Biophysical Stimulation in Delayed Fracture Healing of Hand Phalanx: A Radiographic Evaluation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Biophysical Treatment
2.3. Radiological Evaluation
3. Statistical Analysis
4. Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Crowe, C.S.; Massenburg, B.B.; Morrison, S.D.; Chang, J.; Friedrich, J.B.; Abady, G.G.; Alahdab, F.; Alipour, V.; Arabloo, J.; Asaad, M.; et al. Global trends of hand and wrist trauma: A systematic analysis of fracture and digit amputation using the Global Burden of Disease 2017 study. Inj. Prev. 2020, 26 (Suppl. S1), i115–i124. [Google Scholar] [CrossRef] [Green Version]
- McDaniel, D.J.; Rehman, U.H. Phalanx Fractures of the Hand; StatPearls Publishing: Tampa, FL, USA, 2021. [Google Scholar]
- Chew, E.M.; Chong, A.K. Hand fractures in children: Epidemiology and misdiagnosis in a tertiary referral hospital. J. Hand Surg. Am. 2012, 37, 1684–1688. [Google Scholar] [CrossRef] [PubMed]
- Stern, P.J. Fractures of the metacarpals and phalanges. In Green’s Operative Hand Surgery, 5th ed.; Green, D.P., Hotchkiss, R.N., Pederson, W.C., Wolfe, S.W., Eds.; Elsevier Churchill Livingstone: London, UK, 2005; pp. 286–294. [Google Scholar]
- Karl, J.W.; Olson, P.R.; Rosenwasser, M.P. The epidemiology of upper extremity fractures in the United States, 2009. J. Orthop. Trauma 2015, 29, e242–e244. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.C.; Alderman, A.K. Replantation of the upper extremity: Indications and outcomes. J. Hand Surg. 2002, 2, 78–94. [Google Scholar] [CrossRef] [Green Version]
- van Onselen, E.B.H.; Karim, R.B.; Hage, J.J.; Ritt, M.J.P.F. Prevalence and distribution of hand fractures. J. Hand Surg. Br. 2003, 28, 491–495. [Google Scholar] [CrossRef]
- Sassoon, A.A.; Fitz-Gibbon, P.D.; Harmsen, W.S.; Moran, S.L. Enchondromas of the hand: Factors affecting recurrence, healing, motion, and malignant transformation. J. Hand Surg. Am. 2012, 37, 1229–1234. [Google Scholar] [CrossRef]
- Duncan, R.W.; Freeland, A.E.; Jabaley, M.E.; Meydrech, E.F. Open hand fractures: An analysis of the recovery of active motion and of complications. J. Hand Surg. 1993, 18, 387–394. [Google Scholar] [CrossRef]
- Marx, R.E. Bone and bone graft healing. Oral Maxillofac. Surg. Clin. N. Am. 2007, 19, 455–466. [Google Scholar] [CrossRef] [PubMed]
- Barton, N.J. Fractures of the phalanges of the hand in children. J. Hand Surg. Am. 1997, 11, 134–143. [Google Scholar] [CrossRef]
