Role of Matrix Metalloproteinases in Musculoskeletal Diseases
Abstract
:1. Introduction
2. Extracellular Matrix (ECM) Components
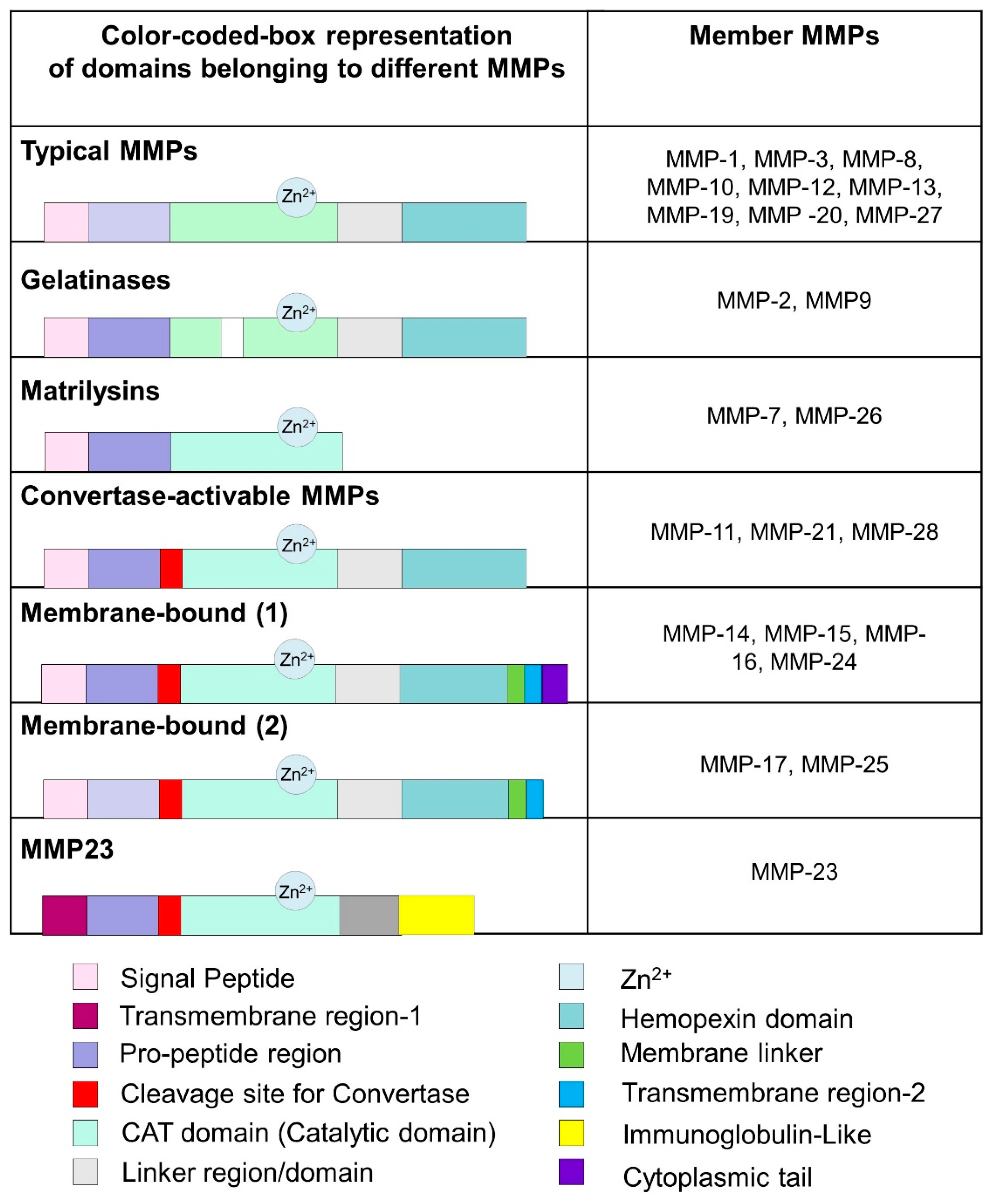
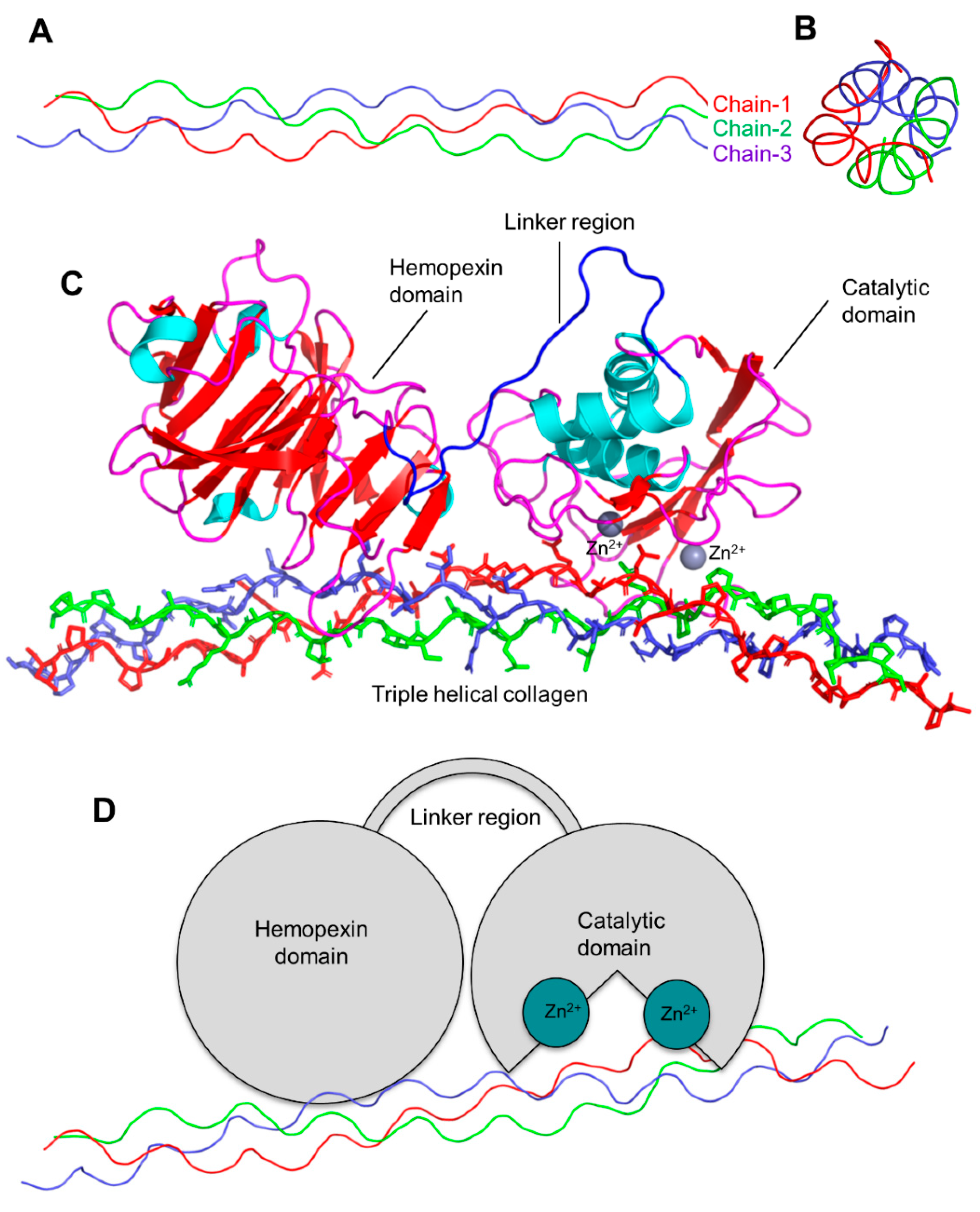
3. Matrix Metalloproteinases (MMPs)
Classes and Structural Features of MMPs
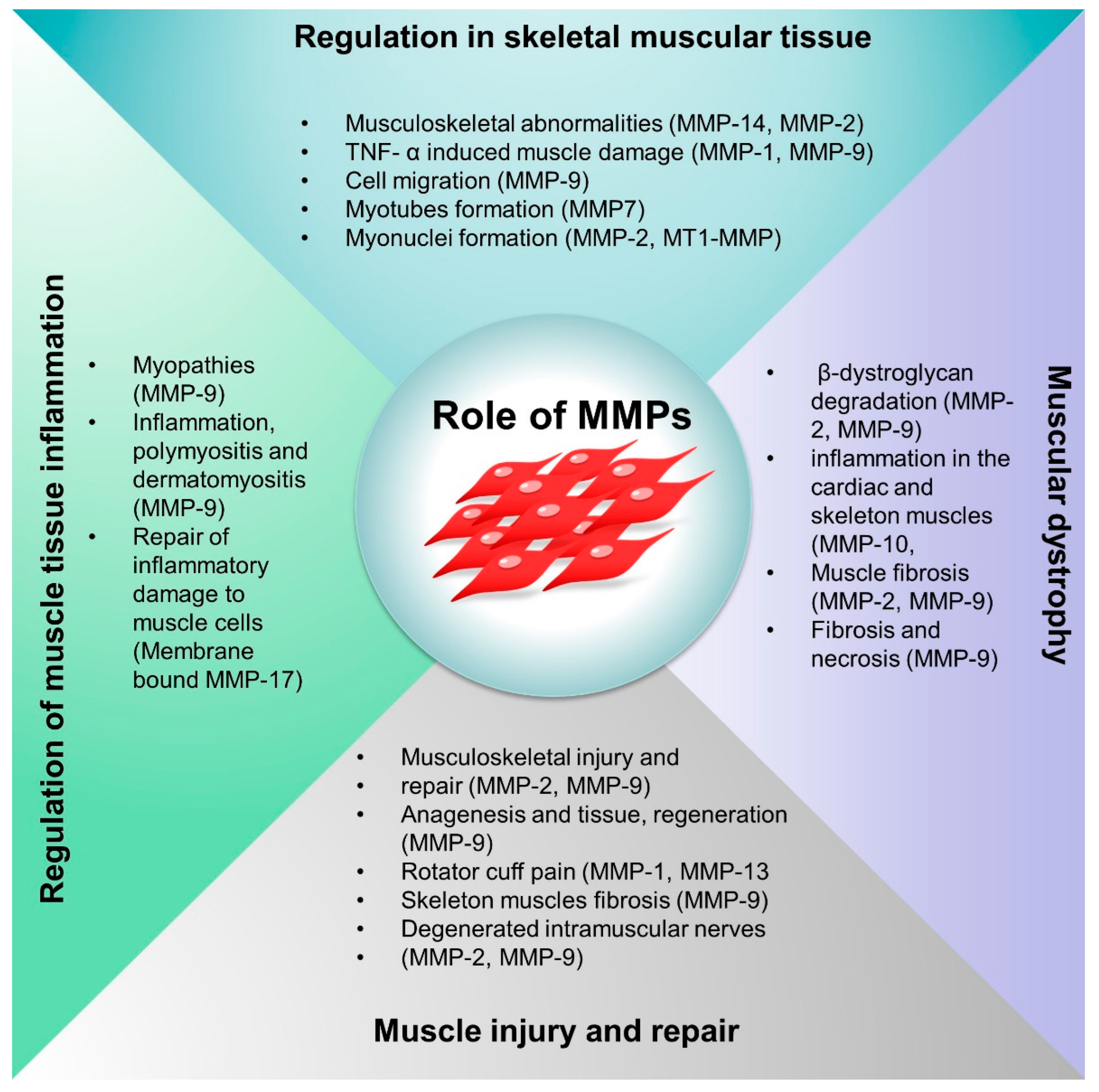
4. Function of MMPs in Musculoskeletal Diseases
4.1. Role of MMPs in the Musculoskeletal System
4.2. MMPs Regulation in Skeletal Muscle Tissue
4.3. MMPs in Musculoskeletal Injury and Repair Mechanism
4.4. Role of MMPs in the Regulation of Inflammation in Muscle Diseases
4.5. MMPs in Muscular Dystrophy (MD)
5. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hu, M.; Ling, Z.; Ren, X. Extracellular matrix dynamics: Tracking in biological systems and their implications. J. Biol. Eng. 2022, 16, 13. [Google Scholar] [CrossRef]
- Brooks, P.M. The burden of musculoskeletal disease—A global perspective. Clin. Rheumatol. 2006, 25, 778–781. [Google Scholar] [CrossRef]
- LeBlanc, K.E.; LeBlanc, L.L. Musculoskeletal disorders. Prim. Care 2010, 37, 389–406. [Google Scholar] [CrossRef]
- Dogba, M.J.; Rauch, F.; Douglas, E.; Bedos, C. Impact of three genetic musculoskeletal diseases: A comparative synthesis of achondroplasia, Duchenne muscular dystrophy and osteogenesis imperfecta. Health Qual. Life Outcomes 2014, 12, 151. [Google Scholar] [CrossRef] [Green Version]
- Brightwell, C.R.; Latham, C.M.; Thomas, N.T.; Keeble, A.R.; Murach, K.A.; Fry, C.S. A glitch in the matrix: The pivotal role for extracellular matrix remodeling during muscle hypertrophy. Am. J. Physiol. Physiol. 2022, 323, C763–C771. [Google Scholar] [CrossRef]
- Sheets, K.; Overbey, J.; Ksajikian, A.; Bovid, K.; Kenter, K.; Li, Y. The pathophysiology and treatment of musculoskeletal fibrosis. J. Cell. Biochem. 2022, 123, 843–851. [Google Scholar] [CrossRef]
- Lu, P.; Takai, K.; Weaver, V.M.; Werb, Z. Extracellular Matrix Degradation and Remodeling in Development and Disease. Cold Spring Harb. Perspect. Biol. 2011, 3, a005058. [Google Scholar] [CrossRef]
- Couch, C.B.; Strittmatter, W.J. Rat myoblast fusion requires metalloendoprotease activity. Cell 1983, 32, 257–265. [Google Scholar] [CrossRef]
- Guérin, C.W.; Holland, P.C. Synthesis and secretion of matrix-degrading metalloproteases by human skeletal muscle satellite cells. Dev. Dyn. 1995, 202, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, Y. Role of matrix metalloproteinases in skeletal muscle: Migration, differentiation, regeneration and fibrosis. Cell Adh. Migr. 2009, 3, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Ogura, Y.; Tajrishi, M.M.; Sato, S.; Hindi, S.M.; Kumar, A. Therapeutic potential of matrix metalloproteinases in Duchenne muscular dystrophy. Front. Cell Dev. Biol. 2014, 2, 11. [Google Scholar] [CrossRef] [Green Version]
- Alameddine, H.S. Matrix metalloproteinases in skeletal muscles: Friends or foes? Neurobiol. Dis. 2012, 48, 508–518. [Google Scholar] [CrossRef]
- Brew, K.; Nagase, H. The tissue inhibitors of metalloproteinases (TIMPs): An ancient family with structural and functional diversity. Biochim. Biophys. Acta 2010, 1803, 55–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raeeszadeh-Sarmazdeh, M.; Do, L.; Hritz, B. Metalloproteinases and Their Inhibitors: Potential for the Development of New Therapeutics. Cells 2020, 9, 1313. [Google Scholar] [CrossRef]
- Karamanos, N.K.; Theocharis, A.D.; Neill, T.; Iozzo, R.V. Matrix modeling and remodeling: A biological interplay regulating tissue homeostasis and diseases. Matrix Biol. 2019, 75–76, 1–11. [Google Scholar] [CrossRef]
