Waist-to-Hip Ratio and Inflammatory Parameters Are Associated with Risk of Non-Alcoholic Fatty Liver Disease in Patients with Morbid Obesity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Study Participants
2.2. Clinical and Biochemical Parameters Evaluated
2.3. Predictors of Hepatic Fibrosis and Steatosis
- ▪
- FLI score: FLI = ey/(1 + ey) × 100, where y = 0.953 × ln(triglycerides, mg/dL) + 0.139 × BMI, kg/m2 + 0.718 × ln(GGT, U/L) + 0.053 × waist circumference, cm–15.745. FLI scores < 30 indicate low risk of hepatic steatosis, 30–60 intermediate risk, and ≥60 high risk [18].
- ▪
- BARD score: BMI ≥ 28 = 1 point; AST/ALT ratio ≥ 0.8 = 2 points, presence of diabetes = 1 point. Low fibrosis risk patients score 0–1 points and higher risk patients score 2–4 points [6].
2.4. Outcomes and Statistical Analysis
3. Results
3.1. Baseline Population Characteristics
3.2. Association between FLI and BARD and Anthropometric Parameters
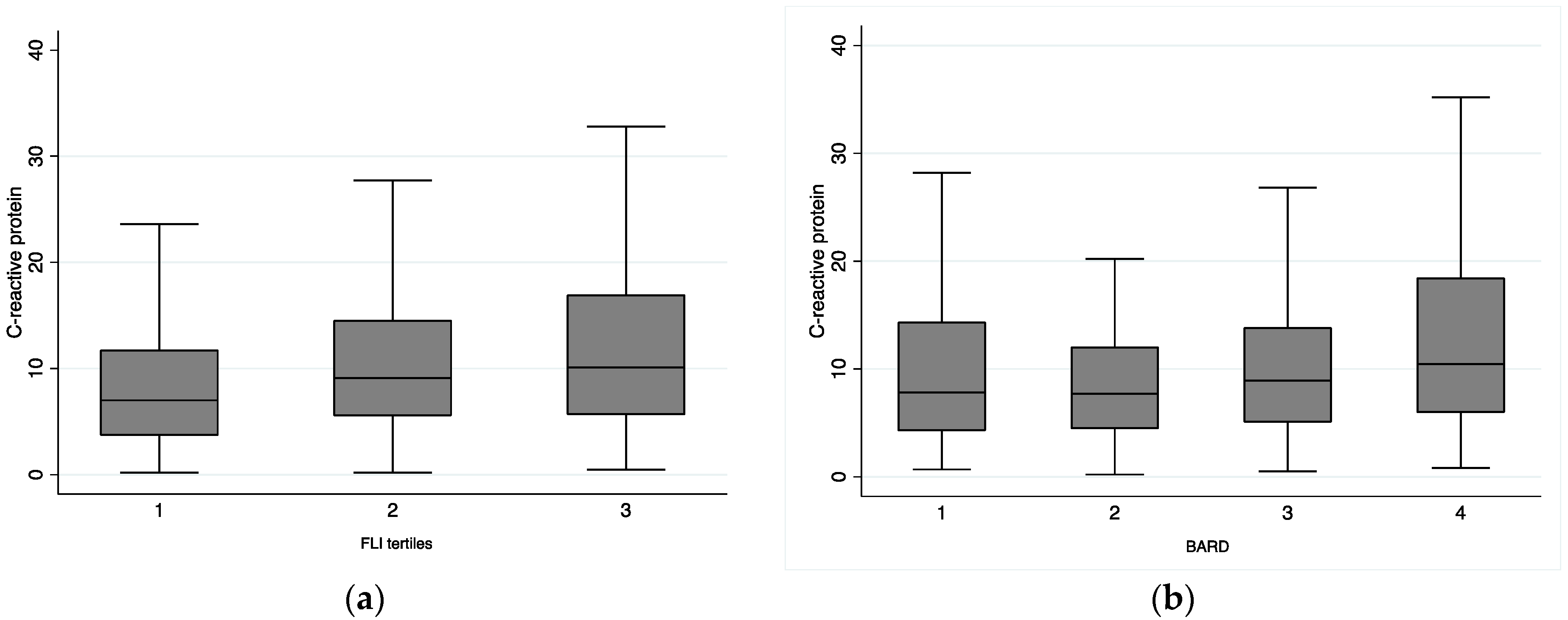
3.3. Association between FLI and BARD and Inflammatory Parameters
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sayiner, M.; Koenig, A.; Henry, L.; Younossi, Z.M. Epidemiology of Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis in the United States and the Rest of the World. Clin. Liver Dis. 2016, 20, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Feng, R.N.; Du, S.S.; Wang, C.; Li, Y.C.; Liu, L.Y.; Guo, F.C.; Sun, C.-H. Lean-non-alcoholic fatty liver disease increases risk for metabolic disorders in a normal weight Chinese population. World J. Gastroenterol. 2014, 20, 17932–17940. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.F.; Zeng, X.; Zou, Z.Y.; Tang, W.; Guo, Y.B.; Yuan, Z.L.; Shi, P.M.; Tan, Y.; Song, Y.; Shi, Y.Q.; et al. The presence of NAFLD in nonobese subjects increased the risk of metabolic abnormalities than obese subjects without NAFLD: A population-based cross-sectional study. Hepatobiliary Surg Nutr. 2021, 10, 811–824. [Google Scholar] [CrossRef] [PubMed]
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Charlton, M.; Cusi, K.; Rinella, M.; Harrison, S.A.; Brunt, E.M.; Sanyal, A.J. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018, 67, 328–357. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef]
- Cheah, M.C.; McCullough, A.J.; Goh, G.B. Current Modalities of Fibrosis Assessment in Non-alcoholic Fatty Liver Disease. J. Clin. Transl. Hepatol. 2017, 5, 261–271. [Google Scholar] [CrossRef]