- Green, D.P.; Rowland, S.P. Fractures and dislocations in the hand. In Fractures; Rockwood, G.A., Green, D.P., Eds.; J.B. Lippincott Co.: Philadelphia, PA, USA, 1975; pp. 265–344. [Google Scholar]
- Smith, F.L.; Rider, D.L. A study of the healing of one hundred consecutive phalangeal fractures. J. Bone Joint Surg. 1935, 17, 91–109. [Google Scholar]
- Jupiter, J.B.; Koniuch, M.P.; Smith, R.J. The management of delayed union and nonunion of the metacarpals and phalanges. J. Hand Surg. 1985, 10, 457–466. [Google Scholar] [CrossRef]
- van Oosterom, F.J.; Brete, G.J.; Ozdemir, C.; Hovius, S.E. Treatment of phalangeal fractures in severely injured hands. J. Hand Surg. Br. 2001, 26, 108–111. [Google Scholar] [CrossRef]
- Mills, L.; Tsang, J.; Hopper, G.; Keenan, G.; Simpson, A.H.R.W. The multifactorial aetiology of fracture nonunion and the importance of searching for latent infection. Bone Joint Res. 2016, 5, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Brinker, M.R. Nonunions: Evaluation and treatment. In Skeletal Trauma: Basic Science, Management, and Reconstruction, 5th ed.; Browner, B.D., Levine, A.M., Jupiter, J.B., Trafton, P.G., Eds.; Saunders WB: Philadelphia, PA, USA, 2015; Chapter 20; pp. 507–604. [Google Scholar]
- Everding, J.; Roßlenbroich, S.; Raschke, M.J. Pseudarthroses of the long bones. Der Chir. 2018, 89, 73–88. [Google Scholar] [CrossRef] [PubMed]
- Jiao, H.; Xiao, E.; Graves, D.T. Diabetes and its effect on bone and fracture healing. Curr. Osteoporos. Res. 2015, 13, 327–335. [Google Scholar] [CrossRef] [Green Version]
- Patel, R.A.; Wilson, R.F.; Patel, P.A.; Palmer, R.M. The effect of smoking on bone healing: A systematic review. Bone Joint Res. 2013, 14, 102–111. [Google Scholar] [CrossRef]
- Heim, U. The treatment of nonunion in the bones of the hand. In Pseudarthroses and Their Treatment; Chapchal, G., Ed.; George Thieme Publishers: Stuttgart, Germany, 1979; pp. 168–169. [Google Scholar]
- Brown, P.W. The management of phalangeal and metacarpal fractures. Surg. Clin. N. Am. 1973, 53, 1393–1437. [Google Scholar] [CrossRef]
- Littler, J.W. Metacarpal reconstruction. J. Bone Joint Surg. Am. 1947, 29, 723–737. [Google Scholar] [PubMed]
- O’Brien, E.T. Fractures of the metacarpals and phalanges. In Operative Hand Surgery; Green, D.P., Ed.; Churchill Livingstone: New York, NY, USA, 1982; pp. 596–598. [Google Scholar]
- Butler, B.; Neviaser, R.J.; Adams, J.P. Complications of treatment of injuries to the hand. In Complications in Orthopaedic Surgery; Epps, C.P., Ed.; J.B. Lippincott Co.: Philadelphia, PA, USA, 1978; pp. 359–361. [Google Scholar]