- Furuno, K.; Goodman, M.N.; Goldberg, A.L. Role of different proteolytic systems in the degradation of muscle proteins during denervation atrophy. J. Biol. Chem. 1990, 265, 8550–8557. [Google Scholar] [CrossRef]
- Carmeli, E.; Moas, M.; Reznick, A.Z.; Coleman, R. Matrix metalloproteinases and skeletal muscle: A brief review. Muscle Nerve 2004, 29, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Candelario-Jalil, E.; Thompson, J.F.; Cuadrado, E.; Estrada, E.Y.; Rosell, A.; Montaner, J.; Rosenberg, G.A. Increased intranuclear matrix metalloproteinase activity in neurons interferes with oxidative DNA repair in focal cerebral ischemia. J. Neurochem. 2010, 112, 134–149. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.; Rajasekaran, N.; Hartenstein, B.; Szabowski, S.; Gajda, M.; Angel, P.; Bräuer, R.; Illges, H. Collagenase-3 (MMP-13) deficiency protects C57BL/6 mice from antibody-induced arthritis. Arthritis Res. Ther. 2013, 15, R222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, H.-C.; Ho, H.-L.; Chang, C.-C.; Chuang, C.-L.; Pun, C.K.; Lee, F.-Y.; Huang, Y.-H.; Hou, M.-C.; Hsu, S.-J. Matrix metalloproteinase-9 inhibition or deletion attenuates portal hypertension in rodents. J. Cell. Mol. Med. 2021, 25, 10073–10087. [Google Scholar] [CrossRef] [PubMed]
- Laronha, H.; Caldeira, J. Structure and Function of Human Matrix Metalloproteinases. Cells 2020, 9, 1076. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liu, Y.; Zhang, H. Extracellular matrix: An important regulator of cell functions and skeletal muscle development. Cell Biosci. 2021, 11, 65. [Google Scholar] [CrossRef] [PubMed]
- Yurchenco, P.D.; McKee, K.K.; Reinhard, J.R.; Rüegg, M.A. Laminin-deficient muscular dystrophy: Molecular pathogenesis and structural repair strategies. Matrix Biol. 2018, 71–72, 174–187. [Google Scholar] [CrossRef] [PubMed]
- Roman, W.; Martins, J.P.; Gomes, E.R. Local Arrangement of Fibronectin by Myofibroblasts Governs Peripheral Nuclear Positioning in Muscle Cells. Dev. Cell 2018, 46, 102–111.e6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- To, W.S.; Midwood, K.S. Plasma and cellular fibronectin: Distinct and independent functions during tissue repair. Fibrogenesis Tissue Repair 2011, 4, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, Q.Q.; McNally, E.M. The Dystrophin Complex: Structure, Function, and Implications for Therapy. Compr. Physiol. 2015, 5, 1223–1239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allen, D.G.; Whitehead, N.P.; Froehner, S.C. Absence of Dystrophin Disrupts Skeletal Muscle Signaling: Roles of Ca2+, Reactive Oxygen Species, and Nitric Oxide in the Development of Muscular Dystrophy. Physiol. Rev. 2016, 96, 253–305. [Google Scholar] [CrossRef] [Green Version]
- Rapraeger, A.; Jalkanen, M.; Bernfield, M. Cell surface proteoglycan associates with the cytoskeleton at the basolateral cell surface of mouse mammary epithelial cells. J. Cell Biol. 1986, 103, 2683–2696. [Google Scholar] [CrossRef]
- Niland, S.; Riscanevo, A.X.; Eble, J.A. Matrix metalloproteinases shape the tumor microenvironment in cancer progression. Int. J. Mol. Sci. 2021, 23, 146. [Google Scholar] [CrossRef]
- Carey, P.; Low, E.; Harper, E.; Stack, M.S. Metalloproteinases in ovarian cancer. Int. J. Mol. Sci. 2021, 22, 3403. [Google Scholar] [CrossRef]