- Blond, E.; Disse, E.; Cuerq, C.; Drai, J.; Valette, P.J.; Laville, M.; Thivolet, C.; Simon, C.; Caussy, C. EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease in severely obese people: Do they lead to over-referral? Diabetologia 2017, 60, 1218–1222. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver; European Association for the Study of Diabete; European Association for the Study of Obesity. EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J. Hepatol. 2016, 64, 1388–1402. [Google Scholar] [CrossRef]
- Bhaskaran, K.; Dos-Santos-Silva, I.; Leon, D.A.; Douglas, I.J.; Smeeth, L. Association of BMI with overall and cause-specific mortality: A population-based cohort study of 3.6 million adults in the UK. Lancet Diabetes Endocrinol. 2018, 6, 944–953. [Google Scholar] [CrossRef]
- Zembic, A.; Eckel, N.; Stefan, N.; Baudry, J.; Schulze, M.B. An Empirically Derived Definition of Metabolically Healthy Obesity Based on Risk of Cardiovascular and Total Mortality. JAMA Netw. Open 2021, 4, e218505. [Google Scholar] [CrossRef]
- April-Sanders, A.K.; Rodriguez, C.J. Metabolically Healthy Obesity Redefined. JAMA Netw. Open 2021, 4, e218860. [Google Scholar] [CrossRef] [PubMed]
- Huxley, R.; Mendis, S.; Zheleznyakov, E.; Reddy, S.; Chan, J. Body mass index, waist circumference and waist:hip ratio as predictors of cardiovascular risk—A review of the literature. Eur. J. Clin. Nutr. 2010, 64, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Rohm, T.V.; Meier, D.T.; Olefsky, J.M.; Donath, M.Y. Inflammation in obesity, diabetes, and related disorders. Immunity 2022, 55, 31–55. [Google Scholar] [CrossRef] [PubMed]
- Colca, J.R.; Scherer, P.E. The metabolic syndrome, thiazolidinediones, and implications for intersection of chronic and inflammatory disease. Mol. Metab. 2022, 55, 101409. [Google Scholar] [CrossRef]
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2019. Diabetes Care 2019, 42 (Suppl. 1), S13–S28. [Google Scholar] [CrossRef]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef]
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA 2001, 285, 2486–2497. [Google Scholar] [CrossRef]
- Bedogni, G.; Bellentani, S.; Miglioli, L.; Masutti, F.; Passalacqua, M.; Castiglione, A.; Tiribelli, C. The Fatty Liver Index: A simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006, 6, 33. [Google Scholar] [CrossRef]
- Von-Hafe, M.; Borges-Canha, M.; Vale, C.; Leite, A.R.; Sergio Neves, J.; Carvalho, D.; Leite-Moreira, A. Nonalcoholic Fatty Liver Disease and Endocrine Axes-A Scoping Review. Metabolites 2022, 12, 298. [Google Scholar] [CrossRef]
