Traumatic Brain Injury (TBI) Detection: Past, Present, and Future
Abstract
:1. Introduction
2. Brief Historical Review
3. Conventional TBI Detection Using EEG
3.1. EEG Signal Preprocessing
3.2. TBI Detection Using EEG
4. TBI Detection Using Artificial Intelligence
4.1. Artificial Intelligence
4.2. Machine Learning for TBI Detection
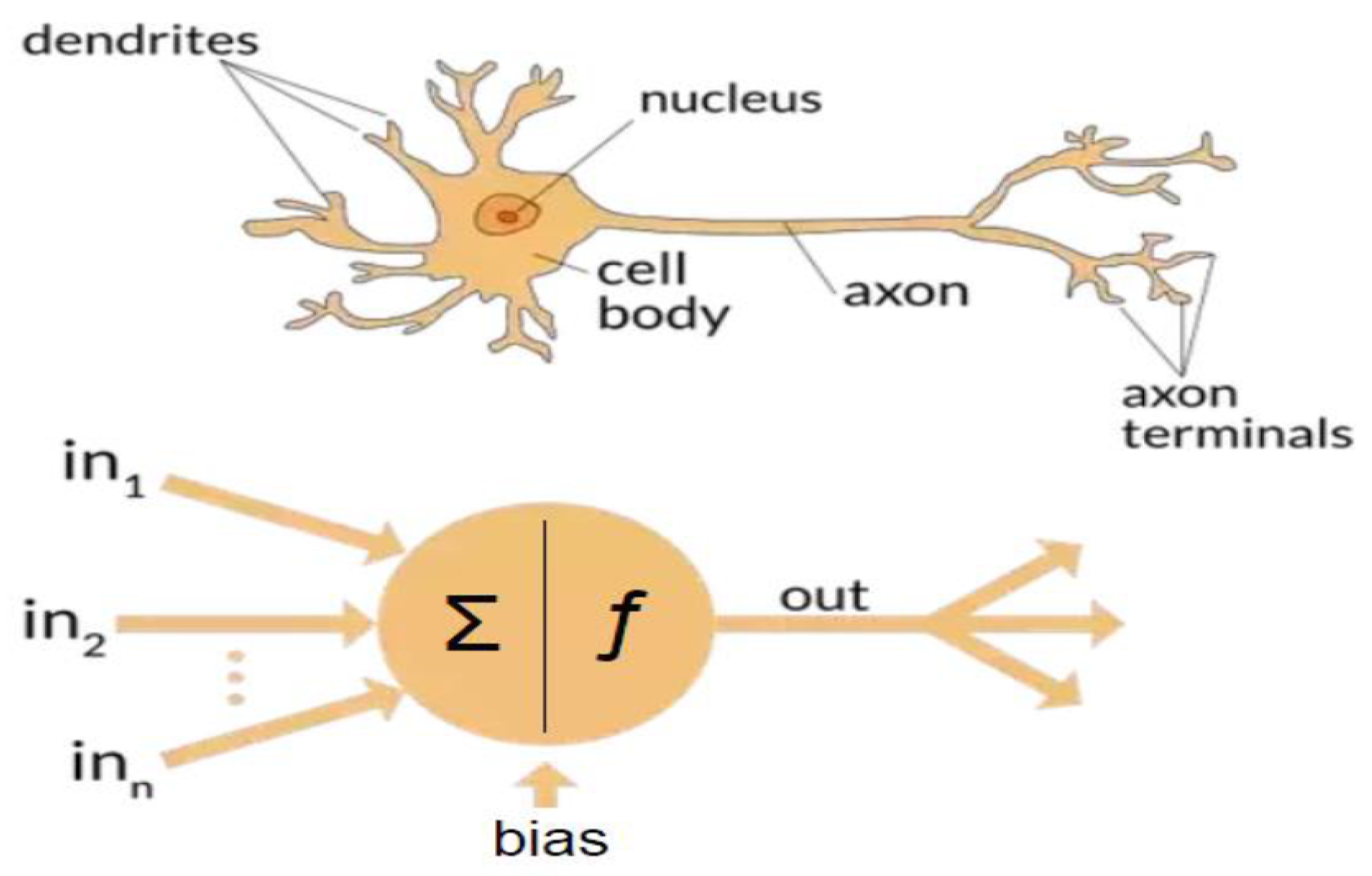
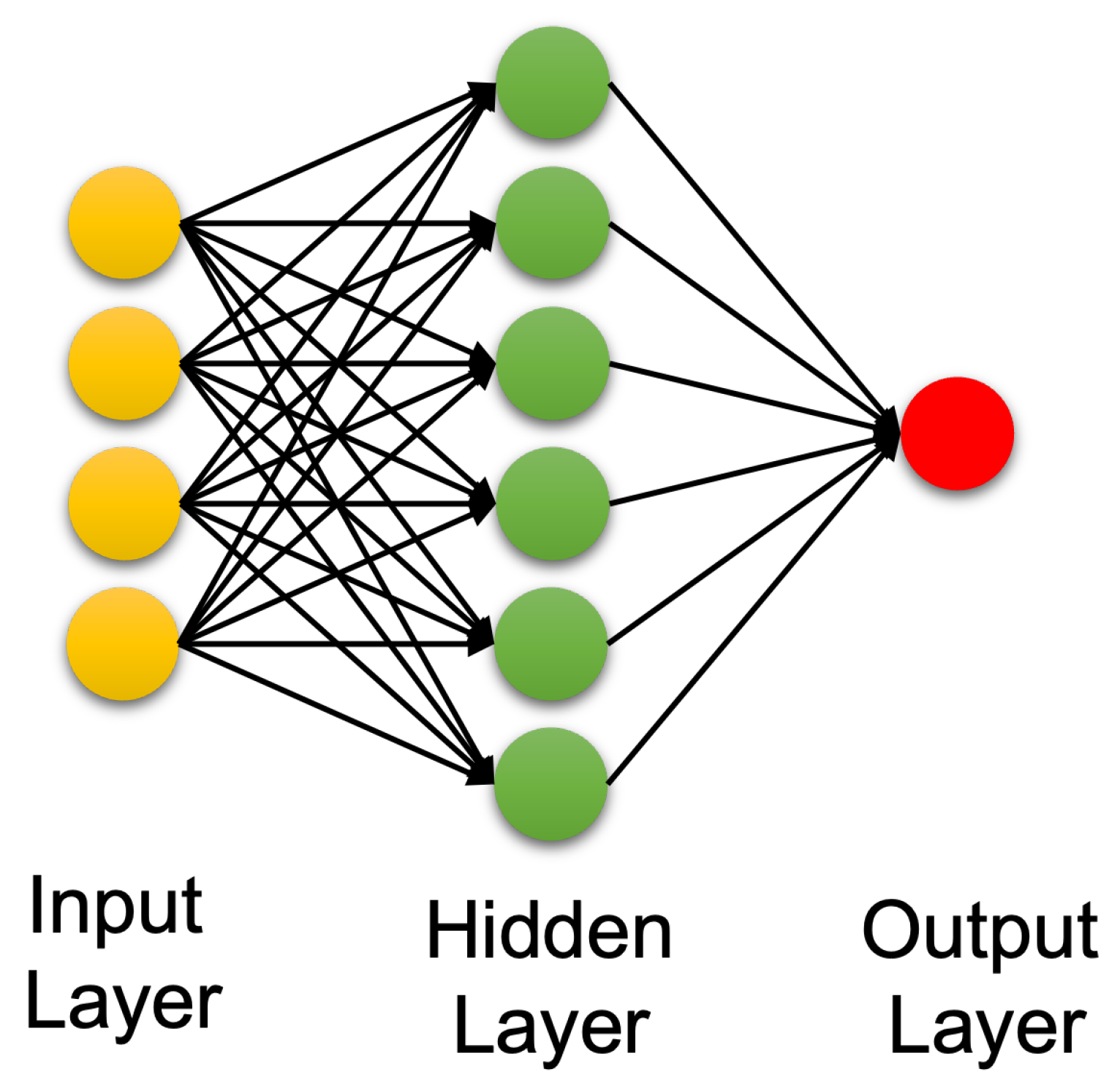
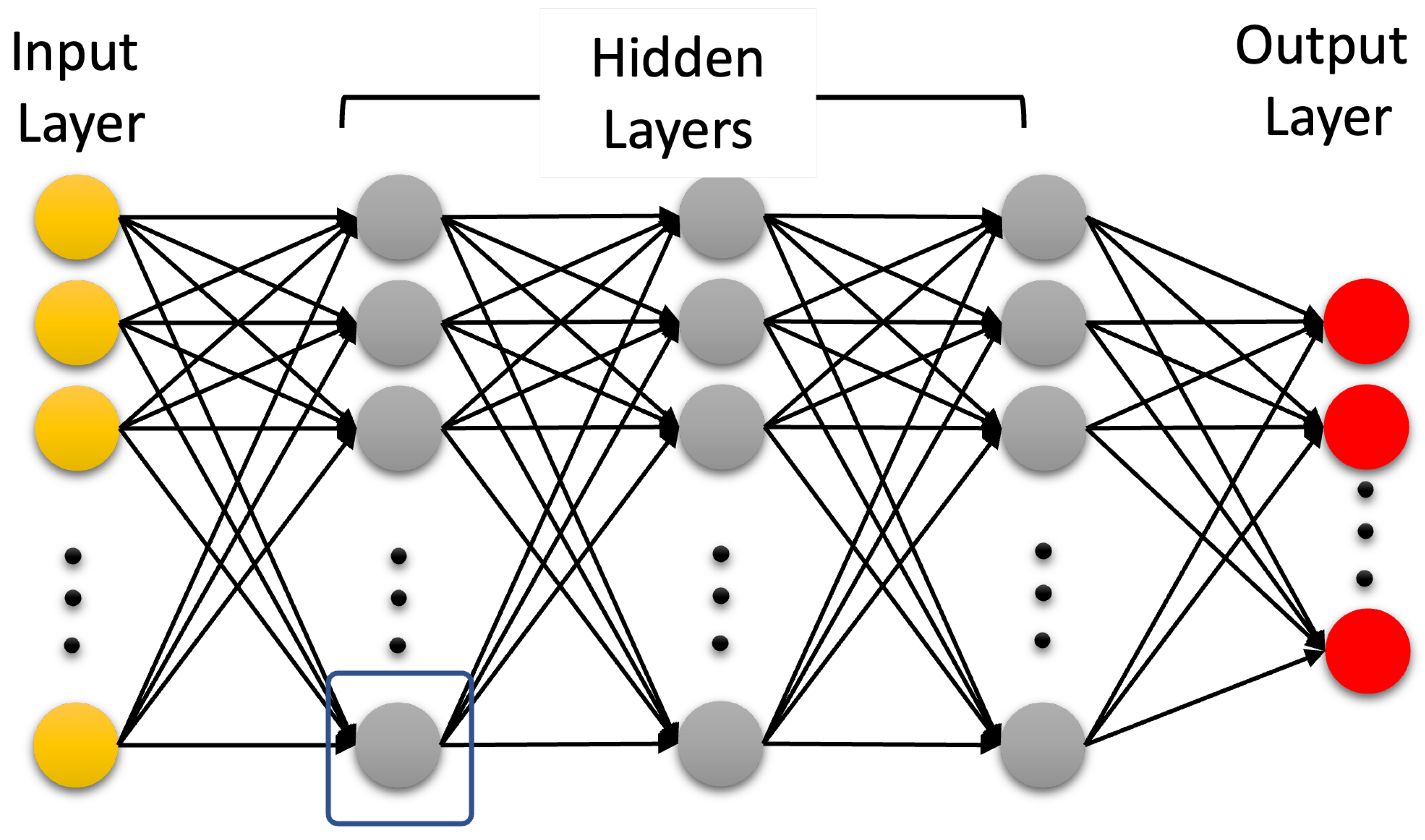
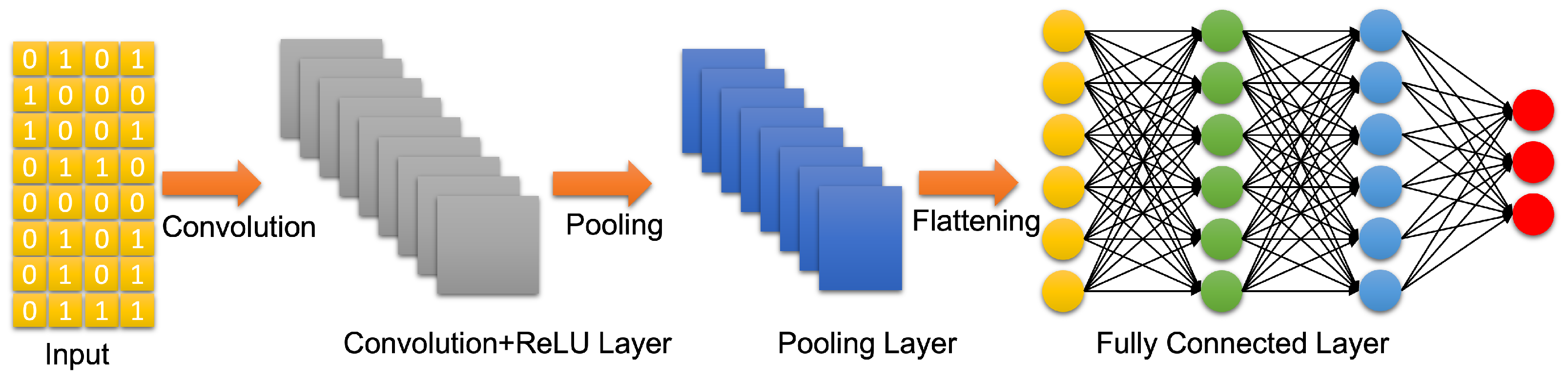
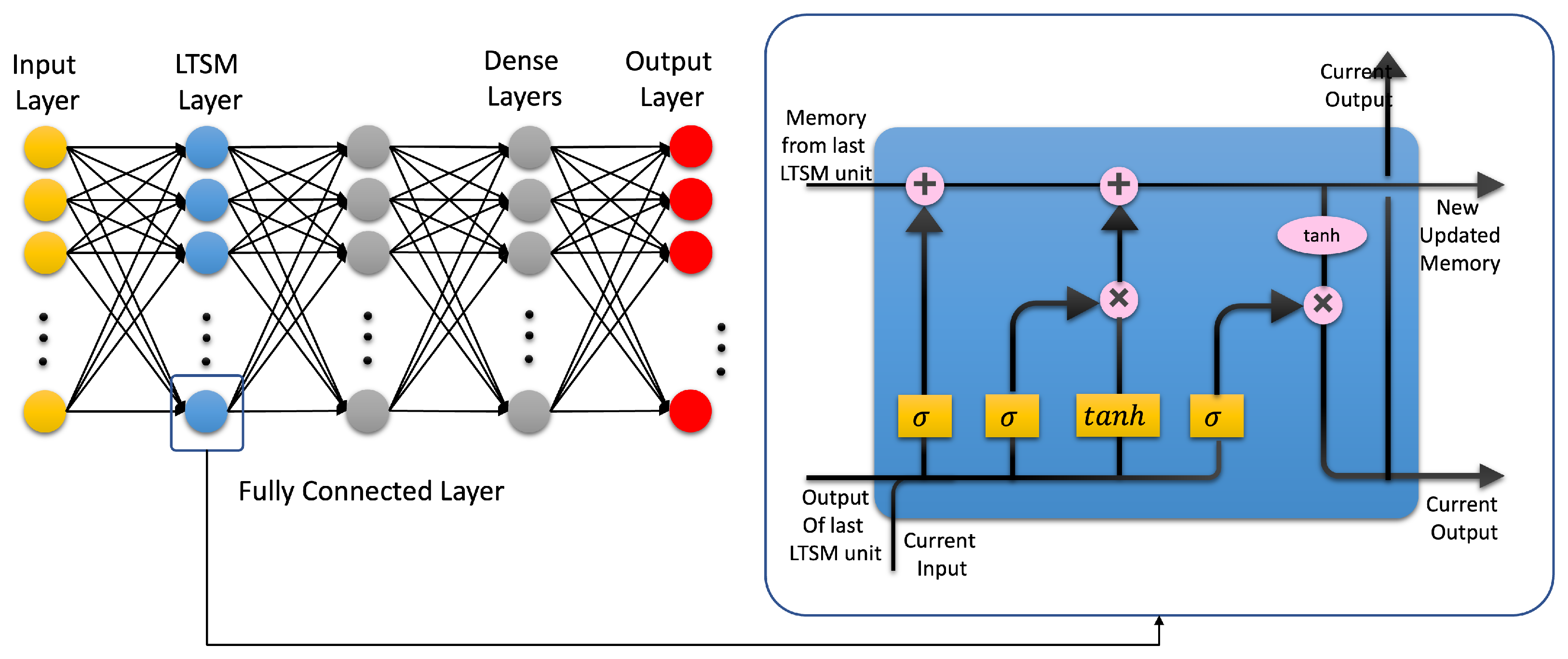
4.3. Artificial Neural Networks: Deep Learning Neural Networks
5. Future of TBI Detection
5.1. Multi Channel EEG Data Collection
5.2. EEG Preprocessing
5.3. Universal qEEG for TBI Detection and Need for Universal Testing
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Miller, J.D.; Murray, L.S.; Teasdale, G.M. Development of a traumatic intracranial hematoma after a “minor” head injury. Neurosurgery 1990, 27, 669–673. [Google Scholar] [CrossRef] [PubMed]
- Kristiansson, H.; Nissborg, E.; Bartek, J., Jr.; Andresen, M.; Reinstrup, P.; Romner, B. Measuring elevated intracranial pressure through noninvasive methods: A review of the literature. J. Neurosurg. Anesthesiol. 2013, 25, 372–385. [Google Scholar] [CrossRef] [PubMed]
- Puffer, R.C.; Yue, J.K.; Mesley, M.; Billigen, J.B.; Sharpless, J.; Fetzick, A.L.; Puccio, A.; Diaz-Arrastia, R.; Okonkwo, D.O. Long-term outcome in traumatic brain injury patients with midline shift: A secondary analysis of the Phase 3 COBRIT clinical trial. J. Neurosurg. 2018, 131, 596–603. [Google Scholar] [CrossRef] [PubMed]
- Dewan, M.C.; Rattani, A.; Gupta, S.; Baticulon, R.E.; Hung, Y.C.; Punchak, M.; Agrawal, A.; Adeleye, A.O.; Shrime, M.G.; Rubiano, A.M.; et al. Estimating the global incidence of traumatic brain injury. J. Neurosurg. 2018, 130, 1080–1097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, G.F.; DePadilla, L.; Xu, L. Costs of nonfatal traumatic brain injury in the United States, 2016. Med. Care 2021, 59, 451–455. [Google Scholar] [CrossRef]
- Agimi, Y.; Regasa, L.E.; Stout, K.C. Incidence of traumatic brain injury in the US Military, 2010–2014. Mil. Med. 2019, 184, e233–e241. [Google Scholar] [CrossRef]