- Jabłońska-Trypuć, A.; Matejczyk, M.; Rosochacki, S. Matrix metalloproteinases (MMPs), the main extracellular matrix (ECM) enzymes in collagen degradation, as a target for anticancer drugs. J. Enzyme Inhib. Med. Chem. 2016, 31, 177–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCarthy, S.M.; Bove, P.F.; Matthews, D.E.; Akaike, T.; van der Vliet, A. Nitric oxide regulation of MMP-9 activation and its relationship to modifications of the cysteine switch. Biochemistry 2008, 47, 5832–5840. [Google Scholar] [CrossRef] [Green Version]
- Magdalena, K.; Magdalena, K.; Grazyna, S. The Role of Matrix Metalloproteinase-3 in the Development of Atherosclerosis and Cardiovascular Events. EJIFCC 2006, 17, 2–5. [Google Scholar]
- Gaffney, J.; Solomonov, I.; Zehorai, E.; Sagi, I. Multilevel regulation of matrix metalloproteinases in tissue homeostasis indicates their molecular specificity in vivo. Matrix Biol. 2015, 44–46, 191–199. [Google Scholar] [CrossRef]
- Kok, H.J.; Barton, E.R. Actions and interactions of IGF-I and MMPs during muscle regeneration. Semin. Cell Dev. Biol. 2021, 119, 11–22. [Google Scholar] [CrossRef]
- Fanjul-Fernández, M.; Folgueras, A.R.; Cabrera, S.; López-Otín, C. Matrix metalloproteinases: Evolution, gene regulation and functional analysis in mouse models. Biochim. Biophys. Acta Mol. Cell Res. 2010, 1803, 3–19. [Google Scholar] [CrossRef] [Green Version]
- Madzharova, E.; Kastl, P.; Sabino, F.; auf dem Keller, U. Post-Translational Modification-Dependent Activity of Matrix Metalloproteinases. Int. J. Mol. Sci. 2019, 20, 3077. [Google Scholar] [CrossRef] [Green Version]
- Tan, R.J.; Liu, Y. Matrix metalloproteinases in kidney homeostasis and diseases. Am. J. Physiol. Physiol. 2012, 302, F1351–F1361. [Google Scholar] [CrossRef] [Green Version]
- Masson, V. Roles of serine proteases and matrix metalloproteinases in tumor invasion and angiogenesis. Bull. Mem. Acad. R. Med. Belg. 2006, 161, 320–326. [Google Scholar]
- Bassiouni, W.; Ali, M.A.M.; Schulz, R. Multifunctional intracellular matrix metalloproteinases: Implications in disease. FEBS J. 2021, 288, 7162–7182. [Google Scholar] [CrossRef]
- Martins, S.G.; Zilhão, R.; Thorsteinsdóttir, S.; Carlos, A.R. Linking oxidative stress and DNA damage to changes in the expression of ECM components. Front. Genet. 2021, 12, 1279. [Google Scholar] [CrossRef] [PubMed]
- Larochelle, J.; Yang, C.; Liu, L.; Candelario-Jalil, E. An Unexplored Role for MMP-7 (Matrix Metalloproteinase-7) in Promoting Gut Permeability After Ischemic Stroke. Stroke 2022, 53, 3238–3242. [Google Scholar] [CrossRef]
- Ciccone, L.; Vandooren, J.; Nencetti, S.; Orlandini, E. Natural marine and terrestrial compounds as modulators of matrix metalloproteinases-2 (MMP-2) and MMP-9 in Alzheimer’s disease. Pharmaceuticals 2021, 14, 86. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, J.; Sun, H.; Zhang, Y.; Zou, D. New insights into fibrosis from the ECM degradation perspective: The macrophage-MMP-ECM interaction. Cell Biosci. 2022, 12, 117. [Google Scholar] [CrossRef]
- Chen, W.; You, W.; Valencak, T.G.; Shan, T. Bidirectional roles of skeletal muscle fibro-adipogenic progenitors in homeostasis and disease. Ageing Res. Rev. 2022, 80, 101682. [Google Scholar] [CrossRef]
- Oh, J.; Takahashi, R.; Adachi, E.; Kondo, S.; Kuratomi, S.; Noma, A.; Alexander, D.B.; Motoda, H.; Okada, A.; Seiki, M.; et al. Mutations in two matrix metalloproteinase genes, MMP-2 and MT1-MMP, are synthetic lethal in mice. Oncogene 2004, 23, 5041–5048. [Google Scholar] [CrossRef] [Green Version]
- Sitia, G.; Isogawa, M.; Iannacone, M.; Campbell, I.L.; Chisari, F.V.; Guidotti, L.G. MMPs are required for recruitment of antigen-nonspecific mononuclear cells into the liver by CTLs. J. Clin. Investig. 2004, 113, 1158–1167. [Google Scholar] [CrossRef] [Green Version]
