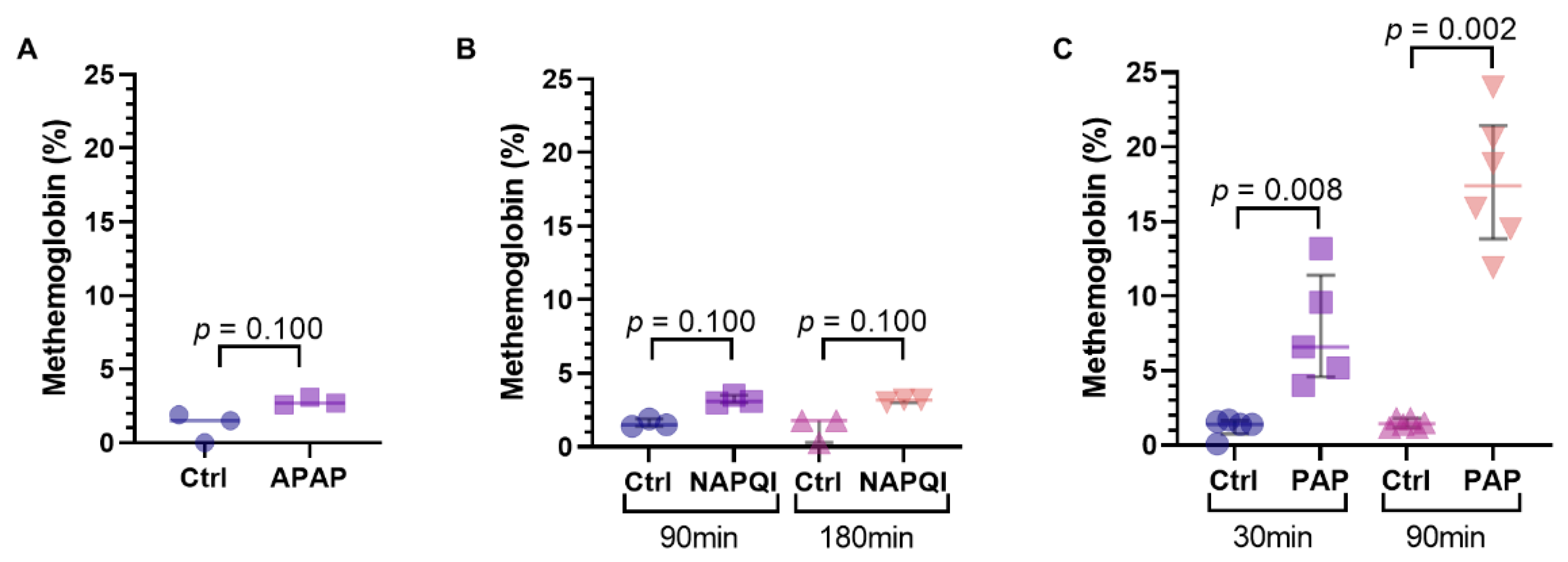
3.5. Methemoglobinemia and Hemolysis
3.6. Carbon Tetrachloride (CCl4)
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, W.M. Etiologies of Acute Liver Failure. Semin. Liver Dis. 2008, 28, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Khashab, M.; Tector, A.J.; Kwo, P.Y. Epidemiology of acute liver failure. Curr. Gastroenterol. Rep. 2007, 9, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Darweesh, S.K.; Ibrahim, M.F.; El-Tahawy, M.A. Effect of N-Acetylcysteine on Mortality and Liver Transplantation Rate in Non-Acetaminophen-Induced Acute Liver Failure: A Multicenter Study. Clin. Drug Investig. 2017, 37, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.M.; Hynan, L.S.; Rossaro, L.; Fontana, R.J.; Stravitz, R.T.; Larson, A.M.; Davern, T.J.; Murray, N.G.; McCashland, T.; Reisch, J.S.; et al. Intravenous N-Acetylcysteine Improves Transplant-Free Survival in Early Stage Non-Acetaminophen Acute Liver Failure. Gastroenterology 2009, 137, 856–864. [Google Scholar] [CrossRef] [Green Version]
- O’Grady, J.G.; Alexander, G.J.; Hayllar, K.M.; Williams, R. Early indicators of prognosis in fulminant hepatic failure. Gastroenterology 1989, 97, 439–445. [Google Scholar] [CrossRef]
- Lidofsky, S.D. Liver transplantation for fulminant hepatic failure. Gastroenterol. Clin. N. Am. 1993, 22, 257–269. [Google Scholar] [CrossRef]
- Ostapowicz, G.; Fontana, R.J.; Schiødt, F.V.; Larson, A.; Davern, T.J.; Han, S.H.; McCashland, T.M.; Shakil, A.O.; Hay, J.E.; Hynan, L.; et al. Results of a Prospective Study of Acute Liver Failure at 17 Tertiary Care Centers in the USA. Ann. Intern. Med. 2002, 137, 947–954. [Google Scholar] [CrossRef]
- McGill, M.R.; Jaeschke, H. Metabolism and Disposition of Acetaminophen: Recent Advances in Relation to Hepatotoxicity and Diagnosis. Pharm. Res. 2013, 30, 2174–2187. [Google Scholar] [CrossRef] [Green Version]
- Mazaleuskaya, L.L.; Sangkuhl, K.; Thorn, C.F.; FitzGerald, G.A.; Altman, R.B.; Klein, T.E. PharmGKB summary: Pathways of acetaminophen metabolism at the therapeutic versus toxic doses. Pharm. Genom. 2015, 25, 416–426. [Google Scholar] [CrossRef] [Green Version]
- Moyer, A.M.; Fridley, B.L.; Jenkins, G.D.; Batzler, A.J.; Pelleymounter, L.L.; Kalari, K.R.; Ji, Y.; Chai, Y.; Nordgren, K.K.; Weinshilboum, R.M. Acetaminophen-NAPQI Hepatotoxicity: A Cell Line Model System Genome-Wide Association Study. Toxicol. Sci. 2011, 120, 33–41. [Google Scholar] [CrossRef]
