Vericiguat in Heart Failure: Characteristics, Scientific Evidence and Potential Clinical Applications
Abstract
1. Introduction
2. Mechanism of Drug Action
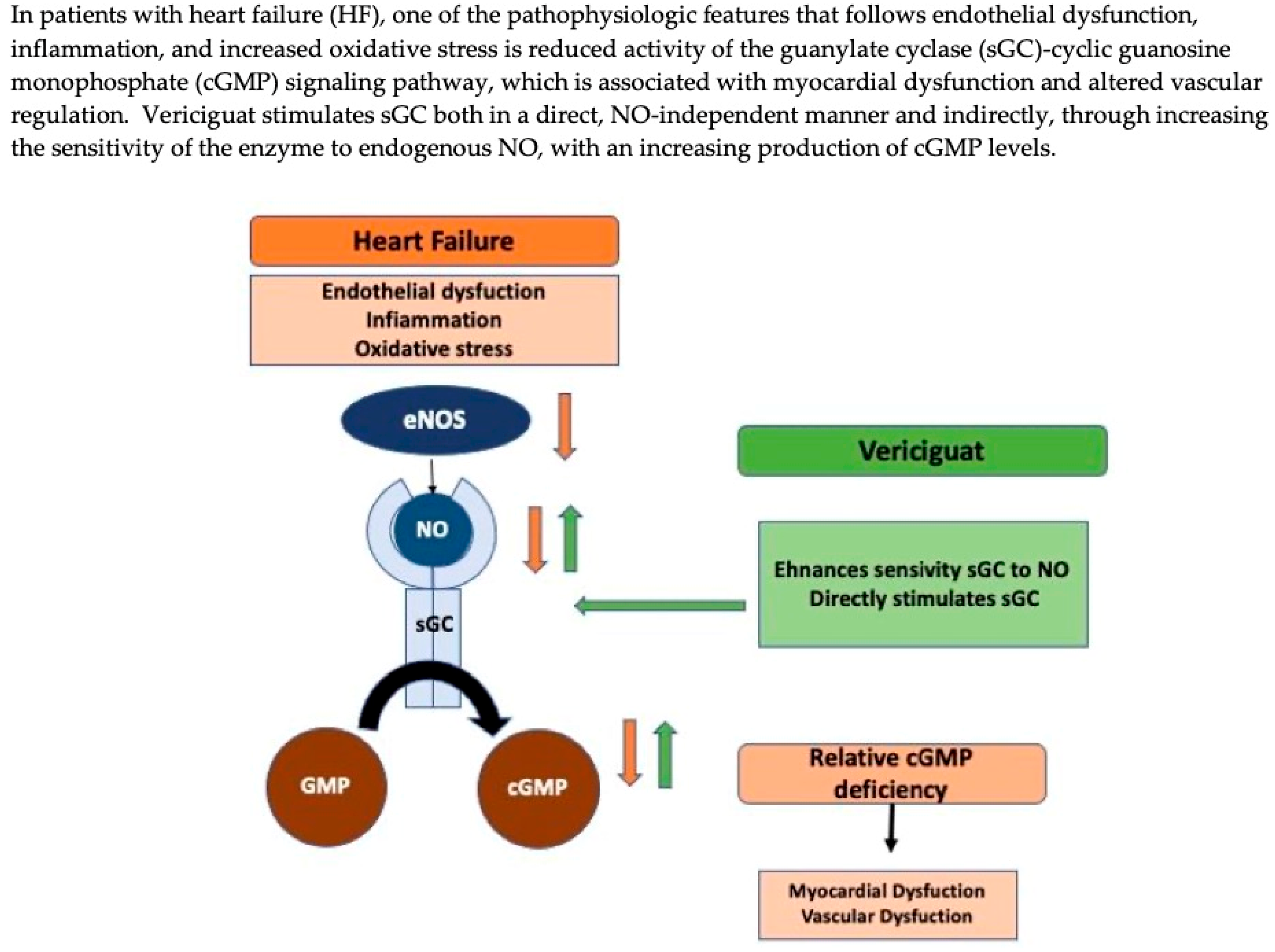
3. Pharmacological Proprieties of Vericiguat
4. Main HF Clinical Trials
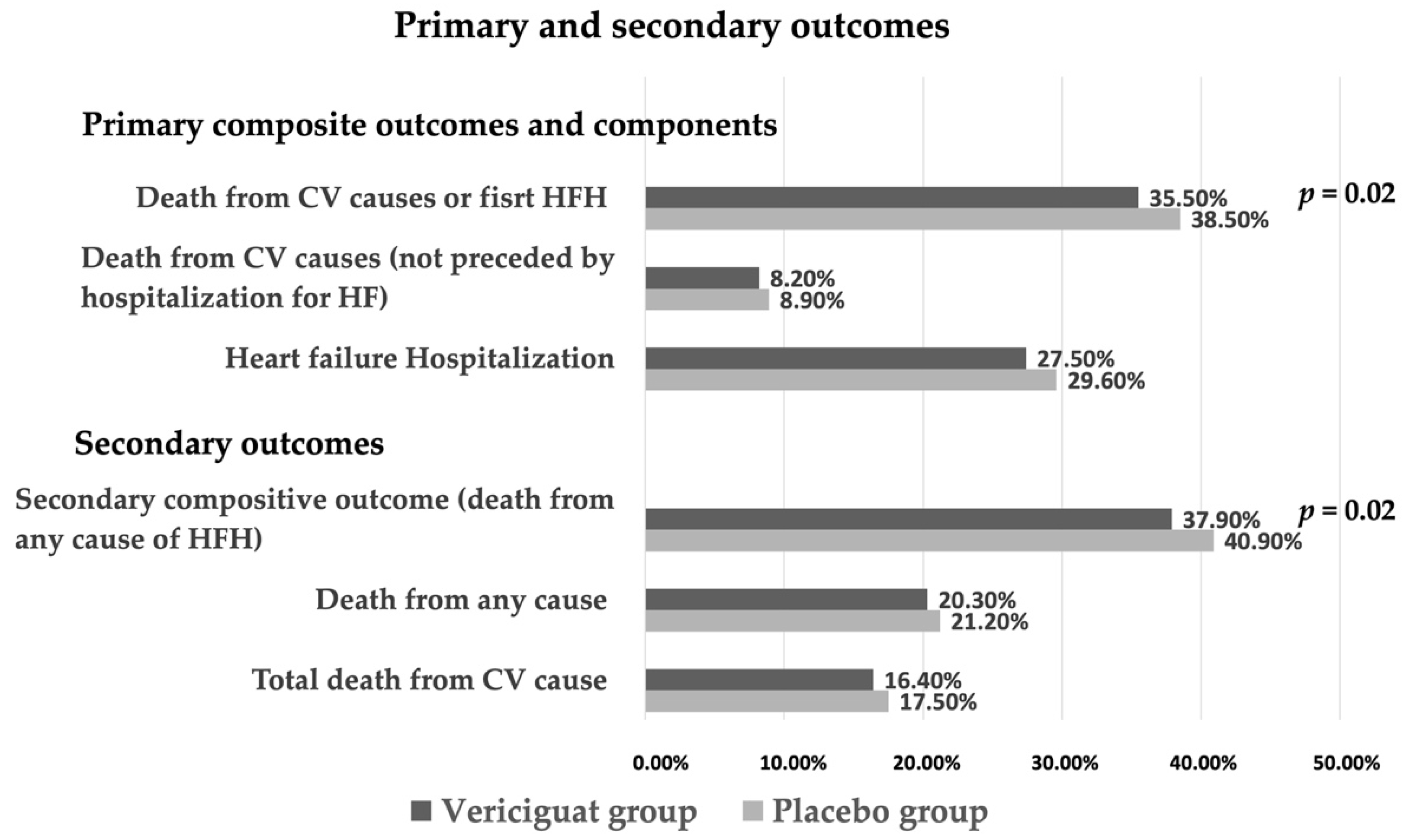
4.1. Vericiguat in HFrEF
4.2. Vericiguat in HFpEF
5. Future Perspectives
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Groenewegen, A.; Rutten, F.H.; Mosterd, A.; Hoes, A.W. Epidemiology of heart failure. Eur. J. Heart Fail. 2020, 22, 1342–1356. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2022, 79, e263–e421. [Google Scholar] [CrossRef]
- Yancy, C.W.; Jessup, M.; Bozkurt, B.; Butler, J.; Casey, D.E., Jr.; Drazner, M.H.; Fonarow, G.C.; Geraci, S.A.; Horwich, T.; Januzzi, J.L.; et al. 2013 ACCF/AHA guideline for the management of heart failure: A report of the American college of cardiology foundation/american heart association task force on practice guidelines. J. Am. Coll. Cardiol. 2013, 62, e147–e239. [Google Scholar] [CrossRef] [PubMed]
- Aimo, A.; Castiglione, V.; Vergaro, G.; Panichella, G.; Senni, M.; Lombardi, C.M.; Emdin, M. The place of vericiguat in the landscape of treatment for heart failure with reduced ejection fraction. Heart Fail. Rev. 2021, 27, 1165–1171. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Hui, N.; Tian, L.; Liang, C.; Zhang, J.; Liu, J.; Wang, J.; Ren, X.; Xie, X.; Wang, K. Development of vericiguat: The first soluble guanylate cyclase (sGC) stimulator launched for heart failure with reduced ejection fraction (HFrEF). Biomed. Pharmacother. 2022, 149, 112894. [Google Scholar] [CrossRef] [PubMed]