- Sabir, N.; Sermez, Y.; Kazil, S.; Zencir, M. Correlation of abdominal fat accumulation and liver steatosis: Importance of ultrasonographic and anthropometric measurements. Eur. J. Ultrasound. 2001, 14, 121–128. [Google Scholar] [CrossRef]
- Shao, C.; Ye, J.; Li, F.; Feng, S.; Wang, W.; Zhong, B. Different predictors of steatosis and fibrosis severity among lean, overweight and obese patients with nonalcoholic fatty liver disease. Dig. Liver Dis. 2019, 51, 1392–1399. [Google Scholar] [CrossRef] [PubMed]
- Sookoian, S.; Pirola, C.J. Systematic review with meta-analysis: Risk factors for non-alcoholic fatty liver disease suggest a shared altered metabolic and cardiovascular profile between lean and obese patients. Aliment. Pharmacol. Ther. 2017, 46, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Sheng, G.; Xie, Q.; Wang, R.; Hu, C.; Zhong, M.; Zou, Y. Waist-to-height ratio and non-alcoholic fatty liver disease in adults. BMC Gastroenterol. 2021, 21, 239. [Google Scholar] [CrossRef] [PubMed]
- Cholongitas, E.; Tsilingiris, D.; Diamantopoulou, P.; Mastrogianni, E.; Tentolouris, A.; Karagiannakis, D.; Moyssakis, I.; Papatheodoridis, G.V.; Tentolouris, N. Association of cardiovascular factors in diabetic patients with non-alcoholic fatty liver disease. Hormones 2022, 21, 133–145. [Google Scholar] [CrossRef]
- Kumar, R.; Porwal, Y.C.; Dev, N.; Kumar, P.; Chakravarthy, S.; Kumawat, A. Association of high-sensitivity C-reactive protein (hs-CRP) with non-alcoholic fatty liver disease (NAFLD) in Asian Indians: A cross-sectional study. J. Fam. Med. Prim. Care 2020, 9, 390–394. [Google Scholar] [CrossRef]
- Yeniova, A.O.; Kucukazman, M.; Ata, N.; Dal, K.; Kefeli, A.; Basyigit, S.; Aktaş, B.; Ağladioğlu, K.; Akin, K.O.; Ertugrul, D.T.; et al. High-sensitivity C-reactive protein is a strong predictor of non-alcoholic fatty liver disease. Hepatogastroenterology 2014, 61, 422–425. [Google Scholar]
- Lee, J.; Yoon, K.; Ryu, S.; Chang, Y.; Kim, H.R. High-normal levels of hs-CRP predict the development of non-alcoholic fatty liver in healthy men. PLoS ONE 2017, 12, e0172666. [Google Scholar] [CrossRef]
- Chen, S.; Guo, H.; Xie, M.; Zhou, C.; Zheng, M. Neutrophil: An emerging player in the occurrence and progression of metabolic associated fatty liver disease. Int. Immunopharmacol. 2021, 97, 107609. [Google Scholar] [CrossRef]
- Hwang, S.; Yun, H.; Moon, S.; Cho, Y.E.; Gao, B. Role of Neutrophils in the Pathogenesis of Nonalcoholic Steatohepatitis. Front. Endocrinol. 2021, 12, 751802. [Google Scholar] [CrossRef]