- Dinh, M.M.; Bein, K.; Roncal, S.; Byrne, C.M.; Petchell, J.; Brennan, J. Redefining the golden hour for severe head injury in an urban setting: The effect of prehospital arrival times on patient outcomes. Injury 2013, 44, 606–610. [Google Scholar] [CrossRef]
- Hu, W.; Freudenberg, V.; Gong, H.; Huang, B. The “Golden Hour” and field triage pattern for road trauma patients. J. Saf. Res. 2020, 75, 57–66. [Google Scholar] [CrossRef]
- Polinder, S.; Cnossen, M.C.; Real, R.G.L.; Covic, A.; Gorbunova, A.; Voormolen, D.C.; Master, C.L.; Haagsma, J.A.; Diaz-Arrastia, R.; von Steinbuechel, N. A Multidimensional Approach to Post-concussion Symptoms in Mild Traumatic Brain Injury. Front. Neurol. 2018, 9, 1113. [Google Scholar] [CrossRef] [Green Version]
- Reith, F.; Van den Brande, R.; Synnot, A.; Gruen, R.; Maas, A.I. The reliability of the Glasgow Coma Scale: A systematic review. Intensive Care Med. 2016, 42, 3–15. [Google Scholar] [CrossRef]
- Shan, R.; Szmydynger-Chodobska, J.; Warren, O.U.; Mohammad, F.; Zink, B.J.; Chodobski, A. A new panel of blood biomarkers for the diagnosis of mild traumatic brain injury/concussion in adults. J. Neurotrauma 2016, 33, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Panteliadis, C.P. Historical Overview of Electroencephalography: From Antiquity to the Beginning of the 21st Century. J. Brain Neurol. Disord. 2021, 3, 1–10. [Google Scholar]
- Maiese, A.; Frati, P.; Manetti, A.C.; De Matteis, A.; Di Paolo, M.; La Russa, R.; Turillazzi, E.; Frati, A.; Fineschi, V. Traumatic Internal Carotid Artery Injuries: Do We Need a Screening Strategy? Literature Review, Case Report, and Forensic Evaluation. Curr. Neuropharmacol. 2022, 20, 1752–1773. [Google Scholar] [CrossRef]
- Gudigar, A.; Raghavendra, U.; Hegde, A.; Menon, G.R.; Molinari, F.; Ciaccio, E.J.; Acharya, U.R. Automated detection and screening of traumatic brain injury (TBI) using computed tomography images: A comprehensive review and future perspectives. Int. J. Environ. Res. Public Health 2021, 18, 6499. [Google Scholar]