- Emdin, M.; Aimo, A.; Castiglione, V.; Vergaro, G.; Georgiopoulos, G.; Saccaro, L.F.; Lombardi, C.M.; Passino, C.; Cerbai, E.; Metra, M.; et al. Targeting Cyclic Guanosine Monophosphate to Treat Heart Failure: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2020, 76, 1795–1807. [Google Scholar] [CrossRef]
- Kassis-George, H.; Verlinden, N.J.; Fu, S.; Kanwar, M. Vericiguat in Heart Failure with a Reduced Ejection Fraction: Patient Selection and Special Considerations. Ther. Clin. Risk Manag. 2022, 18, 315–322. [Google Scholar] [CrossRef]
- Evgenov, O.V.; Pacher, P.; Schmidt, P.M.; Haskó, G.; Schmidt, H.H.; Stasch, J.P. NO-independent stimulators and activators of soluble guanylate cyclase: Discovery and therapeutic potential. Nat. Rev. Drug Discov. 2006, 5, 755–768. [Google Scholar] [CrossRef]
- Gheorghiade, M.; Marti, C.N.; Sabbah, H.N.; Roessig, L.; Greene, S.J.; Böhm, M.; Burnett, J.C.; Campia, U.; Cleland, J.G.F.; Collins, S.P.; et al. Soluble guanylate cyclase: A potential therapeutic target for heart failure. Heart Fail. Rev. 2013, 18, 123–134. [Google Scholar] [CrossRef]
- Burnett, J.C. Vericiguat—Another Victory for Targeting Cyclic GMP in Heart Failure. N. Engl. J. Med. 2020, 382, 1952–1953. [Google Scholar] [CrossRef] [PubMed]
- Mattia, A. Vericiguat in heart failure—Who benefits the most? Cardiovasc. Med. 2021, 24, w10049. [Google Scholar] [CrossRef]