- Yang, N.; Lu, Y.; Cao, L.; Lu, M. The association between non-alcoholic fatty liver disease and serum ferritin levels in American adults. J. Clin. Lab. Anal. 2022, 36, e24225. [Google Scholar] [CrossRef]
- Yoneda, M.; Nozaki, Y.; Endo, H.; Mawatari, H.; Iida, H.; Fujita, K.; Yoneda, K.; Takahashi, H.; Kirikoshi, H.; Inamori, M.; et al. Serum ferritin is a clinical biomarker in Japanese patients with nonalcoholic steatohepatitis (NASH) independent of HFE gene mutation. Dig. Dis. Sci. 2010, 55, 808–814. [Google Scholar] [CrossRef] [PubMed]
- Du, S.X.; Lu, L.L.; Geng, N.; Victor, D.W.; Chen, L.Z.; Wang, C.; Yue, H.-Y.; Xin, Y.-N.; Xuan, S.-Y.; Jin, W.W. Association of serum ferritin with non-alcoholic fatty liver disease: A meta-analysis. Lipids Health Dis. 2017, 16, 228. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Age, years | 42.7 ± 10.5 |
| Feminine sex, n (%) | 1846 (84.6) |
| Weight, kg | 115.1 ± 18.6 |
| Body mass index, kg/m2 | 43.5 ± 5.8 |
| Waist circumference, cm | 123.1 ± 25.1 |
| Hip circumference, cm | 131.5 ± 11.8 |
| Waist-to-hip ratio | 0.9 ± 0.2 |
| Diabetes, n (%) | 521 (33.3) |
| Dyslipidemia, n (%) | 974 (45.6) |
| Hypertension, n (%) | 1136 (66.9) |
| Smoker *, n (%) | 307 (15.3) |
| C-reactive protein, mg/L | 8.4 [4.9, 14.0] |
| Leucocytes, ×109/L | 8.1 ± 3.2 |
| Ferritin, ng/mL | 87 [39, 166] |
| AST, U/L | 22 [18, 28] |
| ALT, U/L | 24 [17, 35] |
| GGT, U/L | 27 [19, 41] |
| BARD, n (%) | |
| 1 | 331 (22.1) |
| 2 | 221 (14.8) |
| 3 | 666 (44.5) |
| 4 | 278 (18.6) |
| FLI | 97.4 [93.9, 99.1] |
| FLI | BARD | |||
|---|---|---|---|---|
| β | p-Value | OR | p-Value | |
| Waist circumference, cm | ||||
| Non-adjusted | 0.10 (0.09, 0.11) | <0.01 | 0.99 (0.98, 0.99) | 0.016 |
| Model 1 | 0.31 (0.29, 0.33) | <0.01 | 1.00 (0.99, 1.01) | 0.329 |
| Model 2 | 0.30 (0.28, 0.32) | <0.01 | 1.00 (0.99, 1.00) | 0.335 |
| Hip circumference, cm | ||||
| Non-adjusted | 0.22 (0.20, 0.25) | <0.01 | 1.00 (0.99, 1.01) | 0.888 |
| Model 1 | 0.22 (0.20, 0.25) | <0.01 | 1.00 (0.99, 1.01) | 0.943 |
| Model 2 | 0.22 (0.19, 0.24) | <0.01 | 1.00 (0.99, 1.01) | 0.752 |
| Waist-to-hip ratio | ||||
| Non-adjusted | 8.68 (6.85, 10.52) | <0.01 | 0.26 (0.09, 0.73) | 0.011 |
| Model 1 | 28.80 (25.06, 32.54) | <0.01 | 2.25 (0.61, 8.26) | 0.220 |
| Model 2 | 27.48 (23.48, 31.48) | <0.01 | 0.57 (0.15, 2.24) | 0.425 |
| FLI | BARD | |||
|---|---|---|---|---|
| β | p-Value | OR | p-Value | |
| C-reactive protein, mg/L | ||||
| Non-adjusted | 0.16 (0.11, 0.21) | <0.01 | 1.02 (1.01, 1.04) | <0.01 |
| Model 1 | 0.17 (0.12, 0.22) | <0.01 | 1.03 (1.01, 1.04) | <0.01 |