- Bardin, J.C.; Fins, J.J.; Katz, D.I.; Hersh, J.; Heier, L.A.; Tabelow, K.; Dyke, J.P.; Ballon, D.J.; Schiff, N.D.; Voss, H.U. Dissociations between behavioural and functional magnetic resonance imaging-based evaluations of cognitive function after brain injury. Brain 2011, 134, 769–782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hassan, M.; Wendling, F. Electroencephalography source connectivity: Toward high time/space resolution brain networks. arXiv 2018, arXiv:1801.02549. [Google Scholar] [CrossRef]
- Arciniegas, D.B. Clinical electrophysiologic assessments and mild traumatic brain injury: State-of-the-science and implications for clinical practice. Int. J. Psychophysiol. 2011, 82, 41–52. [Google Scholar] [CrossRef]
- Li, D.; Yang, Z.; Hou, F.; Kang, Q.; Liu, S.; Song, Y.; Gao, Q.; Dong, E. EEG-Based Emotion Recognition with Haptic Vibration by a Feature Fusion Method. IEEE Trans. Instrum. Meas. 2022, 71, 2504111. [Google Scholar] [CrossRef]
- Wu, D.; Xu, Y.; Lu, B.L. Transfer Learning for EEG-Based Brain–Computer Interfaces: A Review of Progress Made Since 2016. IEEE Trans. Cogn. Dev. Syst. 2022, 14, 4–19. [Google Scholar] [CrossRef]
- Khurana, V.; Gahalawat, M.; Kumar, P.; Roy, P.P.; Dogra, D.P.; Scheme, E.; Soleymani, M. A Survey on Neuromarketing Using EEG Signals. IEEE Trans. Cogn. Dev. Syst. 2021, 13, 732–749. [Google Scholar] [CrossRef]
- Congedo, M.; Gouy-Pailler, C.; Jutten, C. On the blind source separation of human electroencephalogram by approximate joint diagonalization of second order statistics. Clin. Neurophysiol. 2008, 119, 2677–2686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mammone, N.; La Foresta, F.; Morabito, F.C. Automatic Artifact Rejection From Multichannel Scalp EEG by Wavelet ICA. IEEE Sens. J. 2012, 12, 533–542. [Google Scholar] [CrossRef]
- Zou, Y.; Nathan, V.; Jafari, R. Automatic Identification of Artifact-Related Independent Components for Artifact Removal in EEG Recordings. IEEE J. Biomed. Health Inform. 2016, 2, 73–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mannan, M.M.N.; Jeong, M.Y.; Kamran, M.A. Hybrid ICA—Regression: Automatic identification and removal of ocular artifacts from electroencephalographic signals. Front. Hum. Neurosci. 2016, 10, 193. [Google Scholar] [CrossRef] [PubMed]
- Mannan, M.M.N.; Kamran, M.A.; Jeong, M.Y. Identification and Removal of Physiological Artifacts From Electroencephalogram Signals: A Review. IEEE Access 2018, 6, 30630–30652. [Google Scholar] [CrossRef]