- Nougué, H.; Pezel, T.; Picard, F.; Sadoune, M.; Arrigo, M.; Beauvais, F.; Launay, J.; Cohen-Solal, A.; Vodovar, N.; Logeart, D. Effects of sacubitril/valsartan on neprilysin targets and the metabolism of natriuretic peptides in chronic heart failure: A mechanistic clinical study. Eur. J. Heart Fail. 2019, 21, 598–605. [Google Scholar] [CrossRef] [PubMed]
- McMurray, J.J.; Packer, M.; Desai, A.S.; Gong, J.; Lefkowitz, M.P.; Rizkala, A.R.; Rouleau, J.L.; Shi, V.C.; Solomon, S.D.; Swedberg, K.; et al. Angiotensin–Neprilysin Inhibition versus Enalapril in Heart Failure. N. Engl. J. Med. 2014, 371, 993–1004. [Google Scholar] [CrossRef] [PubMed]
- Boettcher, M.; Gerisch, M.; Lobmeyer, M.; Besche, N.; Thomas, D.; Gerrits, M.; Lemmen, J.; Mueck, W.; Radtke, M.; Becker, C. Metabolism and Pharmacokinetic Drug–Drug Interaction Profile of Vericiguat, A Soluble Guanylate Cyclase Stimulator: Results From Preclinical and Phase I Healthy Volunteer Studies. Clin. Pharmacokinet. 2020, 59, 1407–1418. [Google Scholar] [CrossRef]
- Boettcher, M.; Thomas, D.; Mueck, W.; Loewen, S.; Arens, E.; Yoshikawa, K.; Becker, C. Safety, pharmacodynamic and pharmacokinetic characterization of vericiguat: Results from six phase I studies in healthy subjects. Eur. J. Clin. Pharmacol. 2021, 77, 527–537. [Google Scholar] [CrossRef]
- AIFA. Summary of Product Characteristics. 8 December 2021. Available online: https://farmaci.agenziafarmaco.gov.it/aifa/servlet/PdfDownloadServlet?pdfFileName=footer_000689_049614_RCP.pdf&sys=m0b1l3 (accessed on 8 December 2021).