- Pulos, N.; Kakar, S. Hand and wrist injuries: Common problems and solutions. Clin. Sports Med. 2018, 37, 217–243. [Google Scholar] [CrossRef]
- Divelbiss, B.J.; Adams, B.D. Electrical and ultrasound stimulation for scaphoid fractures. Hand Clin. 2001, 17, 697–701. [Google Scholar] [CrossRef]
- Pao, V.S.; Chang, J. Scaphoid nonunion: Diagnosis and treatment. Plast. Reconstr. Surg. 2003, 112, 1666–1676. [Google Scholar] [CrossRef] [PubMed]
- Cadossi, R.; Massari, L.; Racine-Avila, J.; Aaron, R.K. Pulsed electromagnetic field stimulation of bone healing and joint preservation: Cellular mechanisms of skeletal response. J. Am. Acad. Orthop. Surg. Glob. Res. Rev. 2020, 4, e1900155. [Google Scholar] [CrossRef] [PubMed]
- Aaron, R.K.; Ciombor, D.M.; Wang, S.; Simon, B. Clinical biophysics: The promotion of skeletal repair by physical forces. Ann. N. Y. Acad. Sci. 2006, 1068, 513–531. [Google Scholar] [CrossRef]
- Midura, R.J.; Ibiwoye, M.O.; Powell, K.A.; Sakai, Y.; Doehring, T.; Grabiner, M.D.; Patterson, T.E.; Zborowski, M.; Wolfman, A. Pulsed electromagnetic field treatments enhance the healing of fibular osteotomies. J. Orthop. Res. 2005, 23, 1035–1046. [Google Scholar] [CrossRef] [PubMed]
- Canè, V.; Botti, P.; Soana, S. Pulsed magnetic fields improve osteoblast activity during the repair of an experimental osseous defect. J. Orthop. Res. 1993, 11, 664–670. [Google Scholar] [CrossRef]
- Massari, L.; Benazzo, F.; Falez, F.; Perugia, D.; Pietrogrande, L.; Setti, S.; Osti, R.; Vaienti, E.; Ruosi, C.; Cadossi, R. Biophysical stimulation of bone and cartilage: State of the art and future perspectives. Int. Orthop. 2019, 43, 539–551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aaron, R.K.; Bolander, M.E. (Eds.) Physical Regulation of Skeletal Repair; American Academy of Orthopaedic Surgeons: Rosemont, IL, USA, 2005; pp. 37–51. [Google Scholar]
- Huang, A.J.; Gemperli, M.P.; Bergthold, L.; Singer, S.S.; Garber, A. Health plans’ coverage determinations for technology-based interventions: The case of electrical bone growth stimulation. Am. J. Manag. Care 2004, 10, 957–962. [Google Scholar] [PubMed]
- Busse, J.W.; Morton, E.; Lacchetti, C.; Guyatt, G.H.; Bhandari, M. Current management of tibial shaft fractures: A survey of 450 Canadian orthopedic trauma surgeons. Acta Orthop. 2008, 79, 689–694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kleinert, H.E.; Verdan, C. Report of the committee on tendon injuries (International Federation of Societies for Surgery of the Hand). J. Hand Surg. Am. 1983, 8, 794–798. [Google Scholar] [CrossRef]