- Xue, Y.-L.; Zhao, S.-F.; Zhang, Z.-Y.; Wang, Y.-F.; Li, X.-J.; Huang, X.-Q.; Luo, Y.; Huang, Y.-C.; Liu, C.-G. Effects of a bioartificial liver support system on acetaminophen induced acute liver failure canines. World J. Gastroenterol. 1999, 5, 308–311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lauschke, V.M.; Hendriks, D.F.; Bell, C.C.; Andersson, T.B.; Ingelman-Sundberg, M. Novel 3D Culture Systems for Studies of Human Liver Function and Assessments of the Hepatotoxicity of Drugs and Drug Candidates. Chem. Res. Toxicol. 2016, 29, 1936–1955. [Google Scholar] [CrossRef] [PubMed]
- Eberlova, L.; Maleckova, A.; Mik, P.; Tonar, Z.; Jirik, M.; Mirka, H.; Palek, R.; Leupen, S.; Liska, V. Porcine Liver Anatomy Applied to Biomedicine. J. Surg. Res. 2020, 250, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, N.; Blasczyk, R.; Figueiredo, C. Animal Models in Allogenic Solid Organ Transplantation. Transplantology 2021, 2, 412–424. [Google Scholar] [CrossRef]
- Thiel, C.; Thiel, K.; Etspueler, A.; Morgalla, M.; Rubitschek, S.; Schmid, S.; Steurer, W.; Königsrainer, A.; Schenk, M. A Reproducible Porcine Model of Acute Liver Failure Induced by Intrajejunal Acetaminophen Administration. Eur. Surg. Res. 2011, 46, 118–126. [Google Scholar] [CrossRef]
- Newsome, P.N.; Henderson, N.C.; Nelson, L.J.; Dabos, C.; Filippi, C.; Bellamy, C.; Howie, F.; Clutton, R.E.; King, T.; Lee, A.; et al. Development of an invasively monitored porcine model of acetaminophen-induced acute liver failure. BMC Gastroenterol. 2010, 10, 34. [Google Scholar] [CrossRef] [Green Version]
- van Beekum, C.J.; Vilz, T.O.; Glowka, T.R.; von Websky, M.W.; Kalff, J.C.; Manekeller, S. Normothermic Machine Perfusion (NMP) of the Liver—Current Status and Future Perspectives. Ann. Transplant. 2021, 24, e931664. [Google Scholar] [CrossRef]
- Nasralla, D.; for the Consortium for Organ Preservation in Europe; Coussios, C.C.; Mergental, H.; Akhtar, M.Z.; Butler, A.J.; Ceresa, C.D.L.; Chiocchia, V.; Dutton, S.J.; García-Valdecasas, J.C.; et al. A randomized trial of normothermic preservation in liver transplantation. Nature 2018, 557, 50–56. [Google Scholar] [CrossRef]
- Nagrath, D.; Xu, H.; Tanimura, Y.; Zuo, R.; Berthiaume, F.; Avila, M.; Yarmush, R.; Yarmush, M.L. Metabolic preconditioning of donor organs: Defatting fatty livers by normothermic perfusion ex vivo. Metab. Eng. 2009, 11, 274–283. [Google Scholar] [CrossRef] [Green Version]