- Gheorghiade, M.; Greene, S.J.; Butler, J.; Filippatos, G.; Lam, C.S.; Maggioni, A.P.; Ponikowski, P.; Shah, S.J.; Solomon, S.D.; Kraigher-Krainer, E.; et al. Effect of vericiguat, a soluble guanylate cyclase stimulator, on natriuretic peptide levels in patients withworsening chronic heart failure and reduced ejection fraction the SOCRATES-PRESERVED randomized trial. J. Am. Med. Assoc. 2015, 314, 2251–2262. [Google Scholar] [CrossRef]
- Armstrong, P.W.; Pieske, B.; Anstrom, K.J.; Ezekowitz, J.; Hernandez, A.F.; Butler, J.; Lam, C.S.; Ponikowski, P.; Voors, A.A.; Jia, G.; et al. Vericiguat in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2020, 382, 1883–1893. [Google Scholar] [CrossRef]
- Lombardi, C.M.; Cimino, G.; Pagnesi, M.; Dell’Aquila, A.; Tomasoni, D.; Ravera, A.; Inciardi, R.; Carubelli, V.; Vizzardi, E.; Nodari, S.; et al. Vericiguat for Heart Failure with Reduced Ejection Fraction. Curr. Cardiol. Rep. 2021, 23, 144. [Google Scholar] [CrossRef]
- Pieske, B.; Butler, J.; Filippatos, G.; Lam, C.; Maggioni, A.P.; Ponikowski, P.; Shah, S.; Solomon, S.; Kraigher-Krainer, E.; Samano, E.T.; et al. Rationale and design of the soluble guanylate cyclase stimulator in heart failure studies (SOCRATES). Eur. J. Heart Fail. 2014, 16, 1026–1038. [Google Scholar] [CrossRef]
- Armstrong, P.W.; Roessig, L.; Patel, M.J.; Anstrom, K.J.; Butler, J.; Voors, A.A.; Lam, C.S.; Ponikowski, P.; Temple, T.; Pieske, B.; et al. A Multicenter, Randomized, Double-Blind, Placebo-Controlled Trial of the Efficacy and Safety of the Oral Soluble Guanylate Cyclase Stimulator: The VICTORIA Trial. JACC Heart Fail. 2018, 6, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Pieske, B.; Patel, M.J.; Westerhout, C.M.; Anstrom, K.J.; Butler, J.; Ezekowitz, J.; Hernandez, A.F.; Koglin, J.; Lam, C.S.; Ponikowski, P.; et al. Baseline features of the VICTORIA (Vericiguat Global Study in Subjects with Heart Failure with Reduced Ejection Fraction) trial. Eur. J. Heart Fail. 2019, 21, 1596–1604. [Google Scholar] [CrossRef] [PubMed]
- McMurray, J.J.V.; Solomon, S.D.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Bělohlávek, J.; et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2019, 381, 1995–2008. [Google Scholar] [CrossRef]
- Butler, J.; Anstrom, K.J.; Armstrong, P.W. Comparing the Benefit of Novel Therapies across Clinical Trials: Insights from the VICTORIA Trial. Circulation 2020, 142, 717–719. [Google Scholar] [CrossRef] [PubMed]
- González-Juanatey, J.R.; Anguita-Sánchez, M.; Bayes-Genís, A.; Comín-Colet, J.; García-Quintana, A.; Recio-Mayoral, A.; Zamorano-Gómez, J.L.; Cepeda-Rodrigo, J.M.; Manzano, L. Vericiguat in Heart Failure: From Scientific Evidence to Clinical Practice. Rev. Clínica Española 2022, 222, 359–369. Available online: www.elsevier.es/rce (accessed on 23 April 2022). [CrossRef]
- Aimo, A.; Pateras, K.; Stamatelopoulos, K.; Bayes-Genis, A.; Lombardi, C.M.; Passino, C.; Emdin, M.; Georgiopoulos, G. Relative Efficacy of Sacubitril-Valsartan, Vericiguat and SGLT2 Inhibitors in Heart Failure with Reduced Ejection Fraction: A Systematic Review and Network Meta-Analysis. Cardiovasc. Drugs Ther. 2021, 35, 1067–1076. [Google Scholar] [CrossRef] [PubMed]