- Jeffcoat, M.K.; Reddy, M.S.M. Advances in measurements of periodontal bone and attachment loss. Monogr. Oral Sci. 2000, 17, 56–72. [Google Scholar]
- Hren, N.I.; Miljavec, M. Spontaneous bone healing of the large bone defects in the mandible. Int. J. Oral Maxillofac. Surg. 2008, 37, 1111–1116. [Google Scholar] [CrossRef]
- McNemar, T.B.; Howell, J.W.; Chang, E. Management of metacarpal fractures. J. Hand Ther. 2003, 16, 143–151. [Google Scholar] [CrossRef]
- Anakwe, R.E.; Aitken, S.A.; Cowie, J.G.; Middleton, S.D.; Court-Brown, C.M. The epidemiology of fracturers of the hand and the influence of social deprivation. J. Hand Surg. Eur. 2011, 36, 62–65. [Google Scholar] [CrossRef]
- de Jonge, J.J.; Kingma, J.; van der Lei, B.; Klassen, H.J. Phalangeal fractures of the hand. An analysis of gender and age-related incidence and aetiology. J. Hand Surg. Br. 1994, 19, 168–170. [Google Scholar] [CrossRef]
- de Jonge, J.J.; Kingma, J.; van der Lei, B.; Klassen, H.J. Fractures of the metacarpals. A retrospective analysis of incidence and aetiology and a review of the English-language literature. Injury 1994, 25, 365–369. [Google Scholar] [CrossRef]
- Weum, S.; Millerjord, S.; de Weerd, L. The distribution of hand fractures at the university hospital of North Norway. J. Plast. Surg. Hand Surg. 2016, 50, 146–150. [Google Scholar] [CrossRef] [Green Version]
- Giustini, M.; de Leo, A.; Acciaro, A.L.; Pajardi, G.; Mano, C.; Voller, F.; Fadda, F.; Fondi, G.; Pitidis, A. Incidence estimates of hand and upper extremity injuries in Italy. Ann. Ist. Super Sanita. 2015, 51, 305–312. [Google Scholar]
- Mills, L.A.; Aitken, S.A.; Simpson, A.H.R.W. The risk of non-union per fracture: Current myths and revised figures from a population of over 4 million adults. Acta Orthop. 2017, 88, 434–439. [Google Scholar] [CrossRef] [Green Version]
- Sauerbier, M.; Krimmer, H.; Hahn, P.; Lanz, U. Dorsal intra-articular end-phalangeal fractures. Handchir. Mikrochir. Plast. Chir. 1999, 31, 82–87. [Google Scholar] [CrossRef]
- Shehadi, S.I. External fixation of metacarpal and phalangeal fractures. J. Hand Surg. Am. 1991, 16, 544–550. [Google Scholar] [CrossRef]
- Strickland, J.W.; Steichen, J.B.; Kleinman, W.B. Phalangeal fractures. Factors influencing digital performance. Orthop. Rev. 1982, 11, 39–50. [Google Scholar]