- Maybee, D.V.; Ink, N.L.; Ali, M.A.M. Novel Roles of MT1-MMP and MMP-2: Beyond the Extracellular Milieu. Int. J. Mol. Sci. 2022, 23, 9513. [Google Scholar] [CrossRef]
- Chen, W.; Zhou, Z.; Chen, D.; Li, Y.; Zhang, Q.; Su, J. Bone regeneration using MMP-cleavable peptides-based hydrogels. Gels 2021, 7, 199. [Google Scholar] [CrossRef]
- de Almeida, L.G.N.; Thode, H.; Eslambolchi, Y.; Chopra, S.; Young, D.; Gill, S.; Devel, L.; Dufour, A. Matrix Metalloproteinases: From Molecular Mechanisms to Physiology, Pathophysiology, and Pharmacology. Pharmacol. Rev. 2022, 74, 712–768. [Google Scholar] [CrossRef]
- Liu, D.; Viennois, E.; Fang, J.; Merlin, D.; Iyer, S.S. Toward Point-of-Care Diagnostics to Monitor MMP-9 and TNF-α Levels in Inflammatory Bowel Disease. ACS Omega 2021, 6, 6582–6587. [Google Scholar] [CrossRef] [PubMed]
- Kherif, S.; Dehaupas, M.; Lafuma, C.; Fardeau, M.; Alameddine, H.S. Matrix metalloproteinases MMP-2 and MMP-9 in denervated muscle and injured nerve. Neuropathol. Appl. Neurobiol. 1998, 24, 309–319. [Google Scholar] [CrossRef]
- Balcerzak, D.; Querengesser, L.; Dixon, W.T.; Baracos, V.E. Coordinate expression of matrix-degrading proteinases and their activators and inhibitors in bovine skeletal muscle. J. Anim. Sci. 2001, 79, 94–107. [Google Scholar] [CrossRef]
- Sikorska, M.; Dutkiewicz, M.; Zegrocka–Stendel, O.; Kowalewska, M.; Grabowska, I.; Koziak, K. Beneficial effects of β-escin on muscle regeneration in rat model of skeletal muscle injury. Phytomedicine 2021, 93, 153791. [Google Scholar] [CrossRef]
- Lewis, M.P.; Tippett, H.L.; Sinanan, A.C.; Morgan, M.J.; Hunt, N.P. Gelatinase-B (matrix metalloproteinase-9; MMP-9) secretion is involved in the migratory phase of human and murine muscle cell cultures. J. Muscle Res. Cell Motil. 2000, 21, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Caron, N.J.; Asselin, I.; Morel, G.; Tremblay, J.P. Increased myogenic potential and fusion of matrilysin-expressing myoblasts transplanted in mice. Cell Transplant. 1999, 8, 465–476. [Google Scholar] [CrossRef] [Green Version]
- Anguita-Ruiz, A.; Bustos-Aibar, M.; Plaza-Díaz, J.; Mendez-Gutierrez, A.; Alcalá-Fdez, J.; Aguilera, C.M.; Ruiz-Ojeda, F.J. Omics approaches in adipose tissue and skeletal muscle addressing the role of extracellular matrix in obesity and metabolic dysfunction. Int. J. Mol. Sci. 2021, 22, 2756. [Google Scholar] [CrossRef]
- Echizenya, M.; Kondo, S.; Takahashi, R.; Oh, J.; Kawashima, S.; Kitayama, H.; Takahashi, C.; Noda, M. The membrane-anchored MMP-regulator RECK is a target of myogenic regulatory factors. Oncogene 2005, 24, 5850–5857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barnes, B.R.; Szelenyi, E.R.; Warren, G.L.; Urso, M.L. Alterations in mRNA and protein levels of metalloproteinases-2, -9, and -14 and tissue inhibitor of metalloproteinase-2 responses to traumatic skeletal muscle injury. Am. J. Physiol. Physiol. 2009, 297, C1501–C1508. [Google Scholar] [CrossRef] [PubMed]
- Dehne, N.; Kerkweg, U.; Flohé, S.B.; Brüne, B.; Fandrey, J. Activation of Hypoxia-Inducible Factor 1 in Skeletal Muscle Cells after Exposure to Damaged Muscle Cell Debris. Shock 2011, 35, 632–638. [Google Scholar] [CrossRef] [PubMed]
- Sachinis, N.P.; Yiannakopoulos, C.K.; Chalidis, B.; Kitridis, D.; Givissis, P. Biomolecules Related to Rotator Cuff Pain: A Scoping Review. Biomolecules 2022, 12, 1016. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Mittal, A.; Makonchuk, D.Y.; Bhatnagar, S.; Kumar, A. Matrix metalloproteinase-9 inhibition ameliorates pathogenesis and improves skeletal muscle regeneration in muscular dystrophy. Hum. Mol. Genet. 2009, 18, 2584–2598. [Google Scholar] [CrossRef]