- Voors, A.A.; Mulder, H.; Reyes, E.; Cowie, M.R.; Lassus, J.; Hernandez, A.F.; Ezekowitz, J.A.; Butler, J.; O’Connor, C.M.; Koglin, J.; et al. Renal function and the effects of vericiguat in patients with worsening heart failure with reduced ejection fraction: Insights from the VICTORIA (Vericiguat Global Study in Subjects with HFrEF) trial. Eur. J. Heart Fail. 2021, 23, 1313–1321. [Google Scholar] [CrossRef]
- Ezekowitz, J.A.; O’Connor, C.M.; Troughton, R.W.; Alemayehu, W.G.; Westerhout, C.M.; Voors, A.A.; Butler, J.; Lam, C.S.; Ponikowski, P.; Emdin, M.; et al. N-Terminal Pro-B-Type Natriuretic Peptide and Clinical Outcomes: Vericiguat Heart Failure With Reduced Ejection Fraction Study. JACC Heart Fail. 2020, 8, 931–939. [Google Scholar] [CrossRef]
- Pieske, B.; Maggioni, A.P.; Lam, C.S.; Pieske-Kraigher, E.; Filippatos, G.; Butler, J.; Ponikowski, P.; Shah, S.J.; Solomon, S.D.; Scalise, A.V.; et al. Vericiguat in patients with worsening chronicheart failure and preserved ejection fraction:results of the SOluble guanylate CyclasestimulatoR in heArT failurE patientS withPRESERVED EF (SOCRATES-PRESERVED)study. Eur. Heart J. 2017, 38, 1128–1131. [Google Scholar] [CrossRef]
- Armstrong, P.W.; Lam, C.S.P.; Anstrom, K.J.; Ezekowitz, J.; Hernandez, A.F.; O’Connor, C.M.; Pieske, B.; Ponikowski, P.; Shah, S.J.; Solomon, S.D.; et al. Effect of Vericiguat vs. Placebo on Quality of Life in Patients With Heart Failure and Preserved Ejection Fraction. JAMA 2020, 324, 1512. [Google Scholar] [CrossRef]
- Filippatos, G.; Maggioni, A.P.; Lam, C.S.P.; Pieske-Kraigher, E.; Butler, J.; Spertus, J.; Ponikowski, P.; Shah, S.J.; Solomon, S.D.; Scalise, A.-V.; et al. Patient-reported outcomes in the SOluble guanylate Cyclase stimulatoR in heArT failurE patientS with PRESERVED ejection fraction (SOCRATES-PRESERVED) study. Eur. J. Heart Fail. 2017, 19, 782–791. [Google Scholar] [CrossRef] [PubMed]
- Greene, S.J.; Gheorghiade, M.; Borlaug, B.A.; Pieske, B.; Vaduganathan, M.; Burnett, J.C.; Roessig, L.; Stasch, J.; Solomon, S.D.; Paulus, W.J.; et al. The cGMP signaling pathway as a therapeutic target in heart failure with preserved ejection fraction. J. Am. Heart Assoc. 2013, 2, e000536. [Google Scholar] [CrossRef] [PubMed]
- Screever, E.M.; Meijers, W.C.; van Veldhuisen, D.J.; de Boer, R.A. New developments in the pharmacotherapeutic management of heart failure in elderly patients: Concerns and considerations. Expert Opin. Pharmacother. 2017, 18, 645–655. [Google Scholar] [CrossRef] [PubMed][Green Version]
| Inclusion Criteria | Main Exclusion Criteria | |
|---|---|---|
| SOCRATES-REDUCED [18] |
|
|
| VICTORIA [19] |
|
|
| Trial | Inclusion Criteria | Patients | Treatment | Results |
|---|---|---|---|---|
| SOCRATES-REDUCED [18] |
| 456 (351 patients completed treatment) | Vericiguat (1.25 mg, 2.5 mg, 5 mg, 10 mg daily) vs. placebo |
|
| VICTORIA [19] |