- Eastaugh-Waring, S.J.; Joslin, C.C.; Hardy, J.R.W.; Cunningham, J.L. Quantification of fracture healing from radiographs using the maximum callus index. Clin. Orthop. Relat. Res. 2009, 467, 1986–1991. [Google Scholar] [CrossRef] [Green Version]
- Whelan, D.B.; Bhandari, M.; McKee, M.D.; Guyatt, G.H.; Kreder, H.J.; Stephen, D.; Schemitsch, E.H. Interobserver and intraobserver variation in the assessment of the healing of tibial fractures after intramedullary fixation. J. Bone Joint Surg. Br. 2002, 84, 15–18. [Google Scholar] [CrossRef]
- Whelan, D.B.; Bhandari, M.; Stephen, D.; Kreder, H.J.; McKee, M.D.; Zdero, R.; Schemitsch, E.H. Development of the radiographic union score for tibial fractures for the assessment of tibial fracture healing after intramedullary fixation. J. Trauma 2010, 68, 629–632. [Google Scholar] [CrossRef]
- Bhandari, M.; Chiavaras, M.; Ayeni, O.; Chakraverrty, R.; Parasu, N.; Choudur, H.; Bains, S.; Sprague, S.; Petrisor, B. Assessment Group for Radiographic Evaluation and Evidence (AGREE) study group (AGREE Investigators Writing Committee). Assessment of radiographic fracture healing in patients with operatively treated femoral neck fractures. J. Orthop. Trauma 2013, 27, e213–e219. [Google Scholar] [CrossRef]
- Frank, T.; Osterhoff, G.; Sprague, S.; Garibaldi, A.; Bhandari, M.; Slobogean, G.P.; FAITH Investigators. The radiographic union score for hip (RUSH) identifies radiographic nonunion of femoral neck fractures. Clin. Orthop. Relat. Res. 2016, 474, 1396–1404. [Google Scholar] [CrossRef] [Green Version]
- Bhandari, M.; Chiavaras, M.M.; Parasu, N.; Choudur, H.; Ayeni, O.; Chakravertty, R.; Bains, S.; Hak, A.; Sprague, S.; Petrisor, B. Radiographic union score for hip substantially improves agreement between surgeons and radiologists. BMC Musculoskelet disord. 2013, 25, 70. [Google Scholar] [CrossRef] [Green Version]
- Patel, S.P.; Anthony, S.G.; Zurakowski, D.; Didolkar, M.M.; Kim, P.S.; Wu, J.S.; Kung, J.W.; Dolan, M.; Rosental, T.D. Radiopgraphic scoring system to evaluate union of distal radius fractures. J. Hand Surg. Am. 2014, 39, 1471–1479. [Google Scholar]
- Yuan, J.; Xin, F.; Jiang, W. Underlying signaling pathways and therapeutic applications of pulsed electromagnetic fields in bone repair. Cell Physiol. Biochem. 2018, 46, 1581–1594. [Google Scholar] [CrossRef]
- Azi, M.L.; Aprato, A.; Santi, I.; Kfuri, M., Jr.; Masse, A.; Joeris, A. Autologous bone graft in the treatment of post-traumatic bone defects: A systematic review and meta-analysis. BMC Musculoskelet. Disord. 2016, 17, 465. [Google Scholar] [CrossRef] [Green Version]