- Schoser, B.G.H.; Blottner, D.; Stuerenburg, H.-J. Matrix metalloproteinases in inflammatory myopathies: Enhanced immunoreactivity near atrophic myofibers. Acta Neurol. Scand. 2002, 105, 309–313. [Google Scholar] [CrossRef]
- Kieseier, B.C.; Schneider, C.; Clements, J.M.; Gearing, A.J.; Gold, R.; Toyka, K.V.; Hartung, H.P. Expression of specific matrix metalloproteinases in inflammatory myopathies. Brain 2001, 124, 341–351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peck, B.D.; Murach, K.A.; Walton, R.G.; Simmons, A.J.; Long, D.E.; Kosmac, K.; Dungan, C.M.; Kern, P.A.; Bamman, M.M.; Peterson, C.A. A muscle cell-macrophage axis involving matrix metalloproteinase 14 facilitates extracellular matrix remodeling with mechanical loading. FASEB J. 2022, 36, e22155. [Google Scholar] [CrossRef] [PubMed]
- Castrejón-Téllez, V.; Rubio-Ruiz, M.E.; Cano-Martínez, A.; Pérez-Torres, I.; Del Valle-Mondragón, L.; Carreón-Torres, E.; Guarner-Lans, V. High Sucrose Ingestion during a Critical Period of Vessel Development Promotes the Synthetic Phenotype of Vascular Smooth Muscle Cells and Modifies Vascular Contractility Leading to Hypertension in Adult Rats. Int. J. Hypertens. 2022, 2022, 2298329. [Google Scholar] [CrossRef]
- Vasilceac, F.A.; Marqueti, R.d.C.; Neto, I.V.d.S.; Nascimento, D.d.C.; Souza, M.C.d.; Durigan, J.L.Q.; Mattiello, S.M. Resistance training decreases matrix metalloproteinase-2 activity in quadriceps tendon in a rat model of osteoarthritis. Braz. J. Phys. Ther. 2021, 25, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Martín-Alonso, M.; Iqbal, S.; Vornewald, P.M.; Lindholm, H.T.; Damen, M.J.; Martínez, F.; Hoel, S.; Díez-Sánchez, A.; Altelaar, M.; Katajisto, P.; et al. Smooth muscle-specific MMP17 (MT4-MMP) regulates the intestinal stem cell niche and regeneration after damage. Nat. Commun. 2021, 12, 6741. [Google Scholar] [CrossRef] [PubMed]
- Fukai, Y.; Ohsawa, Y.; Ohtsubo, H.; Nishimatsu, S.-I.; Hagiwara, H.; Noda, M.; Sasaoka, T.; Murakami, T.; Sunada, Y. Cleavage of β-dystroglycan occurs in sarcoglycan-deficient skeletal muscle without MMP-2 and MMP-9. Biochem. Biophys. Res. Commun. 2017, 492, 199–205. [Google Scholar] [CrossRef]
- Giovarelli, M.; Arnaboldi, F.; Zecchini, S.; Cornaghi, L.B.; Nava, A.; Sommariva, M.; Clementi, E.G.I.; Gagliano, N. Characterisation of Progressive Skeletal Muscle Fibrosis in the Mdx Mouse Model of Duchenne Muscular Dystrophy: An In Vivo and In Vitro Study. Int. J. Mol. Sci. 2022, 23, 8735. [Google Scholar] [CrossRef]
- Baraibar-Churio, A.; Bobadilla, M.; Machado, F.J.D.; Sáinz, N.; Roncal, C.; Abizanda, G.; Prósper, F.; Orbe, J.; Pérez-Ruiz, A. Deficiency of MMP-10 Aggravates the Diseased Phenotype of Aged Dystrophic Mice. Life 2021, 11, 1398. [Google Scholar] [CrossRef] [PubMed]
- de Souza, L.B.; Maziero, C.; Lazzarin, M.C.; Quintana, H.T.; Tomé, T.d.C.; Baptista, V.I.d.A.; de Oliveira, F. Presence of metalloproteinases 2 and 9 and 8-OHdG in the fibrotic process in skeletal muscle of Mdx mice. Acta Histochem. 2020, 122, 151458. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, S.C.; Hindi, S.M.; Kumar, A.; Marques, M.J. Effects of omega-3 on matrix metalloproteinase-9, myoblast transplantation and satellite cell activation in dystrophin-deficient muscle fibers. Cell Tissue Res. 2017, 369, 591–602. [Google Scholar] [CrossRef] [PubMed]