- Dai, C.; Wang, J.; Xie, J.; Li, W.; Gong, Y.; Li, Y. Removal of ECG Artifacts From EEG Using an Effective Recursive Least Square Notch Filter. IEEE Access 2019, 7, 158872–158880. [Google Scholar] [CrossRef]
- Chang, C.Y.; Hsu, S.H.; Pion-Tonachini, L.; Jung, T.P. Evaluation of Artifact Subspace Reconstruction for Automatic Artifact Components Removal in Multi-Channel EEG Recordings. IEEE Trans. Biomed. Eng. 2020, 67, 1114–1121. [Google Scholar] [CrossRef]
- Robbins, K.A.; Touryan, J.; Mullen, T.; Kothe, C.; Bigdely-Shamlo, N. How Sensitive Are EEG Results to Preprocessing Methods: A Benchmarking Study. IEEE Trans. Neural Syst. Rehabil. Eng. 2020, 28, 1081–1090. [Google Scholar] [CrossRef]
- Blanco, S.; Garcia, H.; Quiroga, R.Q.; Romanelli, L.; Rosso, O.A. Stationarity of the EEG series. IEEE Eng. Med. Biol. Mag. 1995, 14, 395–399. [Google Scholar] [CrossRef] [Green Version]
- Penttonen, M.; Buzsáki, G. Natural logarithmic relationship between brain oscillators. Thalamus Relat. Syst. 2003, 2, 145–152. [Google Scholar] [CrossRef]
- Nuwer, M.R.; Hovda, D.A.; Schrader, L.M.; Vespa, P.M. Routine and quantitative EEG in mild traumatic brain injury. Clin. Neurophysiol. 2005, 116, 2001–2025. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.K.; Rastegarnia, A.; Yang, Z. Methods for artifact detection and removal from scalp EEG: A review. Neurophysiol. Clin. Neurophysiol. 2016, 46, 287–305. [Google Scholar] [CrossRef] [PubMed]
- Albert, B.; Zhang, J.; Noyvirt, A.; Setchi, R.; Sjaaheim, H.; Velikova, S.; Strisland, F. Automatic EEG processing for the early diagnosis of traumatic brain injury. Procedia Comput. Sci. 2016, 96, 703–712. [Google Scholar] [CrossRef] [Green Version]
- Lewine, J.D.; Plis, S.; Ulloa, A.; Williams, C.; Spitz, M.; Foley, J.; Paulson, K.; Davis, J.; Bangera, N.; Snyder, T.; et al. Quantitative EEG biomarkers for mild traumatic brain injury. J. Clin. Neurophysiol. 2019, 36, 298–305. [Google Scholar] [CrossRef]
- Kostarelos, F.; MacNamee, C.; Mullane, B. A hardware implementation of a qEEG-based discriminant function for brain injury detection. In Proceedings of the 2021 IEEE Biomedical Circuits and Systems Conference (BioCAS), Berlin, Germany, 7–9 October 2021; pp. 1–6. [Google Scholar]