- Chao, E.Y.S.; Inoue, N. Biophysical stimulation of bone fracture repair, regeneration and remodelling. Eur. Cell Mater. 2003, 31, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Sharrard, W.J. Pulsed electromagnetic fields. J. Bone Joint Surg. Br. 1992, 74, 630. [Google Scholar] [CrossRef]
- Bassett, C.A.; Mitchell, S.N.; Gaston, S.R. Treatment of ununited tibial diaphyseal fractures with pulsing electromagnetic fields. J. Bone Joint Surg. Am. 1981, 63, 511–523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colson, D.J.; Browett, J.P.; Fiddian, N.J.; Watson, B. Treatment of delayed- and non-union of fractures using pulsed electromagnetic fields. J. Biomed. Eng. 1988, 10, 301–304. [Google Scholar] [CrossRef]
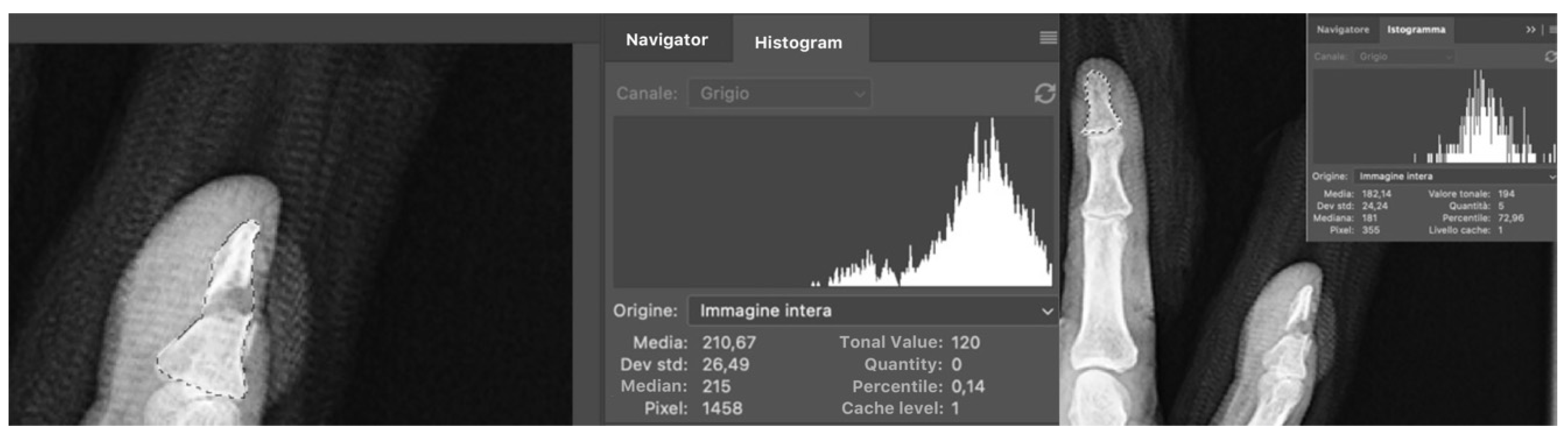



| Defect Tonal Value | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Stimulated | Control | p Value between Groups | |||||||
| Mean | St.Dev | N | p value vs. T1 | Mean | St.Dev. | N | p value vs. T1 | ||
| T1 | 135.2 | 25.2 | 43 | 128.5 | 24.3 | 37 | 0.2300 | ||
| T2 | 141.6 | 26.6 | 33 | 0.0609 | 125.5 | 21.3 | 27 | 0.5495 | 0.0116 |
| T3 | 150.5 | 25.0 | 41 | 0.0033 | 131.6 | 22.3 | 35 | 0.6200 | 0.0008 |
| T6 | 153.1 | 23.3 | 43 | 0.0002 | 125.2 | 32.5 | 36 | 0.6962 | 0.0001 |
| Defect Tonal Value | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Articular Fractures (ART) | Diaphyseal Fractures (DIA) | |||||||||||||
| Stimulated | Control | Stimulated | Control | ART | DIA | |||||||||
| Mean | St.Dev | N | Mean | St.Dev | N | Mean | St.Dev | N | Mean | St.Dev. | N | Stimulated vs. Control | Stimulated vs. Control | |
| T1 | 139.6 | 16.3 | 12 | 116.4 | 31 | 8 | 133.5 | 28.0 | 29 | 133.1 | 21.5 | 27 | 0.0816 | 0.9447 |
| T2 | 142 | 21.6 | 10 | 124.0 | 19.6 | 6 | 141.5 | 28.9 | 23 | 124.2 | 22. | 19 | 0.1142 | 0.0349 |
| T3 | 148.2 | 20.4 | 13 | 126.5 | 25.1 | 8 | 151.6 | 27.1 | 28 | 134.0 | 19.4 | 25 | 0.0599 | 0.0086 |
| T6 | 147.1 | 19.4 | 13 | 114.7 | 37.1 | 8 | 155.7 | 24.6 | 30 | 128.0 | 31.2 | 26 | 0.0468 | 0.0007 |
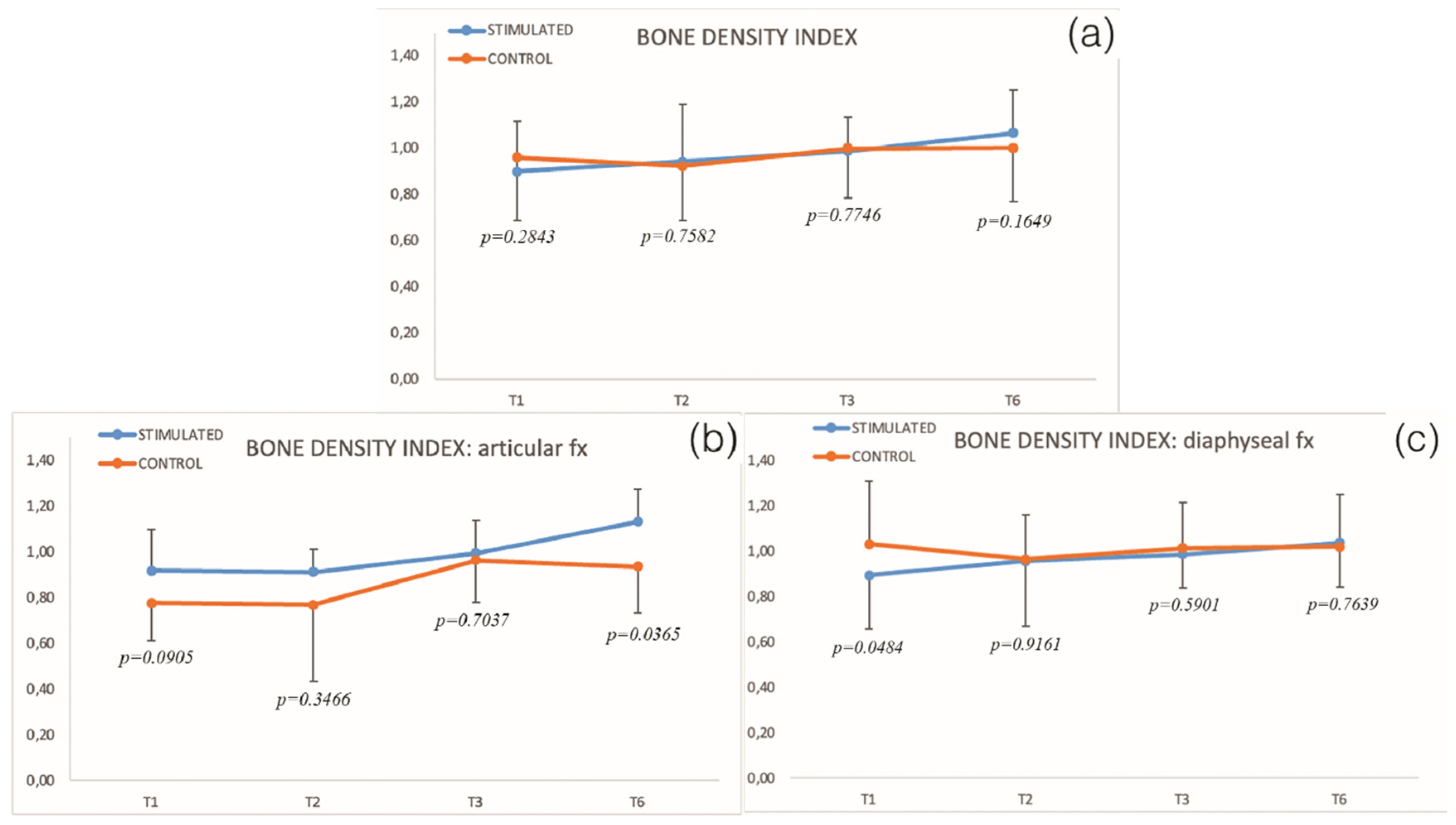
| Relative Bone Density Index | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Stimulated | Control | p Value between Groups | |||||||
| Mean | St.Dev | N | p Value vs. T1 | Mean | St.Dev. | N | p Value vs. T1 | ||
| T1 | 0.90 | 0.22 | 43 | 0.96 | 0.27 | 37 | 0.2843 | ||
| T2 | 0.94 | 0.25 | 33 | 0.5305 | 0.92 | 0.24 | 27 | 0.5197 | 0.7582 |