- Hindi, S.M.; Shin, J.; Ogura, Y.; Li, H.; Kumar, A. Matrix metalloproteinase-9 inhibition improves proliferation and engraftment of myogenic cells in dystrophic muscle of mdx mice. PLoS ONE 2013, 8, e72121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lourbakos, A.; Yau, N.; de Bruijn, P.; Hiller, M.; Kozaczynska, K.; Jean-Baptiste, R.; Reza, M.; Wolterbeek, R.; Koeks, Z.; Ayoglu, B.; et al. Evaluation of serum MMP-9 as predictive biomarker for antisense therapy in Duchenne. Sci. Rep. 2017, 7, 17888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, A.; Monteiro, M.; Barai, A.; Kumar, S.; Sen, S. MMP proteolytic activity regulates cancer invasiveness by modulating integrins. Sci. Rep. 2017, 7, 14219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mengshol, J.A.; Mix, K.S.; Brinckerhoff, C.E. Matrix metalloproteinases as therapeutic targets in arthritic diseases: Bull’s-eye or missing the mark? Arthritis Rheum. 2002, 46, 13–20. [Google Scholar] [CrossRef]
- Winer, A.; Adams, S.; Mignatti, P. Matrix Metalloproteinase Inhibitors in Cancer Therapy: Turning Past Failures Into Future Successes. Mol. Cancer Ther. 2018, 17, 1147–1155. [Google Scholar] [CrossRef] [Green Version]
- Jin, B.; Zhang, L.; Wang, X.; Jin, D. Research on Orientation of Basic Fibroblast Growth Factor with Magnetic Nanoparticles (MNPs) on Regeneration and Recovery of Rats’ Dampened Skeletal Muscle and Expressed Level of Matrix Metalloproteinase. J. Biomed. Nanotechnol. 2022, 18, 557–564. [Google Scholar] [CrossRef]
- Nishimura, T.; Nakamura, K.; Kishioka, Y.; Kato-Mori, Y.; Wakamatsu, J.; Hattori, A. Inhibition of matrix metalloproteinases suppresses the migration of skeletal muscle cells. J. Muscle Res. Cell Motil. 2008, 29, 37–44. [Google Scholar] [CrossRef]
- Ohtake, Y.; Tojo, H.; Seiki, M. Multifunctional roles of MT1-MMP in myofiber formation and morphostatic maintenance of skeletal muscle. J. Cell Sci. 2006, 119, 3822–3832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allen, D.L.; Teitelbaum, D.H.; Kurachi, K. Growth factor stimulation of matrix metalloproteinase expression and myoblast migration and invasion in vitro. Am. J. Physiol. Physiol. 2003, 284, C805–C815. [Google Scholar] [CrossRef] [PubMed]
- Chin, J.R.; Werb, Z. Matrix metalloproteinases regulate morphogenesis, migration and remodeling of epithelium, tongue skeletal muscle and cartilage in the mandibular arch. Development 1997, 124, 1519–1530. [Google Scholar] [CrossRef]
- Zhou, D.; Huang, Z.; Zhu, X.; Hong, T.; Zhao, Y. Combination of endothelial progenitor cells and BB-94 significantly alleviates brain damage in a mouse model of diabetic ischemic stroke. Exp. Ther. Med. 2021, 22, 789. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Wang, G.; Lu, X.; Qiao, P.; Jin, Y.; Yu, J.; Chen, Q.; Wang, H. Elevated matrix metalloproteinase 9 supports peripheral nerve regeneration via promoting Schwann cell migration. Exp. Neurol. 2022, 352, 114020. [Google Scholar] [CrossRef] [PubMed]
- El Fahime, E.; Torrente, Y.; Caron, N.J.; Bresolin, M.D.; Tremblay, J.P. In vivo migration of transplanted myoblasts requires matrix metalloproteinase activity. Exp. Cell Res. 2000, 258, 279–287. [Google Scholar] [CrossRef]
- Pasternak, B.; Aspenberg, P. Metalloproteinases and their inhibitors-diagnostic and therapeutic opportunities in orthopedics. Acta Orthop. 2009, 80, 693–703. [Google Scholar] [CrossRef]
- Oum’hamed, Z.; Garnotel, R.; Josset, Y.; Trenteseaux, C.; Laurent-Maquin, D. Matrix metalloproteinases MMP-2, -9 and tissue inhibitors TIMP-1, -2 expression and secretion by primary human osteoblast cells in response to titanium, zirconia, and alumina ceramics. J. Biomed. Mater. Res. A 2004, 68, 114–122. [Google Scholar] [CrossRef]