- Bishawi, M.; Roan, J.-N.; Milano, C.A.; Daneshmand, M.A.; Schroder, J.N.; Chiang, Y.; Lee, F.H.; Brown, Z.D.; Nevo, A.; Watson, M.; et al. A normothermic ex vivo organ perfusion delivery method for cardiac transplantation gene therapy. Sci. Rep. 2019, 9, 8029. [Google Scholar] [CrossRef]
- Galasso, M.; Feld, J.J.; Watanabe, Y.; Pipkin, M.; Summers, C.; Ali, A.; Qaqish, R.; Chen, M.; Ribeiro, R.V.P.; Ramadan, K.; et al. Inactivating hepatitis C virus in donor lungs using light therapies during normothermic ex vivo lung perfusion. Nat. Commun. 2019, 10, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldaracena, N.; Spetzler, V.N.; Echeverri, J.; Kaths, J.M.; Cherepanov, V.; Persson, R.; Hodges, M.R.; Janssen, H.L.A.; Selzner, N.; Grant, D.R.; et al. Inducing Hepatitis C Virus Resistance After Pig Liver Transplantation-A Proof of Concept of Liver Graft Modification Using Warm Ex Vivo Perfusion. Am. J. Transplant. 2017, 17, 970–978. [Google Scholar] [CrossRef] [PubMed]
- Hefler, J.; Marfil-Garza, B.A.; Dadheech, N.; Shapiro, A.J. Machine Perfusion of the Liver: Applications Beyond Transplantation. Transplantation 2020, 104, 1804–1812. [Google Scholar] [CrossRef] [PubMed]
- Bral, M.; Gala-Lopez, B.; Thiesen, A.; Hatami, S.; Bigam, D.L.; Freed, D.M.; Shapiro, A.J. Determination of Minimal Hemoglobin Level Necessary for Normothermic Porcine Ex Situ Liver Perfusion. Transplantation 2018, 102, 1284–1292. [Google Scholar] [CrossRef]
- Nostedt, J.J.; Churchill, T.; Ghosh, S.; Thiesen, A.; Hopkins, J.; Lees, M.C.; Adam, B.; Freed, D.H.; Shapiro, A.M.J.; Bigam, D.L. Avoiding initial hypothermia does not improve liver graft quality in a porcine donation after circulatory death (DCD) model of normothermic perfusion. PLoS ONE 2019, 14, e0220786. [Google Scholar] [CrossRef] [Green Version]
- Xie, F.; Li, Z.-P.; Wang, H.-W.; Fei, X.; Jiao, Z.-Y.; Tang, W.-B.; Tang, J.; Luo, Y.-K. Evaluation of Liver Ischemia-Reperfusion Injury in Rabbits Using a Nanoscale Ultrasound Contrast Agent Targeting ICAM-1. PLoS ONE 2016, 11, e0153805. [Google Scholar] [CrossRef] [Green Version]
- Davis, B.H.; Jungerius, B.; On behalf of International Council for the Standardization of Haematology (ICSH). International Council for Standardization in Haematology technical report 1-2009: New reference material for haemiglobincyanide for use in standardization of blood haemoglobin measurements. Int. J. Lab. Hematol. 2010, 32, 139–141. [Google Scholar] [CrossRef]
- Dargue, R.; Zia, R.; Lau, C.; Nicholls, A.W.; Dare, T.O.; Lee, K.; Jalan, R.; Coen, M.; Wilson, I.D. Metabolism and Effects on Endogenous Metabolism of Paracetamol (Acetaminophen) in a Porcine Model of Liver Failure. Toxicol. Sci. Off. J. Soc. Toxicol. 2020, 175, 87–97. [Google Scholar] [CrossRef] [Green Version]