- Sjaaheim, H.; Albert, B.; Setchi, R.; Noyvirt, A.; Strisland, F. A portable medical system for the early diagnosis and treatment of traumatic brain injury. In Proceedings of the 2014 IEEE International Conference on Systems, Man, and Cybernetics (SMC), San Diego, CA, USA, 5–8 October 2014; pp. 2529–2534. [Google Scholar]
- Rapp, P.E.; Keyser, D.O.; Albano, A.; Hernandez, R.; Gibson, D.B.; Zambon, R.A.; Hairston, W.D.; Hughes, J.D.; Krystal, A.; Nichols, A.S. Traumatic Brain Injury Detection Using Electrophysiological Methods. Front. Hum. Neurosci. 2015, 9, 11. [Google Scholar] [CrossRef] [Green Version]
- Dingle, A.A.; Jones, R.D.; Carroll, G.J.; Fright, W.R. A multistage system to detect epileptiform activity in the EEG. IEEE Trans. Biomed. Eng. 1993, 40, 1260–1268. [Google Scholar] [CrossRef]
- Chamanzar, A.; George, S.; Venkatesh, P.; Chamanzar, M.; Shutter, L.; Elmer, J.; Grover, P. An algorithm for automated, noninvasive detection of cortical spreading depolarizations based on EEG simulations. IEEE Trans. Biomed. Eng. 2018, 66, 1115–1126. [Google Scholar] [CrossRef]
- Fisher, J.A.; Huang, S.; Ye, M.; Nabili, M.; Wilent, W.B.; Krauthamer, V.; Myers, M.R.; Welle, C.G. Real-time detection and monitoring of acute brain injury utilizing evoked electroencephalographic potentials. IEEE Trans. Neural Syst. Rehabil. Eng. 2016, 24, 1003–1012. [Google Scholar] [CrossRef]
- Jo, T. Machine Learning Foundations; Springer: Berlin/Heidelberg, Germany, 2021. [Google Scholar]
- Alzubi, J.; Nayyar, A.; Kumar, A. Machine learning from theory to algorithms: An overview. Proc. J. Phys. Conf. Ser. 2018, 142, 012012. [Google Scholar] [CrossRef]
- Hastie, T.; Tibshirani, R.; Friedman, J.H.; Friedman, J.H. The Elements of Statistical Learning: Data Mining, Inference, and Prediction; Springer: Berlin/Heidelberg, Germany, 2009; Volume 2. [Google Scholar]
- Sutton, R.S.; Barto, A.G. Reinforcement Learning: An Introduction; MIT Press: Cambridge, MA, USA, 2018. [Google Scholar]
- Hosseini, M.P.; Hosseini, A.; Ahi, K. A review on machine learning for EEG signal processing in bioengineering. IEEE Rev. Biomed. Eng. 2020, 14, 204–218. [Google Scholar] [CrossRef]