| 5050 (1175 discontinued trial regime but included in the analysis) | Vericiguat (1.25 mg, 2.5 mg, 5 mg, 10 mg daily; 10-mg target dose in 89.2%) vs. placebo |
|
| Characteristic | Number of Patients (Vericiguat/Placebo) | Hazard Ratio 0.5–1.0 (Better Vericiguat) 1.0–1.5 (Better Placebo) | 95% Confidence Interval | |
|---|---|---|---|---|
| Age | ||||
| <65 yr | 290/348 | 0.81 | (0.70–0.95) | |
| ≥65 yr | 607/624 | 0.94 | (0.84–1.06) | |
| <75 yr | 579/669 | 0.84 | (0.74–0.94) | |
| ≥75 yr | 318/303 | 1.04 | (0.88–1.21) | |
| Index event | ||||
| IV diuretics < 3 months ago | 96/120 | 0.78 | (0.60–1.02) | |
| HFH < 3 months ago | 660/701 | 0.93 | (0.84–1.04) | |
| HFH 3–6 months ago | 141/151 | 0.85 | (0.67–1.07) | |
| Baseline NYHA class | ||||
| I–II | 445/484 | 0.91 | (0.80–1.04) | |
| III–IV | 451/487 | 0.87 | (0.77–0.99) | |
| Use of sacubitril/valsartan | ||||
| Yes | 134/153 | 0.88 | (0.70–1.11) | |
| NO | 760/818 | 0.90 | (0.81–0.99) | |
| Baseline eGFR | ||||
| ≤30 mL/min/1.73 m2 | 143/128 | 1.06 | (0.83–1.34) | |
| >30 to ≤60 mL/min/1.73 m2 | 392/455 | 0.84 | (0.73–0.96) | |
| >60 mL/min/1.73 m2 | 346/372 | 0.92 | (0.80–1.07) | |
| Baseline NT-proBNP | ||||
| Quartile 1 (≤1556.0 pg/mL) | 128/161 | 0.78 | (0.62–0.99) | |
| Quartile 2 (>1556.0 to ≤2816.0 pg/mL) | 165/201 | 0.73 | (0.60–0.90) | |
| Quartile 3 (>2816.0 to ≤5314.0 pg/mL) | 213/257 | 0.82 | (0.69–0.99) | |
| Quartile 4 (>5314.0 pg/mL) | 355/302 | 1.16 | (0.99–1.35) | |
| Baseline LVEF | ||||
| <35% | 637/704 | 0.88 | (0.79–0.97) | |
| ≥35% | 255/265 | 0.96 | (0.81–1.14) | |
| <40% | 773/851 | 0.88 | (0.80–0.97) | |
| ≥40% | 119-117 | 1.05 | (0.81–1.36) | |
| Trial | Inclusion Criteria | Patients | Treatment | Results |
|---|---|---|---|---|
| SOCRATES-PRESERVED [30] |
| 477 (325 patients completed treatment) | Vericiguat fixed-dose treatment arms (1.25 mg or 2.5 mg) and vericiguat up-titrated treatment arms (2.5–5 mg, 2.5–10 mg daily) vs. placebo |
|
| VITALITY [31] |
| 789 (761 included in primary analysis) | Vericiguat (up-titrated to 15 mg or 10 mg) vs. placebo |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vannuccini, F.; Campora, A.; Barilli, M.; Palazzuoli, A. Vericiguat in Heart Failure: Characteristics, Scientific Evidence and Potential Clinical Applications. Biomedicines 2022, 10, 2471. https://doi.org/10.3390/biomedicines10102471
Vannuccini F, Campora A, Barilli M, Palazzuoli A. Vericiguat in Heart Failure: Characteristics, Scientific Evidence and Potential Clinical Applications. Biomedicines. 2022; 10(10):2471. https://doi.org/10.3390/biomedicines10102471
Chicago/Turabian StyleVannuccini, Francesca, Alessandro Campora, Maria Barilli, and Alberto Palazzuoli. 2022. "Vericiguat in Heart Failure: Characteristics, Scientific Evidence and Potential Clinical Applications" Biomedicines 10, no. 10: 2471. https://doi.org/10.3390/biomedicines10102471
APA StyleVannuccini, F., Campora, A., Barilli, M., & Palazzuoli, A. (2022). Vericiguat in Heart Failure: Characteristics, Scientific Evidence and Potential Clinical Applications. Biomedicines, 10(10), 2471. https://doi.org/10.3390/biomedicines10102471