- McConkey, S.E.; Grant, D.M.; Cribb, A.E. The role of para-aminophenol in acetaminophen-induced methemoglobinemia in dogs and cats. J. Veter- Pharmacol. Ther. 2009, 32, 585–595. [Google Scholar] [CrossRef]
- Henne-Bruns, D.; Artwohl, J.; Broelsch, C.; Kremer, B. Acetaminophen-induced acute hepatic failure in pigs: Controversical results to other animal models. Res. Exp. Med. 1988, 188, 463–472. [Google Scholar] [CrossRef]
- Gazzard, B.G.; Hughes, R.D.; Mellon, P.J.; Portmann, B.; Williams, R. A dog model of fulminant hepatic failure produced by paracetamol administration. Br. J. Exp. Pathol. 1975, 56, 408–411. [Google Scholar] [PubMed]
- Rianprakaisang, T.; Blumenberg, A.; Hendrickson, R.G. Methemoglobinemia associated with massive acetaminophen ingestion: A case series. Clin. Toxicol. 2020, 58, 495–497. [Google Scholar] [CrossRef] [PubMed]
- Ben-Shachar, R.; Chen, Y.; Luo, S.; Hartman, C.; Reed, M.; Nijhout, H.F. The biochemistry of acetaminophen hepatotoxicity and rescue: A mathematical model. Theor. Biol. Med. Model. 2012, 9, 55. [Google Scholar] [CrossRef] [Green Version]
- Warren, O.U.; Blackwood, B. Acquired Methemoglobinemia. N. Engl. J. Med. 2019, 381, 1158. [Google Scholar] [CrossRef]
- Al-Fares, A.; Pettenuzzo, T.; Del Sorbo, L. Extracorporeal life support and systemic inflammation. Intensiv. Care Med. Exp. 2019, 25, 1–14. [Google Scholar] [CrossRef]
- Tingle, S.J.; Ibrahim, I.; Thompson, E.R.; Bates, L.; Sivaharan, A.; Bury, Y.; Figuereido, R.; Wilson, C. Methaemoglobinaemia Can Complicate Normothermic Machine Perfusion of Human Livers. Front. Surg. 2021, 8, 634777. [Google Scholar] [CrossRef] [PubMed]
- Rahman, T.M.; Hodgson, H.J.F. Animal models of acute hepatic failure. Int. J. Exp. Pathol. 2000, 81, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Yuasa, T.; Yamamoto, T.; Rivas-Carrillo, J.D.; Chen, Y.; Navarro-Alvarez, N.; Soto-Guiterrez, A.; Noguchi, H.; Matsumoto, S.; Tanaka, N.; Kobayashi, N. Laparoscopy-Assisted Creation of a Liver Failure Model in Pigs. Cell Transplant. 2008, 17, 187–193. [Google Scholar] [CrossRef] [Green Version]
- Apte, U. Galactosamine. In Encyclopedia of Toxicology, 3rd ed.; Wexler, P., Ed.; Oxford University Press: Oxford, UK, 2014; pp. 689–690. [Google Scholar]
- Wallace, M.C.; Hamesch, K.; Lunova, M.; Kim, Y.; Weiskirchen, R.; Strnad, P.; Friedman, S.L. Standard Operating Procedures in Experimental Liver Research: Thioacetamide model in mice and rats. Lab. Anim. 2015, 49, 21–29. [Google Scholar] [CrossRef] [Green Version]