| Model 2 | 0.14 (0.09, 0.19) | <0.01 | 1.02 (1.00, 1.04) | 0.031 |
| Leucocytes, ×109/L | ||||
| Non-adjusted | 0.11 (−0.01, 0.22) | 0.063 | 0.97 (0.93, 1.01) | 0.109 |
| Model 1 | 0.19 (0.06, 0.32) | <0.01 | 0.99 (0.95, 1.04) | 0.734 |
| Model 2 | 0.24 (0.11, 0.38) | <0.01 | 0.96 (0.01, 1.01) | 0.097 |
| Ferritin, ng/mL | ||||
| Non-adjusted | 0.01 (0.01, 0.02) | <0.01 | 1.00 (1.00, 1.00) | 0.07 |
| Model 1 | 0.01 (−0.00, 0.01) | 0.098 | 1.00 (1.00, 1.00) | 0.611 |
| Model 2 | 0.002 (−0.01, 0.01) | 0.631 | 1.00 (1.00, 1.00) | 0.839 |
| FLI | ||
|---|---|---|
| β | p-Value | |
| C-reactive protein, mg/L | 0.05 (0.01, 0.10) | 0.026 |
| Waist circumference, cm | 0.31 (0.28, 0.37) | <0.01 |
| Sex | 1.05 (−0.28, 2.39) | 0.123 |
| Age, years | 0.03 (−0.02, 0.07) | 0.236 |
| Diabetes | 0.22 (−0.81, 1.24) | 0.679 |
| Dyslipidemia | 1.94 (1.02, 2.86) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borges-Canha, M.; Neves, J.S.; Silva, M.M.; Mendonça, F.; Moreno, T.; Ribeiro, S.; Correa, J.; Vale, C.; Gonçalves, J.; Urbano Ferreira, H.; et al. Waist-to-Hip Ratio and Inflammatory Parameters Are Associated with Risk of Non-Alcoholic Fatty Liver Disease in Patients with Morbid Obesity. Biomedicines 2022, 10, 2416. https://doi.org/10.3390/biomedicines10102416
Borges-Canha M, Neves JS, Silva MM, Mendonça F, Moreno T, Ribeiro S, Correa J, Vale C, Gonçalves J, Urbano Ferreira H, et al. Waist-to-Hip Ratio and Inflammatory Parameters Are Associated with Risk of Non-Alcoholic Fatty Liver Disease in Patients with Morbid Obesity. Biomedicines. 2022; 10(10):2416. https://doi.org/10.3390/biomedicines10102416
Chicago/Turabian StyleBorges-Canha, Marta, João Sérgio Neves, Maria Manuel Silva, Fernando Mendonça, Telma Moreno, Sara Ribeiro, João Correa, Catarina Vale, Juliana Gonçalves, Helena Urbano Ferreira, and et al. 2022. "Waist-to-Hip Ratio and Inflammatory Parameters Are Associated with Risk of Non-Alcoholic Fatty Liver Disease in Patients with Morbid Obesity" Biomedicines 10, no. 10: 2416. https://doi.org/10.3390/biomedicines10102416
APA StyleBorges-Canha, M., Neves, J. S., Silva, M. M., Mendonça, F., Moreno, T., Ribeiro, S., Correa, J., Vale, C., Gonçalves, J., Urbano Ferreira, H., Gil-Santos, S., Guerreiro, V., Sande, A., B. Souto, S., Pedro, J., Freitas, P., Carvalho, D., & CRIO Group. (2022). Waist-to-Hip Ratio and Inflammatory Parameters Are Associated with Risk of Non-Alcoholic Fatty Liver Disease in Patients with Morbid Obesity. Biomedicines, 10(10), 2416. https://doi.org/10.3390/biomedicines10102416