- Chen, W.-J.; Lin, I.-H.; Lee, C.-W.; Chen, Y.-F. Aged Skeletal Muscle Retains the Ability to Remodel Extracellular Matrix for Degradation of Collagen Deposition after Muscle Injury. Int. J. Mol. Sci. 2021, 22, 2123. [Google Scholar] [CrossRef]
- Goncharuk, O.; Savosko, S.; Tykhomyrov, A.; Guzyk, M.; Medvediev, V.; Tsymbaliuk, V.; Chaikovsky, Y. Matrix Metalloproteinase-9 is Involved in the Fibrotic Process in Denervated Muscles after Sciatic Nerve Trauma and Recovery. J. Neurol. Surg. Part A Cent. Eur. Neurosurg. 2021. [Google Scholar] [CrossRef]
- Niedecker, A.; Huhn, R.; Ritz-Timme, S.; Mayer, F. Complex challenges of estimating the age and vitality of muscle wounds: A study with matrix metalloproteinases and their inhibitors on animal and human tissue samples. Int. J. Legal Med. 2021, 135, 1843–1853. [Google Scholar] [CrossRef] [PubMed]
- Simões, G.; Pereira, T.; Caseiro, A. Matrix metaloproteinases in vascular pathology. Microvasc. Res. 2022, 143, 104398. [Google Scholar] [CrossRef] [PubMed]
- Lee-Gannon, T.; Jiang, X.; Tassin, T.C.; Mammen, P.P.A. Biomarkers in Duchenne Muscular Dystrophy. Curr. Heart Fail. Rep. 2022, 19, 52–62. [Google Scholar] [CrossRef]
- González-Jamett, A.; Vásquez, W.; Cifuentes-Riveros, G.; Martínez-Pando, R.; Sáez, J.C.; Cárdenas, A.M. Oxidative Stress, Inflammation and Connexin Hemichannels in Muscular Dystrophies. Biomedicines 2022, 10, 507. [Google Scholar] [CrossRef]
- Duan, D.; Goemans, N.; Takeda, S.; Mercuri, E.; Aartsma-Rus, A. Duchenne muscular dystrophy. Nat. Rev. Dis. Prim. 2021, 7, 13. [Google Scholar] [CrossRef]
- Diaz-Canestro, C.; Puspitasari, Y.M.; Liberale, L.; Guzik, T.J.; Flammer, A.J.; Bonetti, N.R.; Wüst, P.; Costantino, S.; Paneni, F.; Akhmedov, A. MMP-2 knockdown blunts age-dependent carotid stiffness by decreasing elastin degradation and augmenting eNOS activation. Cardiovasc. Res. 2022, 118, 2385–2396. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, P.R.; Nascimento, L.D.; Gerlach, R.F.; Rodrigues, K.E.; Prado, A.F. Matrix Metalloproteinase 2 as a Pharmacological Target in Heart Failure. Pharmaceuticals 2022, 15, 920. [Google Scholar] [CrossRef]
- Dahiya, S.; Bhatnagar, S.; Hindi, S.M.; Jiang, C.; Paul, P.K.; Kuang, S.; Kumar, A. Elevated levels of active matrix metalloproteinase-9 cause hypertrophy in skeletal muscle of normal and dystrophin-deficient mdx mice. Hum. Mol. Genet. 2011, 20, 4345–4359. [Google Scholar] [CrossRef]

| Collagen Types | Classes |
|---|---|
| Fibrillar collagen | Type-I, -II, -III, -V, and -XI |
| MACIT- membrane-associated collagens | Type-XIII and -XVII |
| Basement membrane collagen | Type-IV |
| Facit-fibril | Type-IX, -XII, -XIV, -XIX, and -XXI |
| Short chain collagen | Type-VIII and -X |
| Multiplexin collagen | Type-XV and -XVIII |
| Other types | Type-VI, -VII, and -VIII |
| MMPs | Condition/Model | Role/Function | References |
|---|---|---|---|
| Regulation in skeletal muscular tissue | |||
| MMP-14 | Craniofacial dysmorphism, decreased angiogenesis | Significant abnormalities of skeletal muscles growth and function | [35,45] |
| MMP-2 and MMP-14 | Double knock-out mice | Musculoskeletal abnormalities | [46] |
| MMP-1 and MMP-9 | Muscle damage | Induced by TNF- α, phorbol ester, and muscle tissue damaging factors | [51,52] |
| MMP-1 and MMP-9 | Myoblasts of limb muscles | Differential expression (biomarker signature) | [53] |
| MMP-9 | Mouse myoblasts | 30-fold increase in MMP-9 expression with TNF-α | [54] |