- Matkowskyj, K.A.; Marrero, J.A.; Carroll, R.E.; Danilkovich, A.V.; Green, R.M.; Benya, R.V. Azoxymethane-induced fulminant hepatic failure in C57BL/6J mice: Characterization of a new animal model. Am. J. Physiol.-Gastrointest. Liver Physiol. 1999, 277, G455–G462. [Google Scholar] [CrossRef]
- Ferriero, R.; Nusco, E.; De Cegli, R.; Carissimo, A.; Manco, G.; Brunetti-Pierri, N. Pyruvate dehydrogenase complex and lactate dehydrogenase are targets for therapy of acute liver failure. J. Hepatol. 2018, 69, 325–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takada, Y.; Ishiguro, S.; Fukunaga, K.; Gu, M.; Taniguchi, H.; Seino, K.I.; Yuzawa, K.; Otsuka, M.; Todoroki, T.; Fukao, K. Increased intracranial pressure in a porcine model of fulminant hepatic failure using amatoxin and endotoxin. J. Hepatol. 2001, 34, 825–831. [Google Scholar] [CrossRef]
- Zhou, P.; Xia, J.; Guo, G.; Huang, Z.X.; Lu, Q.; Li, L.; Li, H.X.; Shi, Y.J.; Bu, H. A Macaca mulatta model of fulminant hepatic failure. World J. Gastroenterol. 2012, 18, 435–444. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hefler, J.; Hatami, S.; Thiesen, A.; Olafson, C.; Durand, K.; Acker, J.; Karvellas, C.J.; Bigam, D.L.; Freed, D.H.; Shapiro, A.M.J. Model of Acute Liver Failure in an Isolated Perfused Porcine Liver—Challenges and Lessons Learned. Biomedicines 2022, 10, 2496. https://doi.org/10.3390/biomedicines10102496
Hefler J, Hatami S, Thiesen A, Olafson C, Durand K, Acker J, Karvellas CJ, Bigam DL, Freed DH, Shapiro AMJ. Model of Acute Liver Failure in an Isolated Perfused Porcine Liver—Challenges and Lessons Learned. Biomedicines. 2022; 10(10):2496. https://doi.org/10.3390/biomedicines10102496
Chicago/Turabian StyleHefler, Joshua, Sanaz Hatami, Aducio Thiesen, Carly Olafson, Kiarra Durand, Jason Acker, Constantine J. Karvellas, David L. Bigam, Darren H. Freed, and Andrew Mark James Shapiro. 2022. "Model of Acute Liver Failure in an Isolated Perfused Porcine Liver—Challenges and Lessons Learned" Biomedicines 10, no. 10: 2496. https://doi.org/10.3390/biomedicines10102496
APA StyleHefler, J., Hatami, S., Thiesen, A., Olafson, C., Durand, K., Acker, J., Karvellas, C. J., Bigam, D. L., Freed, D. H., & Shapiro, A. M. J. (2022). Model of Acute Liver Failure in an Isolated Perfused Porcine Liver—Challenges and Lessons Learned. Biomedicines, 10(10), 2496. https://doi.org/10.3390/biomedicines10102496