| T3 | 0.99 | 0.15 | 41 | 0.0431 | 1.00 | 0.21 | 34 | 0.5524 | 0.7746 |
| T6 | 1.06 | 0.19 | 43 | 0.0010 | 1.00 | 0.23 | 35 | 0.4957 | 0.1649 |
| Relative Bone Density Index | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Articular Fractures (ART) | Diaphyseal Fractures (DIA) | |||||||||||||
| Stimulated | Control | Stimulated | Control | ART | DIA | |||||||||
| Mean | St.Dev. | N | Mean | St.Dev. | N | Mean | St.Dev. | N | Mean | St.Dev. | N | Stimulated vs. Control | Stimulated vs. Control | |
| T1 | 0.92 | 0.18 | 12 | 0.78 | 0.17 | 8 | 0.89 | 0.23 | 31 | 1.03 | 0.28 | 27 | 0.0905 | 0.0484 |
| T2 | 0.91 | 0.10 | 10 | 0.77 | 0.33 | 6 | 0.96 | 0.29 | 23 | 0.96 | 0.19 | 19 | 0.3466 | 0.9161 |
| T3 | 0.99 | 0.14 | 13 | 0.96 | 0.18 | 8 | 0.99 | 0.15 | 28 | 1.01 | 0.20 | 24 | 0.7037 | 0.5901 |
| T6 | 1.13 | 0.14 | 13 | 0.94 | 0.20 | 8 | 1.04 | 0.20 | 30 | 1.02 | 0.23 | 25 | 0.0365 | 0.7639 |
| Total Active Motion (TAM) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Stimulated | Control | p Value between Groups | |||||||
| Mean | St.Dev | N | p Value vs. TAM T2 | Mean | St.Dev. | N | p Value vs. TAM T2 | ||
| TAM T2 | 39.2 | 20.1 | 37 | 36.5 | 21.6 | 35 | 0.5883 | ||
| TAM T3 | 45.1 | 17.7 | 35 | 0.0003 | 50.9 | 28.9 | 31 | 0.0003 | 0.3367 |
| TAM T6 | 60.1 | 27.0 | 32 | 0.0000 | 45.7 | 30.0 | 15 | 0.0178 | 0.0534 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Francesco, F.; Gravina, P.; Varagona, S.; Setti, S.; Gigante, A.; Riccio, M. Biophysical Stimulation in Delayed Fracture Healing of Hand Phalanx: A Radiographic Evaluation. Biomedicines 2022, 10, 2519. https://doi.org/10.3390/biomedicines10102519
De Francesco F, Gravina P, Varagona S, Setti S, Gigante A, Riccio M. Biophysical Stimulation in Delayed Fracture Healing of Hand Phalanx: A Radiographic Evaluation. Biomedicines. 2022; 10(10):2519. https://doi.org/10.3390/biomedicines10102519
Chicago/Turabian StyleDe Francesco, Francesco, Pasquale Gravina, Stefano Varagona, Stefania Setti, Antonio Gigante, and Michele Riccio. 2022. "Biophysical Stimulation in Delayed Fracture Healing of Hand Phalanx: A Radiographic Evaluation" Biomedicines 10, no. 10: 2519. https://doi.org/10.3390/biomedicines10102519
APA StyleDe Francesco, F., Gravina, P., Varagona, S., Setti, S., Gigante, A., & Riccio, M. (2022). Biophysical Stimulation in Delayed Fracture Healing of Hand Phalanx: A Radiographic Evaluation. Biomedicines, 10(10), 2519. https://doi.org/10.3390/biomedicines10102519