- Rothmann, M.; Porrmann, M. A Survey of Domain-Specific Architectures for Reinforcement Learning. IEEE Access 2022, 10, 13753–13767. [Google Scholar] [CrossRef]
- Roh, Y.; Heo, G.; Whang, S.E. A survey on data collection for machine learning: A big data-ai integration perspective. IEEE Trans. Knowl. Data Eng. 2019, 33, 1328–1347. [Google Scholar] [CrossRef]
- Jordan, M.I.; Mitchell, T.M. Machine learning: Trends, perspectives, and prospects. Science 2015, 349, 255–260. [Google Scholar] [CrossRef]
- Bonaccorso, G. Machine Learning Algorithms; Packt Publishing Ltd.: Birmingham, UK, 2017. [Google Scholar]
- Zhou, L.; Pan, S.; Wang, J.; Vasilakos, A.V. Machine learning on big data: Opportunities and challenges. Neurocomputing 2017, 237, 350–361. [Google Scholar] [CrossRef] [Green Version]
- L’heureux, A.; Grolinger, K.; Elyamany, H.F.; Capretz, M.A. Machine learning with big data: Challenges and approaches. IEEE Access 2017, 5, 7776–7797. [Google Scholar] [CrossRef]
- Cao, C.; Tutwiler, R.L.; Slobounov, S. Automatic classification of athletes with residual functional deficits following concussion by means of EEG signal using support vector machine. IEEE Trans. Neural Syst. Rehabil. Eng. 2008, 16, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.Q.; Abdullah, M.Z.; Abdullah, J.M.; Azman, A.; Ibrahim, H. Screening of moderate traumatic brain injury from power feature of resting state electroencephalography using support vector machine. In Proceedings of the 2019 2nd International Conference on Electronics and Electrical Engineering Technology, Penang, Malaysia, 25–27 September 2019; pp. 99–103. [Google Scholar]
- Schmid, E.; Fan, Y.; Chi, T.; Golanov, E.; Regnier-Golanov, A.S.; Austerman, R.J.; Podell, K.; Cherukuri, P.; Bentley, T.; Steele, C.T.; et al. Review of wearable technologies and machine learning methodologies for systematic detection of mild traumatic brain injuries. J. Neural Eng. IOP Publ. 2021, 18, 041006. [Google Scholar] [CrossRef]
- Vivaldi, N.; Caiola, M.; Solarana, K.; Ye, M. Evaluating performance of eeg data-driven machine learning for traumatic brain injury classification. IEEE Trans. Biomed. Eng. 2021, 68, 3205–3216. [Google Scholar] [CrossRef]