| MMP-9 | Cell migration | Enhanced cell migration | [55] |
| TIMP-1 | Cell fusion | Reduced cell migration | |
| MMP-7 | Myotubes formation | Regulates tendency toward myotubes formation | [56,57] |
| MMP-2 and MT1-MMP | Co-transfection of C2C12 cells MMP-2/MT1-MMP | Myonuclei formation | [35,58] |
| Muscle injury and repair | |||
| MMP-2 and MMP-9 | Muscle injury repair in animal models | Musculoskeletal injury and repair process | [59] |
| MMP-2 and MMP-9 | Cardiotoxin-induced injury | Prolonged expression during muscle destruction | [10] |
| MMP-9 | Cellular model (C2C12 cells) | Anagenesis and tissue regeneration | [60] |
| MMP-1 and MMP-13 | Rotator cuff pain (Human patients) | Increased MMP-1 and MMP-13 expression | [61] |
| MMP-9 | Skeletal muscle fibrosis | High expression of MMPs during muscle fibrosis | [62] |
| MMP-2, MMP-9 | Degenerated intramuscular nerves | MMP-9 (degenerated nerves) MMP-2 (neuromuscular junctions junctions) | [52] |
| TIMP-1 | Postmortem-inflicted wounds | Biomarker for wound age | |
| Regulation of muscle tissue inflammation | |||
| MMP-9 | Myopathies | MMP-9 regulates inflammation | [63] |
| MMP-1 and MMP-9 | Myositis, polymyositis, and dermatomyositis patients | High expression of MMP-1 and MMP-9 linked with inflammation | [64] |
| MMP-1, MMP-9 | Polymyositis and Dermatomyositis | Overexpression of MMPs | [64] |
| MMP-14 | Exercise incentive in mice | ECM remodeling at the interface of macrophages and muscle cell | [65] |
| MMP-2, MMP-9 | Rat model of Cardiometabolic disease | Vascular smooth muscle fiber integrity | [66] |
| MMP-2 | Resistance training (RT) | MMP-2 downregulation in quadriceps tendon of rats | [67] |
| Membrane-bound MMP-17 | Repair of inflammatory damage to muscle cells | inflammation-induced damaged smooth muscle cells | [68] |
| Muscular dystrophy | |||
| MMP-2, MMP-9 | Muscular dystrophy | Unable to prevent cleavage of β-dystroglycan degradation | [69] |
| Tissue inhibitors of metalloproteinases (TIMPs) | Duchenne muscular dystrophy | High expression of TIMPs in vivo | [70] |
| MMP-10 | Aged dystrophic mice | The deficiency of MMP-10 caused inflammation in the cardiac and skeletal muscles | [71] |
| MMP-2 and MMP-9 | Muscle fibrosis | Muscle inflammation and oxidative stress | [72] |
| MMP-9 | Mouse model (mdx) of DMD | Reduction in MMP-9 expression by omega-3 caused muscle regeneration | [73] |
| MMP-9 | Dystrophinopathy | Inhibition of MMP-9 led to reduced fibrosis, macrophage infiltration, and decreased necrosis | [62,74] |
| MMP-9 | MD patients | MMP-9 levels are not indicative of treatment response in DMD | [75] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, L.; Bisen, M.; Khan, A.; Kumar, P.; Patel, S.K.S. Role of Matrix Metalloproteinases in Musculoskeletal Diseases. Biomedicines 2022, 10, 2477. https://doi.org/10.3390/biomedicines10102477
Kumar L, Bisen M, Khan A, Kumar P, Patel SKS. Role of Matrix Metalloproteinases in Musculoskeletal Diseases. Biomedicines. 2022; 10(10):2477. https://doi.org/10.3390/biomedicines10102477
Chicago/Turabian StyleKumar, Lokender, Monish Bisen, Azhar Khan, Pradeep Kumar, and Sanjay Kumar Singh Patel. 2022. "Role of Matrix Metalloproteinases in Musculoskeletal Diseases" Biomedicines 10, no. 10: 2477. https://doi.org/10.3390/biomedicines10102477
APA StyleKumar, L., Bisen, M., Khan, A., Kumar, P., & Patel, S. K. S. (2022). Role of Matrix Metalloproteinases in Musculoskeletal Diseases. Biomedicines, 10(10), 2477. https://doi.org/10.3390/biomedicines10102477