- Noor, N.S.E.M.; Ibrahim, H. Machine learning algorithms and quantitative electroencephalography predictors for outcome prediction in traumatic brain injury: A systematic review. IEEE Access 2020, 8, 102075–102092. [Google Scholar] [CrossRef]
- Gravesteijn, B.Y.; Nieboer, D.; Ercole, A.; Lingsma, H.F.; Nelson, D.; Van Calster, B.; Steyerberg, E.W.; Åkerlund, C.; Amrein, K.; Andelic, N.; et al. Machine learning algorithms performed no better than regression models for prognostication in traumatic brain injury. J. Clin. Epidemiol. 2020, 122, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Nti, I.K.; Quarcoo, J.A.; Aning, J.; Fosu, G.K. A mini-review of machine learning in big data analytics: Applications, challenges, and prospects. Big Data Min. Anal. 2022, 5, 81–97. [Google Scholar] [CrossRef]
- Hussein, S.; Kandel, P.; Bolan, C.W.; Wallace, M.B.; Bagci, U. Lung and Pancreatic Tumor Characterization in the Deep Learning Era: Novel Supervised and Unsupervised Learning Approaches. IEEE Trans. Med. Imaging 2019, 38, 1777–1787. [Google Scholar] [CrossRef]
- Roy, S.; Menapace, W.; Oei, S.; Luijten, B.; Fini, E.; Saltori, C.; Huijben, I.; Chennakeshava, N.; Mento, F.; Sentelli, A.; et al. Deep Learning for Classification and Localization of COVID-19 Markers in Point-of-Care Lung Ultrasound. IEEE Trans. Med. Imaging 2020, 39, 2676–2687. [Google Scholar] [CrossRef]
- White, J.; Kameneva, T.; McCarthy, C. Vision Processing for Assistive Vision: A Deep Reinforcement Learning Approach. IEEE Trans. Hum. Mach. Syst. 2022, 52, 123–133. [Google Scholar] [CrossRef]
- Wei, X.S.; Song, Y.Z.; Mac Aodha, O.; Wu, J.; Peng, Y.; Tang, J.; Yang, J.; Belongie, S. Fine-Grained Image Analysis with Deep Learning: A Survey. IEEE Trans. Pattern Anal. Mach. Intell. 2021. [Google Scholar] [CrossRef] [PubMed]
- Khodayar, M.; Liu, G.; Wang, J.; Khodayar, M.E. Deep learning in power systems research: A review. CSEE J. Power Energy Syst. 2021, 7, 209–220. [Google Scholar] [CrossRef]
- Tang, C.; Yu, C.; Gao, Y.; Chen, J.; Yang, J.; Lang, J.; Liu, C.; Zhong, L.; He, Z.; Lv, J. Deep learning in nuclear industry: A survey. Big Data Min. Anal. 2022, 5, 140–160. [Google Scholar] [CrossRef]
- Hale, A.T.; Stonko, D.P.; Lim, J.; Guillamondegui, O.D.; Shannon, C.N.; Patel, M.B. Using an artificial neural network to predict traumatic brain injury. J. Neurosurgery Pediatr. PED 2019, 23, 219–226. [Google Scholar] [CrossRef] [Green Version]
- Gong, S.; Xing, K.; Cichocki, A.; Li, J. Deep learning in EEG: Advance of the last ten-year critical period. IEEE Trans. Cogn. Dev. Syst. 2021, 14, 348–365. [Google Scholar] [CrossRef]
- Rashed-Al-Mahfuz, M.; Moni, M.A.; Uddin, S.; Alyami, S.A.; Summers, M.A.; Eapen, V. A deep convolutional neural network method to detect seizures and characteristic frequencies using epileptic electroencephalogram (EEG) data. IEEE J. Transl. Eng. Health Med. 2021, 9, 2000112. [Google Scholar] [CrossRef] [PubMed]
- Hussein, R.; Palangi, H.; Ward, R.K.; Wang, Z.J. Optimized deep neural network architecture for robust detection of epileptic seizures using EEG signals. Clin. Neurophysiol. 2019, 130, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Başar, E. Containing original chapters. In Chaos in Brain Function; Basar, E., Bullock, T.H., Eds.; Brain Dynamics; Springer Science & Business Media: New York, NY, USA, 2012. [Google Scholar]
- Frei, M.G.; Osorio, I. Intrinsic time-scale decomposition: Time–frequency–energy analysis and real-time filtering of non-stationary signals. Proc. R. Soc. A Math. Phys. Eng. Sci. 2007, 463, 321–342. [Google Scholar] [CrossRef]
- Šeba, P. Random matrix analysis of human EEG data. Phys. Rev. Lett. 2003, 91, 198104. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alouani, A.T.; Elfouly, T. Traumatic Brain Injury (TBI) Detection: Past, Present, and Future. Biomedicines 2022, 10, 2472. https://doi.org/10.3390/biomedicines10102472
Alouani AT, Elfouly T. Traumatic Brain Injury (TBI) Detection: Past, Present, and Future. Biomedicines. 2022; 10(10):2472. https://doi.org/10.3390/biomedicines10102472
Chicago/Turabian StyleAlouani, Ali T., and Tarek Elfouly. 2022. "Traumatic Brain Injury (TBI) Detection: Past, Present, and Future" Biomedicines 10, no. 10: 2472. https://doi.org/10.3390/biomedicines10102472
APA StyleAlouani, A. T., & Elfouly, T. (2022). Traumatic Brain Injury (TBI) Detection: Past, Present, and Future. Biomedicines, 10(10), 2472. https://doi.org/10.3390/biomedicines10102472






